Published online Feb 6, 2023. doi: 10.12998/wjcc.v11.i4.844
Peer-review started: August 24, 2022
First decision: December 13, 2022
Revised: December 31, 2022
Accepted: January 16, 2023
Article in press: January 16, 2023
Published online: February 6, 2023
Processing time: 157 Days and 17.2 Hours
Viral pleurisy is a viral infected disease with exudative pleural effusions. It is one of the causes for pleural effusions. Because of the difficult etiology diagnosis, clinically pleural effusions tend to be misdiagnosed as tuberculous pleurisy or idiopathic pleural effusion. Here, we report a case of pleural effusion secondary to viral pleurisy which is driven by infection with epstein-barr virus. Viral infection was identified by metagenomic next-generation sequencing (mNGS).
A 40-year-old male with a history of dermatomyositis, rheumatoid arthritis, and secondary interstitial pneumonia was administered with long-term oral pred
Viral infection should be considered in cases of idiopathic pleural effusion unex
Core Tip: Pleural effusion is a common clinical symptom, and infectious pleurisy is one of the reasons. Its pathogen is difficult to be found by microbiological examination, and the diagnosis of viral pleural effusion is particularly difficult. Epstein-barr virus is latent infection in most adults, and it is easy to be reactivated in people with immune deficiency, which may cause infection in all parts of the body. When idiopathic pleural effusion is not clearly diagnosed through routine examination, the possibility of viral infection should be considered, and early improvement of metagenomic next-generation sequencing examination is helpful for the diagnosis.
- Citation: Liu XP, Mao CX, Wang GS, Zhang MZ. Metagenomic next-generation sequencing for pleural effusions induced by viral pleurisy: A case report. World J Clin Cases 2023; 11(4): 844-851
- URL: https://www.wjgnet.com/2307-8960/full/v11/i4/844.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i4.844
Pleural effusion is a relatively common clinical condition. Even after invasive procedures, such as thoracoscopy, are used, the cause of the pleural effusion cannot be established in up to 15% of patients[1]. It has been reported that virus infection is one of the causes of pleural effusion. Due to the difficulty in virus separation and culture, clinical diagnosis is challenged. Next-generation sequencing (NGS) technology makes it possible to comprehensively analyze the sequence data of nucleic acids in samples in a single assay. Therefore, untargeted metagenomic NGS (mNGS) of clinical samples has been applied for the comprehensive diagnosis of infections, including viruses, bacteria, fungi, and parasites. To our knowledge, this is the first report of diagnosis of pleural effusion induced by viral pleurisy by mNGS.
In May, 2021, a male, 40-year-old was transferred to the Institute of Respiratory Disease of Xinqiao Hospital, Third Military Medical University for pleural effusion of unknown etiology.
On May 6, he had fever after exposure to cold, accompanied by generalized sore and weakness, night sweats, sometimes cough, and few sputums. He felt chest tightness and shortness of breath after exercise. The symptoms were improved after infusion in a local clinic. On May 10, COVID-19 vaccine was administered, and he then developed fever with the highest temperature of 38 ℃. In the meantime, the above symptoms relapsed, along with the concomitant chest pain. There was no evidence of chills, purulent sputum, bloody expectoration, headache, dizziness, abdominal pain or diarrhea.
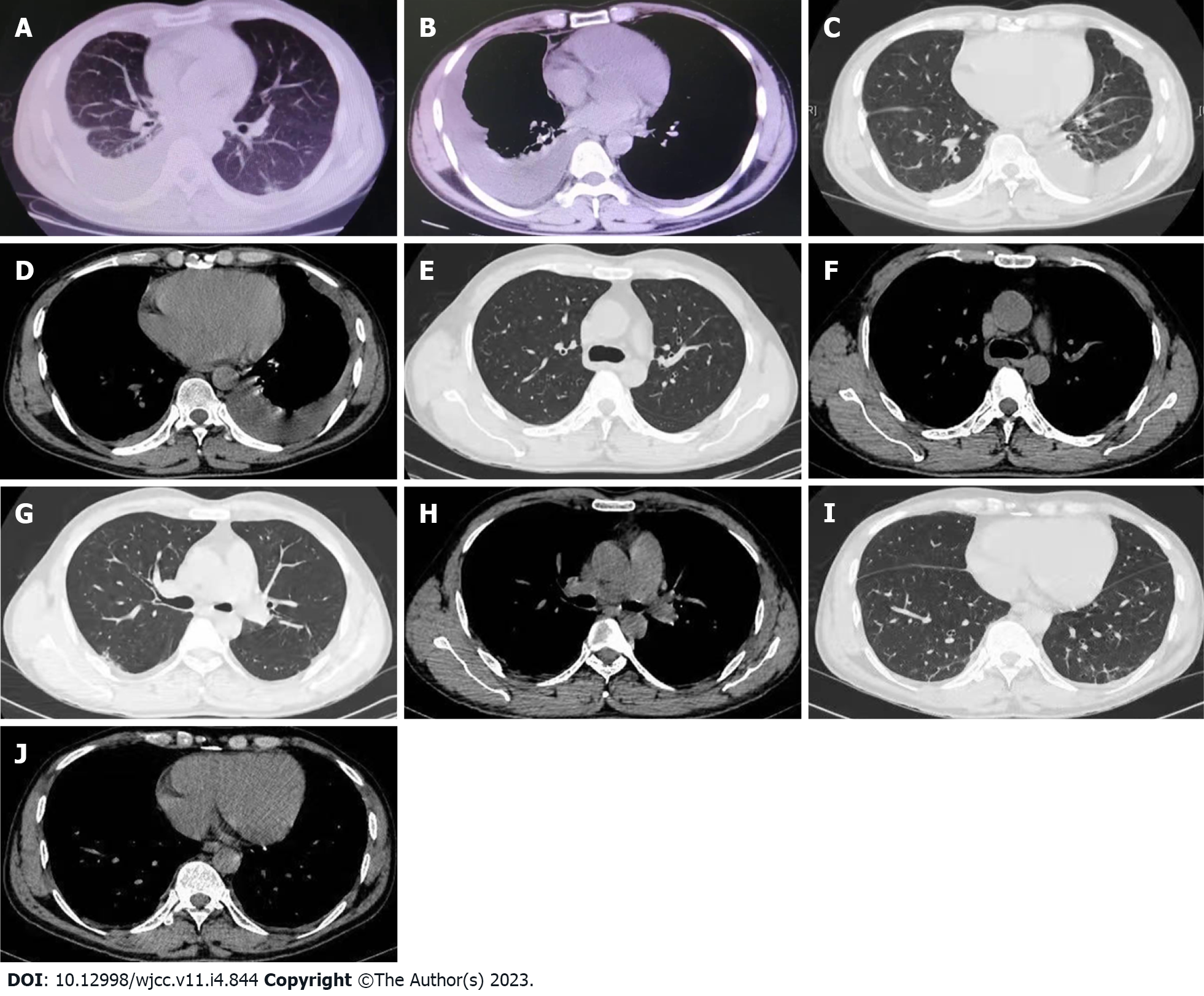
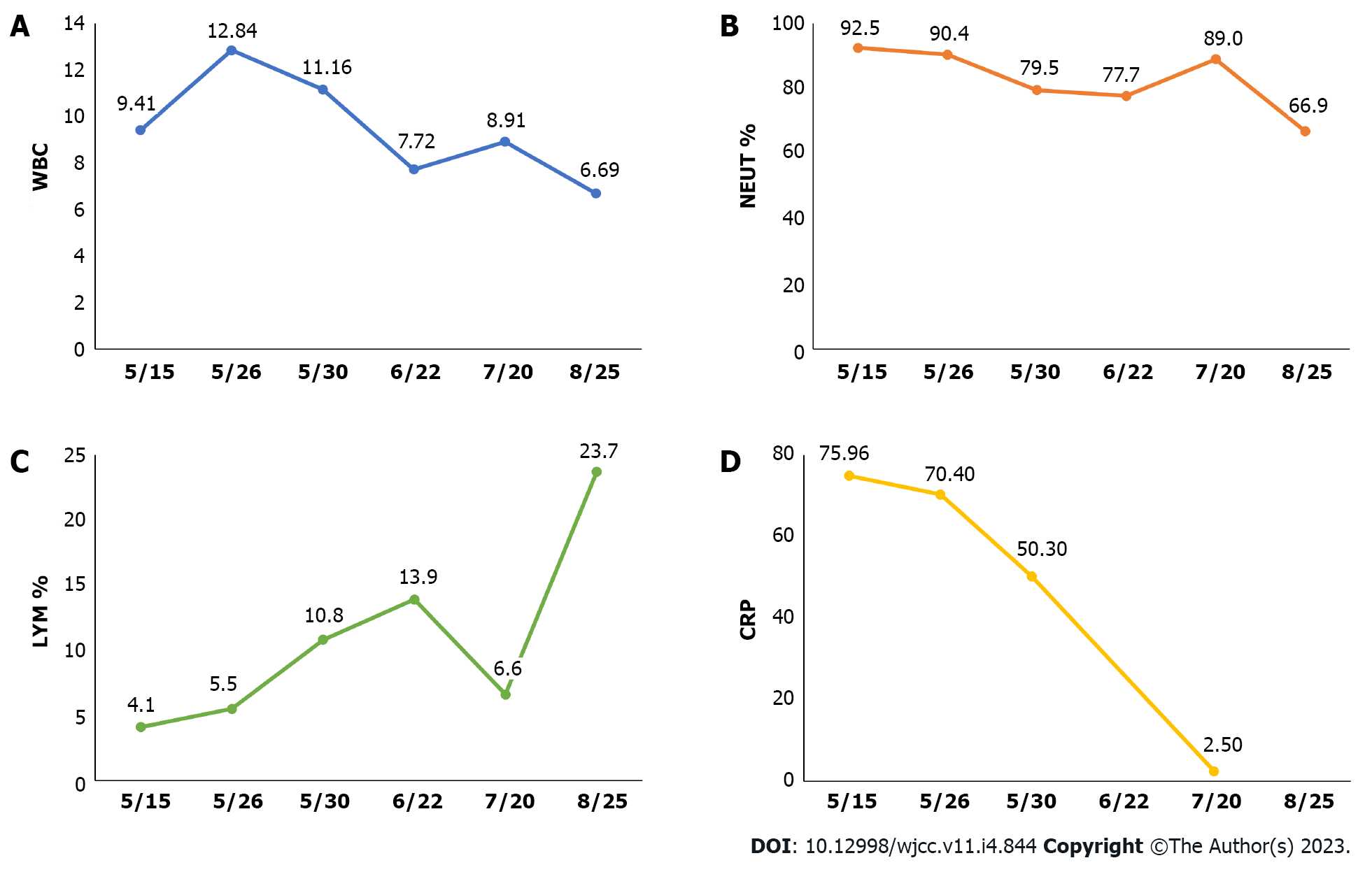
On May 14, he visited the local hospital. Blood routine tests showed white blood cell (WBC) 9.41 × 109/L, hemoglobin 146 g/L, platelet 279 × 109/L, neutrophils (NEUT) 92.5%, lymphocytes (LYM) 4.1% and eosinophil (EO) 0.2%. Inflammatory indices increased, as demonstrated by C-reactive protein (CRP) 74.96 mg/L, erythrocyte sedimentation rate (ESR) 64 mm/H and procalcitonin 0.24 ng/mL. On chest computed tomography (CT) (plain scan), there was bilateral pleural effusions, with more effusions on the right side, and atelectasis of the partial right lower lobe was revealed (Figure 1A and B). Right pleural catheterization was performed. Approximately 600 mL effusions were drained, and they were in yellow and slightly cloudy with some clots. Pleural fluid routine examination revealed positive Rivalta test, red blood cell (RBC) 0, WBC 3292 × 106/L, multinuclear cells 44.7%, monocyte 55.3%, adenosine deaminase (ADA) 55 U/L, lactate dehydrogenase (LDH) 1132U/L, carcinoembryonic antigen (CEA) 3.3 ng/mL, neuron-specificenolase (NSE) 43.39 ng/mL, cytokeratin-19-fragment 56.4 ng/mL, and squamous cell carcinoma associated antigen 2.1 ng/mL. Antibody negative for Mycobacterium tuberculosis was obtained. The patient was assigned to take symptomatic treatment. Cough remained to occur intermittently while the feelings of tired and panting after exercise were milder.
The patient was diagnosed with dermatomyositis, rheumatoid arthritis, and secondary interstitial pneumonia one year before and had a long-term history of oral Prednison Tablet (12.5 mg). He was diagnosed with hypothyroidism 5 years before and confirmed to have autoimmune thyroiditis 4 years later. Long-term oral Euthyrox at 100 μg once daily was understood. Additionally, he was diagnosed with vitiligo 2 mo before the admission.
He had a 20-year history of smoking (1 packet daily) and had no history of alcohol abuse.
Physical examination was insignificant.
Blood routine presented increasing WBC count (WBC 12.84 × 109/L, NEUT% 90.4%, LYM% 5.5%, and EO% 0.1%). Inflammatory indices were CRP 70.4 mg/L and ESR 19 mm/H. Positive Rivalta test was obtained in pleural fluid analysis, along with proteins 54.90 g/L, WBC 10141 × 106/L (NEUT% 68.40%, LYM% 21.90%, monocyte-macrophage 8.80%, EO% 0.90%), multinuclear cells 54.0%, monocyte 46.0%, ADA 53U/L, LDH 1717.4 U/L, and CEA 3.79 ng/mL. Acid-fast staining, bacterial culture and fungal culture in pleural fluid were all negative.
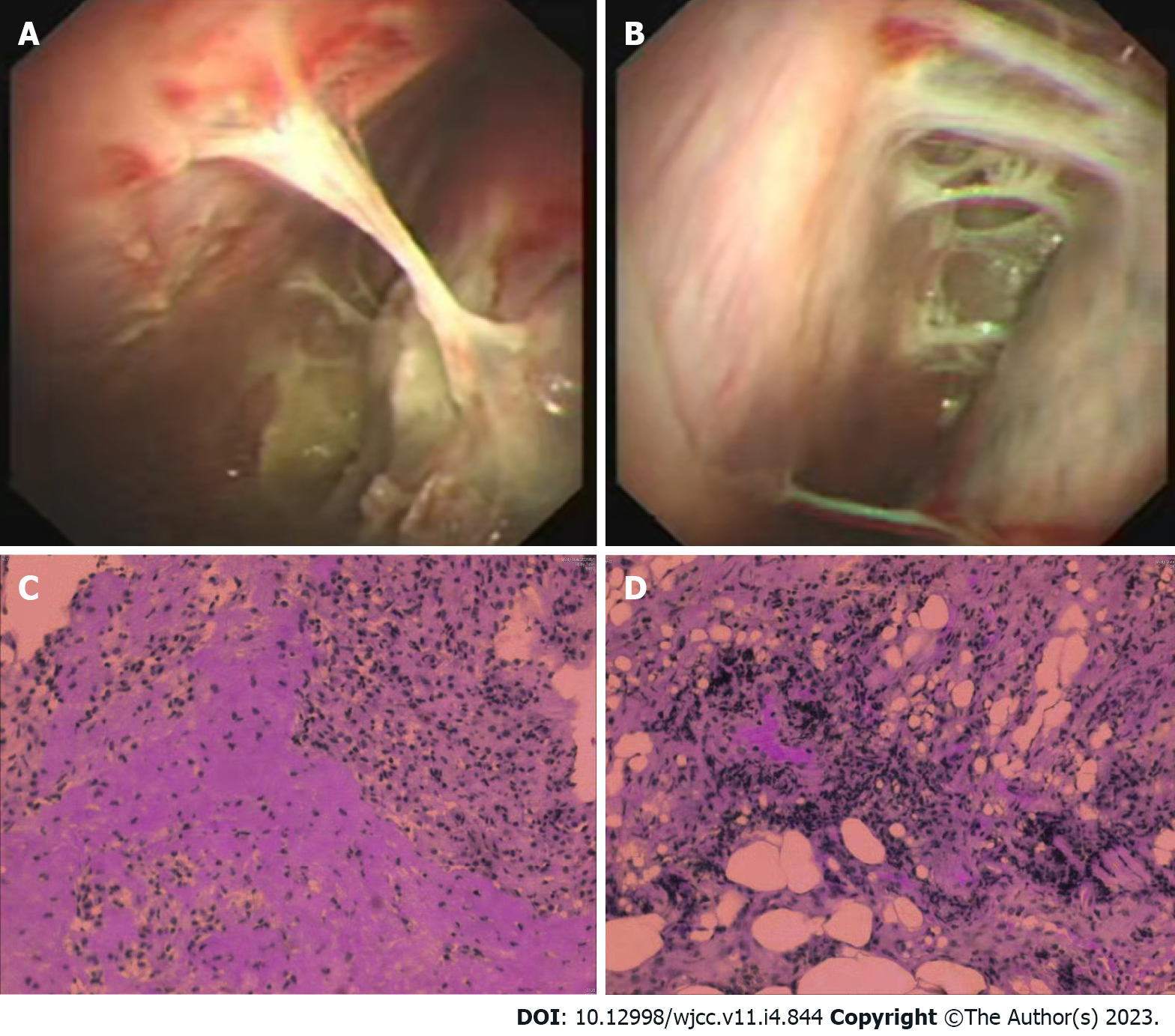
On May 26, he was transferred to the Respiratory Department of our hospital. Chest CT revealed bilateral pleural thickening and adhesion, bilateral pleural effusions while with more effusions on the left side (Figure 1C and D). Left pleural catheterization was therefore scheduled to find light yellow and slightly cloudy fluid. On the thoracoscopy, the left pleural cavity had more fibrillar adhesion bands, extensive auto-pleurodesis, cavity wall pleural surface thickening, and dispersed carbon deposition on the visceral pleural surface (Figure 2A and B).
The pleural fluid was sent to an independent clinical laboratory (ChongQing KingMed Center for Clinical Laboratory) for mNGS analysis using Illumina platform. There was one sequencing read of epstein-barr virus (EBV) and one sequencing read of Staphylococcus epidermidis (S. epidermidis) detected.
The empyema caused by infection with S. epidermidis was not supported by the patient clinical symptoms and thoracoscopic findings. Combining the results in pleural fluid analysis and mNGS, and the previous long-term use of prednison which could induce immunosuppression, the patient was diagnosed as viral infection-induced pleural effusion.
Oral routine dose aciclovir tablet was scheduled on May 30.
On May 30, blood routine tests showed WBC 11.16 × 109/L, NEUT% 79.5%, LYM% 10.8%, EO% 0.6%, and CRP 50.3 mg/L. On June 1, pleural biopsy was performed and revealed dominant infiltration of lymphocytes (major in small B lymphocytes) and tissue cells, slightly increasing CD8+ T lymphocytes and no natural killer (NK) cells (Figure 2C and D). Immunohistochemistry for cytomegalovirus and in situ hybridization for EBV were negative. Given the remarkably decreasing pleural effusions and the self-limited property of viral pleurisy, the patient was allowed to discharge on June 3.
On June 22, the blood routine examination showed that the WBC decreased: WBC 7.72 × 109/L, NEUT% 77.7%, LYM% 13.9%, and EO% 1.4%. On July 20, the blood routine tests showed WBC 8.91 × 109/L, NEUT% 89.0%, LYM% 6.6%, EO% 0.4%, CRP 2.5 mg/L. Chest CT plain scan showed decreasing bilateral pleural effusions and reducing atelectasis of the left lower lobe (Figure 1E-J). The results of blood routine examination on August 25 returned to normal levels: WBC 6.69 × 109/L, NEUT% 66.9%, LYM% 23.7%, and EO% 1.5% (Figure 3).
Pleural effusion is a common clinical manifestation of multiple diseases involved in pleura, the lung and the whole body. Predisposing factors mainly include heart failure, infectious causes, malignancy, pulmonary embolism, liver cirrhosis, subdiaphragmatic abscess and pancreatitis. Due to the non-specific symptoms, such as cough, dyspnoea and chest pain, the diagnosis of pleural effusion is required by both physical examination and laboratory tests[2]. In most cases, the etiology can be identified by clinical information, imaging techniques and pleural fluid analysis[3]. However, there is still a part that cannot be explained, despite extensive workup, including thoracoscopy or invasive tests (such as pleural biopsy)[1]. Generally, cases which fail to be explained by routine clinical evaluation is defined as idiopathic pleural effusions. Infectious pleurisy is the most common cause of exudative pleural effusion, while the responsible pathogen might be hard to detect by routine microbiologic test.
The viral infection-associated pleural effusion can be a result of the viral inflammation in adjacent tissue which extends to the pleura or the allergic reaction induced by viral infection. In those ways, the pleural effusions associated with viral pleurisy can occur independently without intrapulmonary infiltrates foci. As viral pleurisy is self-limited, the related pleural effusion can resolve spontaneously within two weeks. Additionally, some cases have less effusion, which can be absorbed rapidly, making it relatively concealed in clinic. Cohen et al[4] reported that the incidence of pleural effusion after viral pneumonia was 18% according to the radiological findings in the lateral recumbent position, while 2%-9% as recognized.
It has been reported that a variety of viruses could induce viral pleurisy, especially in immunocompromised patients, including influenza viruses[5], coxsackievirus, respiratory syncytial virus[6], cytomegalovirus[7], herpes simplex virus[8], EBV, adenovirus[9], human herpesvirus-8. In this viral-infected population of pleural effusion, clinical diagnosis is challenged. Reasons can be the wide variety of viruses, and difficulty in virus separation and culture. Under these limitations, only a small number of viruses are detectable in specific antibody test and nucleic acid PCR, which, to some extent, makes clinical diagnosis harder. In a prospective study[10], the authors reported that the EBV-positive rate was 40% in the pleural fluid samples of patients with pleural effusions, and the EBV-positive rate reached 59% among patients with unexplained effusions according to the PCR tests.
EBV is widespread, and more than 90% of the worldwide adult population are infected with EBV[11]. EBV is mostly transmitted through saliva, and it could establish a lifelong latent infection that reactivates intermittently to lytic replication. Infancy and childhood are usually the times when EBV primary infections occur subclinically. After primary infection, EBV uses latent infection as an immune evasion strategy to prevent cytotoxic T-cell elimination of infected cells[12]. The amount of EBV latently infected cells remains stable over years, but may vary depending on the individuals[13]. EBV viral loads in normal adults (healthy carriers) are usually undetectable, with 0.1-24.0 Latently infected B-cells per million peripheral blood mononuclear cells (PBMC) in the circulation[14] and low numbers of viral genomes per infected cell[15].
EBV systemic reactivation is possible when the cellular immune response is compromised, for example, in patients who are given bone marrow transplantation[16], patients with solid transplants[17], patients infected with HIV[18] or patients with chronic active EBV infections[19]. In addition, local reactivation of EBV takes place periodically in the oropharynx in EBV-infected healthy individuals[10], this is probably due to insufficient T-cell control in the saliva[20]. It is possible for EBV to infect almost any organ, and complications may result from infection. EBV infection has been found in the pleural space in association with B-cell lymphomas, including primary effusion lymphoma[21,22] and phyotorax-associated lymphoma[23]. However, the role of EBV in nonlymphoma pleural effusions has not been extensively studied. Interstitial pneumonitis has been associated with chronic active EBV infection and primary infection, both in children and in adults, and pleural effusion has been observed as a rare complication of EBV infection[24,25].
In the present case, mNGS detected one EBV sequence whereas the in situ hybridization test for EBV in pleural tissue was negative. Possible reason might be the low viral load in pleural fluid and the limited diagnostic sensitivity of the in situ hybridization test. According to the previous report, the majority of EBV-positive pleural fluid has a low viral load[10]. Here, the pleural effusion in the patient was gradually resolved in 1 mo, along with recovered WBC and CRP levels, consistent with the self-limited characteristic of the viral pleurisy.
Current reports have shown that viral infection might be the main culprit of unexplained pleural effusion. Hence, viral infection should be considered in cases of idiopathic pleural effusion unexplained by routine examination, so as to prevent misdiagnosis and missed diagnosis, in the meantime, decrease repeated pleural fluid analysis and avoid additional invasive tests.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Respiratory system
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kirkik D, Turkey S-Editor: Chen YL L-Editor: A P-Editor: Chen YL
| 1. | Light RW. Clinical practice. Pleural effusion. N Engl J Med. 2002;346:1971-1977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 458] [Cited by in RCA: 405] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 2. | Rahman NM, Chapman SJ, Davies RJ. Pleural effusion: a structured approach to care. Br Med Bull. 2004;72:31-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 55] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 3. | Collins TR, Sahn SA. Thoracocentesis. Clinical value, complications, technical problems, and patient experience. Chest. 1987;91:817-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 150] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 4. | Cohen M, Sahn SA. Resolution of pleural effusions. Chest. 2001;119:1547-1562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 62] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 5. | Choi MJ, Lee YS, Lee JY, Lee KS. Novel influenza A (H1N1) virus infection in children: chest radiographic and CT evaluation. Korean J Radiol. 2010;11:656-664. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Vieira SE, Stewien KE, Queiroz DA, Durigon EL, Török TJ, Anderson LJ, Miyao CR, Hein N, Botosso VF, Pahl MM, Gilio AE, Ejzenberg B, Okay Y. Clinical patterns and seasonal trends in respiratory syncytial virus hospitalizations in São Paulo, Brazil. Rev Inst Med Trop Sao Paulo. 2001;43:125-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Franquet T, Lee KS, Müller NL. Thin-section CT findings in 32 immunocompromised patients with cytomegalovirus pneumonia who do not have AIDS. AJR Am J Roentgenol. 2003;181:1059-1063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 84] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 8. | Aquino SL, Dunagan DP, Chiles C, Haponik EF. Herpes simplex virus 1 pneumonia: patterns on CT scans and conventional chest radiographs. J Comput Assist Tomogr. 1998;22:795-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 42] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Hong JY, Lee HJ, Piedra PA, Choi EH, Park KH, Koh YY, Kim WS. Lower respiratory tract infections due to adenovirus in hospitalized Korean children: epidemiology, clinical features, and prognosis. Clin Infect Dis. 2001;32:1423-1429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 156] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 10. | Thijsen SF, Luderer R, van Gorp JM, Oudejans SJ, Bossink AW. A possible role for Epstein-Barr virus in the pathogenesis of pleural effusion. Eur Respir J. 2005;26:662-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Cohen JI. Epstein-Barr virus infection. N Engl J Med. 2000;343:481-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1156] [Cited by in RCA: 1170] [Article Influence: 46.8] [Reference Citation Analysis (0)] |
| 12. | Thorley-Lawson DA, Gross A. Persistence of the Epstein-Barr virus and the origins of associated lymphomas. N Engl J Med. 2004;350:1328-1337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 735] [Cited by in RCA: 739] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 13. | Wagner HJ, Bein G, Bitsch A, Kirchner H. Detection and quantification of latently infected B lymphocytes in Epstein-Barr virus-seropositive, healthy individuals by polymerase chain reaction. J Clin Microbiol. 1992;30:2826-2829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 90] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 14. | Yang J, Tao Q, Flinn IW, Murray PG, Post LE, Ma H, Piantadosi S, Caligiuri MA, Ambinder RF. Characterization of Epstein-Barr virus-infected B cells in patients with posttransplantation lymphoproliferative disease: disappearance after rituximab therapy does not predict clinical response. Blood. 2000;96:4055-4063. [RCA] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 106] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 15. | Miyashita EM, Yang B, Lam KM, Crawford DH, Thorley-Lawson DA. A novel form of Epstein-Barr virus latency in normal B cells in vivo. Cell. 1995;80:593-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1291] [Cited by in RCA: 1227] [Article Influence: 40.9] [Reference Citation Analysis (0)] |
| 16. | van Esser JW, van der Holt B, Meijer E, Niesters HG, Trenschel R, Thijsen SF, van Loon AM, Frassoni F, Bacigalupo A, Schaefer UW, Osterhaus AD, Gratama JW, Löwenberg B, Verdonck LF, Cornelissen JJ. Epstein-Barr virus (EBV) reactivation is a frequent event after allogeneic stem cell transplantation (SCT) and quantitatively predicts EBV-lymphoproliferative disease following T-cell--depleted SCT. Blood. 2001;98:972-978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 280] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 17. | Baldanti F, Grossi P, Furione M, Simoncini L, Sarasini A, Comoli P, Maccario R, Fiocchi R, Gerna G. High levels of Epstein-Barr virus DNA in blood of solid-organ transplant recipients and their value in predicting posttransplant lymphoproliferative disorders. J Clin Microbiol. 2000;38:613-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 124] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 18. | Ling PD, Vilchez RA, Keitel WA, Poston DG, Peng RS, White ZS, Visnegarwala F, Lewis DE, Butel JS. Epstein-Barr virus DNA loads in adult human immunodeficiency virus type 1-infected patients receiving highly active antiretroviral therapy. Clin Infect Dis. 2003;37:1244-1249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Kimura H, Hoshino Y, Kanegane H, Tsuge I, Okamura T, Kawa K, Morishima T. Clinical and virologic characteristics of chronic active Epstein-Barr virus infection. Blood. 2001;98:280-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 284] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 20. | van Kooij B, Thijsen SF, Meijer E, Niesters HG, van Esser JW, Cornelissen JJ, Verdonck LF, van Loon AM. Sequence analysis of EBV DNA isolated from mouth washings and PBMCs of healthy individuals and blood of EBV-LPD patients. J Clin Virol. 2003;28:85-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 21. | Cesarman E, Chang Y, Moore PS, Said JW, Knowles DM. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N Engl J Med. 1995;332:1186-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2214] [Cited by in RCA: 2151] [Article Influence: 71.7] [Reference Citation Analysis (0)] |
| 22. | Renne R, Zhong W, Herndier B, McGrath M, Abbey N, Kedes D, Ganem D. Lytic growth of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) in culture. Nat Med. 1996;2:342-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 817] [Cited by in RCA: 808] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 23. | Androulaki A, Drakos E, Hatzianastassiou D, Vgenopoulou S, Gazouli M, Korkolopoulou P, Patsouris E, Dosios T. Pyothorax-associated lymphoma (PAL): a western case with marked angiocentricity and review of the literature. Histopathology. 2004;44:69-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | Cloney DL, Kugler JA, Donowitz LG, Lohr JA. Infectious mononucleosis with pleural effusion. South Med J. 1988;81:1441-1442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 25. | Chen J, Konstantinopoulos PA, Satyal S, Telonis J, Blair DC. Just another simple case of infectious mononucleosis? Lancet. 2003;361:1182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |