Published online Feb 6, 2023. doi: 10.12998/wjcc.v11.i4.788
Peer-review started: November 18, 2022
First decision: December 13, 2022
Revised: December 27, 2022
Accepted: January 12, 2023
Article in press: January 12, 2023
Published online: February 6, 2023
Processing time: 79 Days and 17.6 Hours
Cervical pregnancies, interstitial tubal pregnancies, and cesarean scar preg
To examine the efficacy and safety of ultrasound-guided topical absolute ethanol injection in ectopic pregnancies with an intrauterine implantation site.
In this study, we retrospectively examined the medical records of 21 patients who were diagnosed with an ectopic pregnancy with an intrauterine implantation site at our hospital, between April 2010 and December 2018, and underwent tran
There were 21 total cases comprising 10 cervical pregnancies, 10 interstitial tubal pregnancies, and 1 cesarean scar pregnancy. All patients completed treatment with this method. No massive hemorrhaging or serious adverse reactions were observed during treatment. The mean gestation ages at the time of diagnosis were 5.9 wk (SD, ± 0.9 wk) for cervical and 6.9 wk (SD, ± 2.1 wk) for interstitial tubal pregnancies. The total ethanol doses were 4.8 mL (SD, ± 2.2 mL) for cervical pregnancies and 3.3 mL (SD, ± 2.2 mL) for interstitial pregnancies. The treatment period was 28.5 days (SD, ± 11.7 d) for cervical pregnancies and 30.0 ± 8.1 d for interstitial pregnancies. Positive correlations were observed between the blood β- human chorionic gonadotropin level at the beginning of treatment and the total ethanol dose (r = 0.75; P = 0.00008), as well as between the total ethanol dose and treatment period (r = 0.48; P = 0.026).
Transvaginal ultrasound-guided local injections of absolute ethanol could become a new option for intrauterine ectopic pregnancies when fertility preservation is desired.
Core Tip: Transvaginal ultrasound-guided local injections of absolute ethanol for ectopic pregnancies such as cervical pregnancies, interstitial tubal pregnancies, and cesarean scar pregnancies can preserve the uterus without serious adverse reactions. This treatment avoids the complications caused by methotrexate therapy and uterine artery embolization. This treatment may become a new treatment option for intrauterine ectopic pregnancy when fertility preservation is desired.
- Citation: Kakinuma T, Kakinuma K, Matsuda Y, Yanagida K, Ohwada M, Kaijima H. Efficacy of transvaginal ultrasound-guided local injections of absolute ethanol for ectopic pregnancies with intrauterine implantation sites. World J Clin Cases 2023; 11(4): 788-796
- URL: https://www.wjgnet.com/2307-8960/full/v11/i4/788.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i4.788
Ectopic pregnancy occurs in approximately 1% of all pregnancies and the majority of these are tubal pregnancies. Although cervical, interstitial tubal, and cesarean scar pregnancy, which are ectopic pregnancies with intrauterine implantation sites, are rare, they have exhibited an increasing trend with the recent widespread use of assisted reproductive technologies and the increased rate of cesarean delivery[1-3]. Ectopic pregnancies are serious conditions prone to massive hemorrhaging, and surgical treatments such as total hysterectomy and focal excision have been the primary standard treatment options. However, early diagnosis has become possible with the development of high-sensitivity human chorionic gonadotropin (hCG) testing reagents and the increased precision of transvaginal ultrasonic tomography, which enable treatment before clinical manifestation. Therefore, uterine preservation for the purpose of fertility preservation has become more feasible after early therapeutic intervention. There have been reports of ectopic pregnancy removal using methotrexate (MTX) therapy and uterine artery embolization (UAE), or a combination of these, when fertility preservation is desired[4-8]. However, for patients desiring fertility preservation, MTX therapy is concerning because of the associated delay in resumption of infertility treatment to avoid the possibility of decreased ovarian function and the necessary washout period[9]. Moreover, increased rates of miscarriage and placenta accreta during the next pregnancy have been associated with UAE[10]. Furthermore, MTX administration is often unsuccessful when there is fetal heart movement or when the blood hCG value is high[11-14]. Additionally, there are concerns that massive hemorrhaging may occur during treatment[15-17]. Therefore, it is important to consider the effects of treatment on fertility for those who desire uterine preservation and future pregnancies.
We have previously reported the efficacy and safety of ultrasound-guided topical injection of absolute ethanol as an alternative to topical MTX treatment for ectopic pregnancy[18]. Because this treatment has a local effect, there is no effect on ovarian function and no need for a washout period, which is required with MTX therapy. Moreover, this therapy can help the avoid negative effects on subsequent pregnancies that are otherwise associated with UAE.
The purpose of this study was to examine the efficacy and safety of ultrasound-guided topical injection of absolute ethanol in ectopic pregnancies with an implantation site within the uterus.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This study was approved by the International University of Health and Welfare Hospital Ethics Committee (referral number: 21-B-34). Written informed consent was obtained from all individual participants included in the study. We retrospectively collected and examined the medical records of patients diagnosed with ectopic pregnancy with an implantation site within the uterus (interstitial tubal pregnancy, cervical pregnancy, cesarean scar pregnancy) and who underwent transvaginal ultrasound-guided local injections of absolute ethanol at our hospital from April 2010 to December 2018. Transvaginal ultrasound-guided local injections of absolute ethanol were administered after obtaining informed consent from the patients.
The diagnostic methods for ectopic pregnancies with intrauterine implantation sites included the confirmation of fetal heart movement using transvaginal ultrasonography, evaluation of the blood flow around the gestation sac using color Doppler imaging, and performing a β-hCG assay. We evaluated the treatment methods, treatment outcomes, presence of bleeding requiring hemostasis measures and blood transfusion, complications, and treatment periods. Successful treatment was defined as the completion of treatment using transvaginal ultrasound-guided local injections of absolute ethanol alone. The treatment period was defined as the time from the initiation of treatment until a negative blood β-hCG level (detection sensitivity limit: 1.2 mIU/mL) was confirmed.
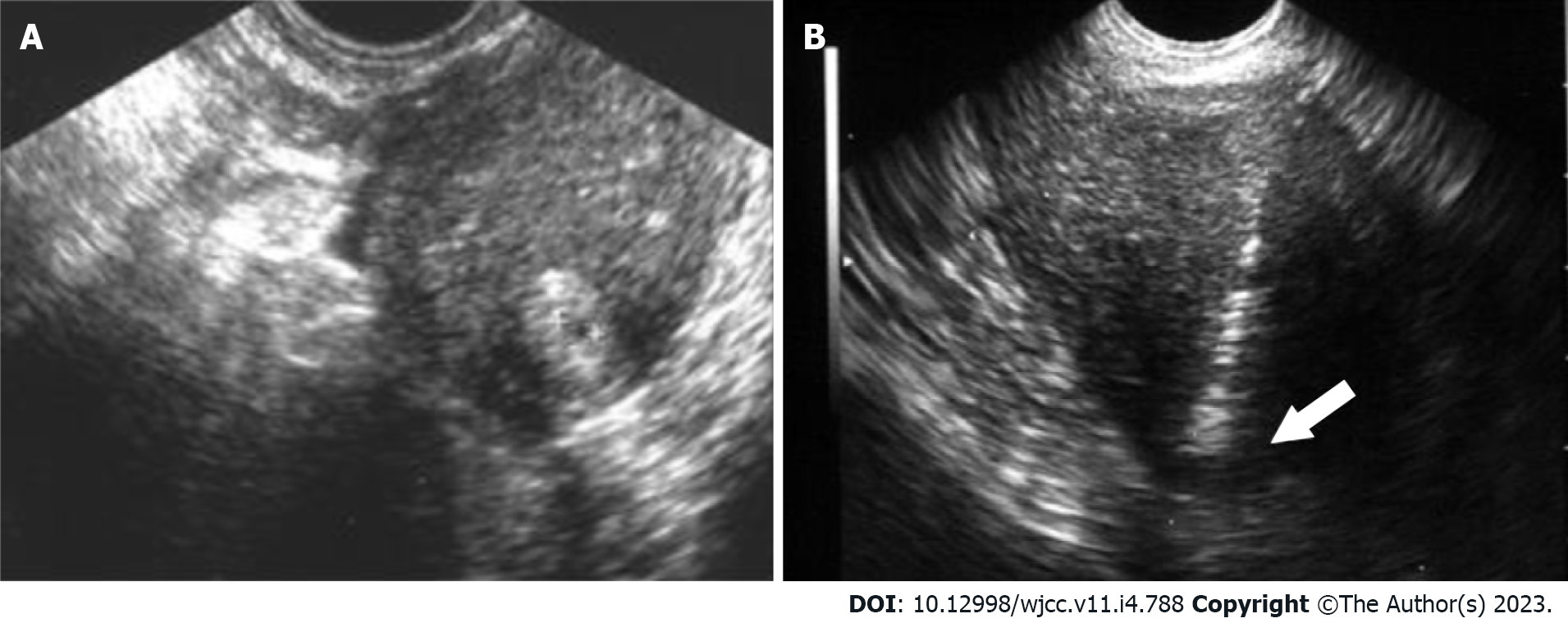
Transvaginal ultrasound-guided local injections of absolute ethanol were administered to inpatients, and analgesia was administered using a nonsteroidal anti-inflammatory drug suppository or peri-cervical block 15 min before treatment. Using transvaginal ultrasound guidance, anhydrous ethanol (anhydrous ethanol injection, Fuso; Fuso Pharmaceutical Industries, Ltd., Osaka, Japan) was locally injected through the myometrium into the guide sheath (GS) periphery of the intrauterine ectopic pregnancy site using a 21-gauge oocyte collection needle (KITAZATO OPU NEEDLE; Kitazato Corporation, Tokyo, Japan). Absolute ethanol was locally injected in the site, where the GS peripheral blood flow was confirmed using the transvaginal ultrasound color Doppler method, until blood flow was interrupted and the GS periphery changed to a highly echoic image (Figure 1). When the blood β-hCG level at 2 h after local ethanol injection exhibited a 10% to 30% decrease compared to that before treatment, the treatment was deemed effective. Additionally, when the blood β-hCG level had decreased after transvaginal ultrasound-guided local injections of absolute ethanol but increased again at a later date, an inadequate response was considered; thereafter, additional local injections of absolute alcohol were administered at a suitable time. The blood β-hCG level was measured using the chemiluminescent immunoassay method (CLIA method; Abbott Japan LLC, Chiba, Japan).
Continuous variables are presented as the mean ± standard deviation (SD). To investigate the correlations among the blood β-hCG level at the beginning of treatment, the treatment period, and the total absolute ethanol dose, Pearson’s product-moment correlation coefficient was derived (Microsoft Excel 2019; Microsoft, Redmond, WA, United States) and P < 0.05 was considered significant.
There were 21 total cases comprising 10 cervical pregnancies, 10 interstitial tubal pregnancies, and 1 cesarean scar pregnancy (Table 1). All these cases were treated with transvaginal ultrasound-guided local injections of absolute ethanol. The mean patient age for those with cervical pregnancies was 37.4 years (SD, ± 3.9 years). The mean patient age for those with interstitial tubal pregnancies was 36.4 years (SD, ± 5.5 years). Of the women with cervical pregnancies, eight were nulliparous and two were parous. Nine of the 10 women with interstitial tubal pregnancies were nulliparous. Of the 10 cervical pregnancies, 8 (80%) were attributable to in vitro fertilization with embryo transfer. Seven (70%) of the 10 interstitial tubal pregnancies were attributable to in vitro fertilization with embryo transfer. The mean gestation ages at the time of diagnosis were 5.9 wk (SD, ± 0.9 wk) for cervical pregnancies and 6.9 wk (SD, ± 2.1 wk) for interstitial tubal pregnancies. The blood β-hCG levels at the beginning of treatment were 13352.0 mIU/mL (SD, ± 8004.1 mIU/mL) for cervical pregnancies and 7485.7 mIU/mL (SD, ± 9647.8 mIU/mL) for interstitial pregnancies. Fetal heart movement was observed during two of the cervical pregnancies and one cesarean scar pregnancy. For cervical pregnancies, 1.9 (SD, ± 0.9) transvaginal ultrasound-guided local injections of absolute ethanol were administered. For interstitial pregnancies, 2.6 (SD, ± 1.1) transvaginal ultrasound-guided local injections of absolute ethanol were administered. The total ethanol doses were 4.8 mL (SD, ± 2.2 mL) for cervical pregnancies and 3.3 mL (SD, ± 2.2 mL) for interstitial pregnancies. The treatment period was 28.5 d (SD, ± 11.7 d) for cervical pregnancies; for interstitial pregnancies, it was 30.0 ± 8.1 d. Positive correlations were observed between the blood β-hCG level (mIU/mL) at the beginning of treatment and the total ethanol dose (r = 0.75; P = 0.00008), as well as between the total ethanol dose and the treatment period (r = 0.48; P = 0.026). No correlation was observed between the blood β-hCG level (mIU/mL) at the beginning of treatment and the treatment period (r = 0.31; P = 1.41).
| Case | Implantation site | Age, yr | Pregnancies, no. | Live birth, no. | Gestation age at diagnosis | Fetal heartbeat | Blood β-hCG (mIU/mL) at the beginning of treatment | Blood β-hCG (mIU/mL) at the final visit | Local ethanol injections, no. | Total ethanol dose, mL | Treatment period, d | |
| 1 | Cervix | 32 | 2 | 0 | IVF-ET | Week 5 Day 0 | - | 17760 | < 1.2 | 3 | 5 | 51 |
| 2 | Cervix | 37 | 1 | 0 | IVF-ET | Week 5 Day 6 | - | 20795 | < 1.2 | 1 | 4.8 | 16 |
| 3 | Cervix | 36 | 0 | 0 | Spontaneous pregnancy | Week 6 Day 5 | - | 12585 | < 1.2 | 3 | 6.8 | 39 |
| 4 | Cervix | 36 | 1 | 1 | IVF-ET | Week 6 Day 1 | - | 2326 | < 1.2 | 2 | 3 | 36 |
| 5 | Cervix | 39 | 0 | 0 | IVF-ET | Week 5 Day 1 | - | 6480 | < 1.2 | 2 | 4 | 19 |
| 6 | Cervix | 35 | 1 | 0 | IVF-ET | Week 5 Day 2 | - | 4835 | < 1.2 | 1 | 3.2 | 22 |
| 7 | Cervix | 39 | 1 | 0 | IVF-ET | Week 5 Day 5 | + | 16346 | < 1.2 | 3 | 10 | 39 |
| 8 | Cervix | 41 | 1 | 0 | IVF-ET | Week 5 Day 3 | - | 7807 | < 1.2 | 1 | 2 | 22 |
| 9 | Cervix | 33 | 1 | 1 | Spontaneous pregnancy | Week 7 Day 6 | + | 26930 | < 1.2 | 1 | 5 | 21 |
| 10 | Cervix | 37 | 1 | 0 | IVF-ET | Week 5 Day 3 | - | 19356 | < 1.2 | 2 | 4.5 | 20 |
| 11 | Fallopian tube interstitium | 41 | 0 | 0 | IVF-ET | Week 8 Day 2 | - | 2384 | < 1.2 | 4 | 4.7 | 28 |
| 12 | Fallopian tube interstitium | 35 | 3 | 0 | IVF-ET | Week 6 Day 1 | - | 853 | < 1.2 | 1 | 0.6 | 16 |
| 13 | Fallopian tube interstitium | 39 | 1 | 0 | IVF-ET | Week 5 Day 4 | - | 2933 | < 1.2 | 3 | 4 | 24 |
| 14 | Fallopian tube interstitium | 37 | 3 | 0 | IVF-ET | Week 7 Day 0 | - | 1170 | < 1.2 | 2 | 1.5 | 26 |
| 15 | Fallopian tube interstitium | 36 | 0 | 0 | IVF-ET | Week 7 Day 3 | - | 2198 | < 1.2 | 4 | 4.9 | 30 |
| 16 | Fallopian tube interstitium | 25 | 0 | 0 | Spontaneous pregnancy | Week 12 Day 0 | - | 2420 | < 1.2 | 2 | 1.5 | 26 |
| 17 | Fallopian tube interstitium | 38 | 2 | 0 | IVF-ET | Week 7 Day 0 | - | 11147 | < 1.2 | 3 | 3.6 | 31 |
| 18 | Fallopian tube interstitium | 33 | 1 | 0 | Spontaneous pregnancy | Week 5 Day 2 | - | 6715 | < 1.2 | 3 | 4.6 | 45 |
| 19 | Fallopian tube interstitium | 34 | 0 | 0 | Spontaneous pregnancy | Week 5 Day 1 | - | 12836 | < 1.2 | 1 | 1.3 | 36 |
| 20 | Fallopian tube interstitium | 46 | 2 | 1 | IVF-ET | Week 5 Day 3 | - | 32201 | < 1.2 | 3 | 8 | 38 |
| 21 | Cesarean delivery scar | 35 | 1 | 1 | Spontaneous pregnancy | Week 7 Day 1 | + | 91798 | < 1.2 | 3 | 12 | 41 |
No massive hemorrhaging occurred during observation, and no patients required blood transfusions. Additionally, no complications attributable to this treatment were observed. Menses resumed for all women after treatment. Furthermore, case 1 achieved spontaneous pregnancy 5 mo after undergoing ultrasound-guided local injections of absolute ethanol. The course of her pregnancy was normal, the placental attachment site was problem-free, and normal delivery occurred at 41+0 wk of gestation. There was no abnormal postpartum bleeding and the postpartum course was good.
In this study, we studied the efficacy and safety of transvaginal ultrasound-guided local injections of absolute ethanol as a new conservative treatment for ectopic pregnancy with an implantation site within the uterus.
Although cervical pregnancy, interstitial tubal pregnancy, and cesarean scar pregnancy, which are ectopic pregnancies with intrauterine implantation sites, are serious conditions prone to massive hemorrhaging, early diagnosis has become possible with the development of high-sensitivity hCG testing reagents and the increased precision of transvaginal ultrasonic tomography. However, there is no consensus regarding uterus-preserving treatments for ectopic pregnancies when the attachment site is within the uterus.
MTX therapy is associated with the possibility of decreased ovarian function and delayed resumption of infertility treatment because of the washout period[9]. Therefore, MTX therapy has possible negative effects on fertility preservation. An investigation on oocyte yields before and after MTX therapy for 35 cervical pregnancy patients with a history of MTX therapy reported that the oocyte yield of in vitro fertilization was 7.8 (SD, ± 3.6) after treatment; however, it was 10.1 (SD, ± 3.9) before treatment[9]. Therefore, MTX therapy was considered to reduce the oocyte yield. This reduction in oocytes was associated with a subsequent decrease in the number of eggs collected[9]. Therefore, the possible negative effects of MTX treatment on the ovaries must be considered for women who desire future pregnancies.
Additionally, because a washout period is necessary when MTX is used, a contraception period of 4 to 6 mo postoperatively is recommended[19,20] before infertility therapy can be resumed. Most of the cases that have been studied involved pregnancy as a result of in vitro fertilization. Therefore, a treatment method that enables early resumption of infertility treatments is desirable because the patients are often of advanced age. Furthermore, it has been reported that MTX administration is often unsuccessful when there is either fetal heart movement or high blood hCG values[11-14], and there are concerns that massive hemorrhaging may occur during treatment[15-17].
Complications after UAE include fever, pain, endometriosis, intrauterine adhesions, uterine necrosis, and reduced ovarian function[21-23]. Regarding postoperative fertility, Hardeman et al[24] studied 14 of 53 patients who had undergone UAE for obstetrical bleeding and desired to become pregnant and reported that 12 were able to achieve pregnancy and live birth. Therefore, fertility after UAE is considered relatively good. However, pregnancies after UAE are affected by significantly increased rates of miscarriage, postpartum hemorrhage, premature birth, and malpresentation; furthermore, intrauterine growth restriction has been observed after UAE[10]. Therefore, careful management during the perinatal period is necessary for pregnancies after UAE.
In this study, we performed transvaginal ultrasound-guided local injections of absolute ethanol as an alternative to MTX therapy and UAE. We previously reported the efficacy and safety of this treatment method for ectopic pregnancy[18]. This treatment method involves local injections of absolute ethanol into the GS periphery of the pregnancy site under ultrasonic guidance. The therapeutic effect of absolute ethanol can be judged within a shorter time than that of MTX therapy because it is possible to confirm a blood hCG decrease of 10% to 30% at 2 h after local injection. Absolute ethanol is thought to dewater and denature the chorionic tissue, resulting in acute tissue changes that reduce the blood hCG within a short time. Therefore, among the cases that we have studied, transvaginal ultrasound-guided local injections of absolute ethanol have been considered effective even with high blood hCG levels and fetal heart movement, which cannot be successfully treated with MTX therapy. Additionally, because transvaginal ultrasound-guided local injections of absolute ethanol did not result in massive hemorrhaging, it was surmised that hemostatic action is involved; therefore, it is likely an effective treatment for intrauterine ectopic pregnancies accompanied by genital bleeding, even when fertility preservation is desired. Additionally, because of the characteristics of absolute ethanol, including its low probability of associated infection, it is effective for transvaginal procedures. Moreover, because a fine-gauge needle is used, there is little blood loss and pain, and general anesthesia is unnecessary. Because absolute ethanol is less expensive than MTX, there are fewer economic burdens on the patients. When repeated administration is required for persistent trophoblastic disease, local treatment with absolute ethanol is suitable because it produces a local effect, whereas anticancer drugs such as MTX produce adverse reactions. Multiple local injections of absolute ethanol were administered for persistent trophoblastic disease among 15 of the cases examined, and no adverse reactions attributable to this treatment were observed.
Although we observed a cervical pregnancy after spontaneous conception and normal delivery after this treatment among our target cases, few reports have described the pregnancy prognosis and uterine preservation for those who desire to have children and have experienced an intrauterine ectopic pregnancy. No consensus has been reached regarding such cases. Pregnancy and delivery courses were recorded for women with cervical pregnancies who desired pregnancy and fertility-preserving treatment and underwent MTX therapy and UAE[17,25-29]. Of the 110 women examined, approximately half (n = 51) wished to become pregnant; of those 110 women, 32 (62.7%) became pregnant and 22 (43.1%) achieved live birth[17,25-29]. Although the background characteristics of the individual women differed, approximately half became pregnant; therefore, it is important to proactively study conservative treatments for cervical pregnancies of women who desire fertility preservation. Additionally, there have been reports of an increased risk of uterine rupture during pregnancies after surgical treatment of the pregnancy site of interstitial tubal pregnancies[30-32]. Therefore, conservative treatments are thought to be highly significant from the perspective of fertility preservation. For women who have undergone treatment for cesarean scar pregnancies, approximately half became pregnant after cesarean scar pregnancy treatment, and their outcomes varied. Although some women have achieved live birth by cesarean delivery at full term, others experienced another cesarean scar pregnancy, stillbirth, or maternal death caused by uterine rupture even though implantation occurred in the uterine body, and some required a total hysterectomy because of massive hemorrhaging caused by placenta accreta[7,33]. During pregnancy management after cesarean scar pregnancy treatment, careful examination is necessary during early pregnancy. Even in cases of normal pregnancy, it may be necessary to be cautious of uterine rupture and placenta accreta.
Since intrauterine ectopic pregnancy is a relatively rare disease, further examination of an accumulated number of cases and long-term follow-up after transvaginal ultrasound-guided local injections of absolute ethanol are required. There are many unknowns regarding the effects of fertility preservation treatments on future pregnancies. Therefore, investigations of the treatment methods used for intrauterine ectopic pregnancies and long-term follow-up are necessary.
Transvaginal ultrasound-guided local injections of absolute ethanol for ectopic pregnancies with an intrauterine implantation site can preserve the uterus without serious adverse reactions. This treatment method avoids the complications caused by MTX therapy and UAE. Therefore, it may become a new treatment option for intrauterine ectopic pregnancy when fertility preservation is desired.
Ectopic pregnancy at cervical pregnancy, caesarean scar pregnancy, and interstitial pregnancy are rare; therefore, it is challenging to say that a standard treatment has been established.
Removal of ectopic pregnancies using methotrexate therapy and/or uterine artery embolization has been reported. However, delayed resumption of infertility treatments after methotrexate therapy is indicated, and negative effects on the next pregnancy after uterine artery embolization have been reported. To avoid these problems, we will establish a new treatment method for Cervical pregnancies, interstitial tubal pregnancies, and cesarean scar pregnancies, which are ectopic pregnancies with intrauterine implantation sites.
The purpose of this study was to examine the efficacy and safety of ultrasound-guided topical injection of absolute ethanol in ectopic pregnancies with an implantation site within the uterus.
We retrospectively examined the medical records of 21 patients who were diagnosed with an ectopic pregnancy with an intrauterine implantation site at our hospital, between April 2010 and December 2018, and underwent transvaginal ultrasound-guided local injections of absolute ethanol to determine the treatment outcomes. We evaluated the treatment methods, treatment outcomes, presence of bleeding requiring hemostasis measures and blood transfusion, complications, and treatment periods.
All patients completed treatment with transvaginal ultrasound-guided local injections of absolute ethanol. No massive hemorrhaging or serious adverse reactions were observed during treatment.
Transvaginal ultrasound-guided local injections of absolute ethanol could become a new option for intrauterine ectopic pregnancies when fertility preservation is desired.
Ectopic pregnancy at cervical pregnancy, caesarean scar pregnancy, and interstitial pregnancy are rare; therefore, it is challenging to say that a standard treatment has been established. Transvaginal ultrasound-guided local injections of absolute ethanol for ectopic pregnancies such as cervical pregnancies, interstitial tubal pregnancies, and cesarean scar pregnancies can preserve the uterus without serious adverse reactions. This treatment avoids the complications caused by methotrexate therapy and uterine artery embolization. This treatment may become a new treatment option for intrauterine ectopic pregnancy when fertility preservation is desired.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Obstetrics and gynecology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Maglic R, Serbia; Zhang F, China S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Londra L, Moreau C, Strobino D, Garcia J, Zacur H, Zhao Y. Ectopic pregnancy after in vitro fertilization: differences between fresh and frozen-thawed cycles. Fertil Steril. 2015;104:110-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 2. | Clayton HB, Schieve LA, Peterson HB, Jamieson DJ, Reynolds MA, Wright VC. Ectopic pregnancy risk with assisted reproductive technology procedures. Obstet Gynecol. 2006;107:595-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 151] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 3. | Bouyer J, Coste J, Fernandez H, Pouly JL, Job-Spira N. Sites of ectopic pregnancy: a 10 year population-based study of 1800 cases. Hum Reprod. 2002;17:3224-3230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 389] [Cited by in RCA: 355] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 4. | Adabi K, Nekuie S, Rezaeei Z, Rahimi-Sharbaf F, Banifatemi S, Salimi S. Conservative management of cervical ectopic pregnancy: systemic methotrexate followed by curettage. Arch Gynecol Obstet. 2013;288:687-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Martinelli P, Maruotti GM, Oppedisano R, Agangi A, Mazzarelli LL, Votino C, Quarantelli M, Iaccarino V. Is uterine artery embolization for cervical ectopic pregnancy always safe? J Minim Invasive Gynecol. 2007;14:758-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Ushakov FB, Elchalal U, Aceman PJ, Schenker JG. Cervical pregnancy: past and future. Obstet Gynecol Surv. 1997;52:45-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 145] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 7. | Ash A, Smith A, Maxwell D. Caesarean scar pregnancy. BJOG. 2007;114:253-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 349] [Cited by in RCA: 331] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 8. | Hafner T, Aslam N, Ross JA, Zosmer N, Jurkovic D. The effectiveness of non-surgical management of early interstitial pregnancy: a report of ten cases and review of the literature. Ultrasound Obstet Gynecol. 1999;13:131-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 55] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | McLaren JF, Burney RO, Milki AA, Westphal LM, Dahan MH, Lathi RB. Effect of methotrexate exposure on subsequent fertility in women undergoing controlled ovarian stimulation. Fertil Steril. 2009;92:515-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | Goldberg J, Pereira L, Berghella V. Pregnancy after uterine artery embolization. Obstet Gynecol. 2002;100:869-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 83] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 11. | Hung TH, Shau WY, Hsieh TT, Hsu JJ, Soong YK, Jeng CJ. Prognostic factors for an unsatisfactory primary methotrexate treatment of cervical pregnancy: a quantitative review. Hum Reprod. 1998;13:2636-2642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 96] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 12. | Lipscomb GH, McCord ML, Stovall TG, Huff G, Portera SG, Ling FW. Predictors of success of methotrexate treatment in women with tubal ectopic pregnancies. N Engl J Med. 1999;341:1974-1978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 205] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 13. | Watanabe K, Chigusa Y, Kondoh E, Mogami H, Horie A, Baba T, Mandai M. Human chorionic gonadotropin value and its change prior to methotrexate treatment can predict the prognosis in ectopic tubal pregnancies. Reprod Med Biol. 2019;18:51-56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Pulatoglu C, Dogan O, Basbug A, Kaya AE, Yildiz A, Temizkan O. Predictive factors of methotrexate treatment success in ectopic pregnancy: A single-center tertiary study. North Clin Istanb. 2018;5:227-231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Cok T, Kalayci H, Ozdemir H, Haydardedeoglu B, Parlakgumus AH, Tarim E. Transvaginal ultrasound-guided local methotrexate administration as the first-line treatment for cesarean scar pregnancy: Follow-up of 18 cases. J Obstet Gynaecol Res. 2015;41:803-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Zhang S, Yan H, Ji WT. Uterine artery embolization combined with intra-arterial MTX infusion: its application in treatment of cervical pregnancy. Arch Gynecol Obstet. 2016;293:1043-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Yamaguchi M, Honda R, Erdenebaatar C, Monsur M, Honda T, Sakaguchi I, Okamura Y, Ohba T, Katabuchi H. Treatment of cervical pregnancy with ultrasound-guided local methotrexate injection. Ultrasound Obstet Gynecol. 2017;50:781-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | Kaijima H, Osada H, Kato K, Segawa T, Takehara Y, Teramoto S, Kato O. The efficacy and safety of managing ectopic pregnancies with transvaginal ultrasound-guided local injections of absolute ethanol. J Assist Reprod Genet. 2006;23:293-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Gougeon A. Dynamics of follicular growth in the human: a model from preliminary results. Hum Reprod. 1986;1:81-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 443] [Cited by in RCA: 423] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 20. | Strauss J, Barbieri R, Gargiulo A. The ovarian life cycle. In: Yen & Jaffe’s reproductive endocrinology: physiology, pathophysiology, and clinical management. 5th edn. Philadelphia: Elsevier Saunders, 2004; 213. |
| 21. | Badawy SZ, Etman A, Singh M, Murphy K, Mayelli T, Philadelphia M. Uterine artery embolization: the role in obstetrics and gynecology. Clin Imaging. 2001;25:288-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 107] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 22. | Vegas G, Illescas T, Muñoz M, Pérez-Piñar A. Selective pelvic arterial embolization in the management of obstetric hemorrhage. Eur J Obstet Gynecol Reprod Biol. 2006;127:68-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 63] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 23. | Hong TM, Tseng HS, Lee RC, Wang JH, Chang CY. Uterine artery embolization: an effective treatment for intractable obstetric haemorrhage. Clin Radiol. 2004;59:96-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 79] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 24. | Hardeman S, Decroisette E, Marin B, Vincelot A, Aubard Y, Pouquet M, Maubon A. Fertility after embolization of the uterine arteries to treat obstetrical hemorrhage: a review of 53 cases. Fertil Steril. 2010;94:2574-2579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 63] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 25. | Xiaolin Z, Ling L, Chengxin Y, Yiqing T, Jun W, Yan C, Guangxi T. Transcatheter intraarterial methotrexate infusion combined with selective uterine artery embolization as a treatment option for cervical pregnancy. J Vasc Interv Radiol. 2010;21:836-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Wang Y, Xu B, Dai S, Zhang Y, Duan Y, Sun C. An efficient conservative treatment modality for cervical pregnancy: angiographic uterine artery embolization followed by immediate curettage. Am J Obstet Gynecol. 2011;204:31.e1-31.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 27. | Krissi H, Hiersch L, Stolovitch N, Nitke S, Wiznitzer A, Peled Y. Outcome, complications and future fertility in women treated with uterine artery embolization and methotrexate for non-tubal ectopic pregnancy. Eur J Obstet Gynecol Reprod Biol. 2014;182:172-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 28. | Uludag SZ, Kutuk MS, Aygen EM, Sahin Y. Conservative management of cervical ectopic pregnancy: Single-center experience. J Obstet Gynaecol Res. 2017;43:1299-1304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 29. | Chen H, Yang S, Fu J, Song Y, Xiao L, Huang W, Zhang H. Outcomes of Bilateral Uterine Artery Chemoembolization in Combination with Surgical Evacuation or Systemic Methotrexate for Cervical Pregnancy. J Minim Invasive Gynecol. 2015;22:1029-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 30. | Imai S, Kondoh E, Kawasaki K, Mogami H, Ueda A, Umeoka S, Konishi I. Placental blood flow disappears coincident with a fall in human chorionic gonadotropin to undetectable levels in conservative management of placenta accreta. Eur J Obstet Gynecol Reprod Biol. 2014;180:199-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 31. | Weissman A, Fishman A. Uterine rupture following conservative surgery for interstitial pregnancy. Eur J Obstet Gynecol Reprod Biol. 1992;44:237-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 32. | Downey GP, Tuck SM. Spontaneous uterine rupture during subsequent pregnancy following non-excision of an interstitial ectopic gestation. Br J Obstet Gynaecol. 1994;101:162-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 41] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 33. | Ben Nagi J, Helmy S, Ofili-Yebovi D, Yazbek J, Sawyer E, Jurkovic D. Reproductive outcomes of women with a previous history of Caesarean scar ectopic pregnancies. Hum Reprod. 2007;22:2012-2015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 90] [Article Influence: 5.0] [Reference Citation Analysis (0)] |