Published online Feb 6, 2023. doi: 10.12998/wjcc.v11.i4.764
Peer-review started: September 21, 2022
First decision: November 30, 2022
Revised: December 14, 2022
Accepted: January 12, 2023
Article in press: January 12, 2023
Published online: February 6, 2023
Processing time: 137 Days and 18.9 Hours
Various immune-mediated inflammatory diseases consisting of inflammatory bowel disease (IBD) and rheumatoid arthritis (RA), are found to have a subs
To identify the prevalence level and temporal trends of depression in hospitalized IBD-RA patients.
All adult hospitalized patients from January 2000 to December 2019 in the nationwide inpatient sample (NIS) were captured. The study population included all patients with a primary or secondary IBD-RA overlap disease using corresponding international classification of diseases (ICD)-9 and ICD-10 codes. IBD includes Crohn’s disease and ulcerative colitis. The study population was divided into IBD-RA without MDD (controls) and IBD-RA with MDD (cases). For group comparison between MDD vs no MDD, we used Student's t-test for continuous variables and Rao-Scott Chi-square tests for categorical variables. For univariate analyses, we used logistic regression, and for multivariate analysis, we used a weighted multi-level mixed-effects model. We attested all hypotheses with two-tailed significance level of 0.05 (P < 0.05 was considered significant). The outcome is to examine the temporal trends and prevalence of depression in patients with IBD-RA by gender, race, and age.
A total of 133315 records were identified with IBD-RA overlap, of which 26155 patients (19.62%) had MDD. Among the IBD-RA patients, those who had MDD were younger [mean age of 56 years (SD ± 15)] to IBD-RA without MDD patients with a P < 0.0001, more females (80% among cases vs 73% among controls) than males with a P < 0.0001, frequent in the white race (79% among cases vs 73% among controls) than black race. Over the 19 years, the number of patients with MDD in IBD-RA increased from 153 (the year 2000) to 2880 (the year 2019) in weighted NIS, representing a 1782% increase compared to the year 2000 with a P < 0.001. Factors associated with higher MDD included younger age, female gender, white race, alcohol, opioids, esophageal disorders, peptic ulcer disease, chronic pancreatitis, paralysis, dementia, menopausal disorders, obesity, nutritional deficiencies, diabetes mellitus with chronic complications, and osteoarthritis.
There is a rise in the prevalence of depression in younger patients with IBD-RA combined compared to their counterparts. These patients are also at higher risk for the increased cost of care and poor treatment compliance. It is crucial to educate the involved clinicians to identify the early signs and symptoms of depression in patients with IBD or RA or IBD-RA combined and treat them to have a better overall prognosis.
Core Tip: Our study is a nationwide inpatient sample-based study of the two decades in which we aim to analyze inflammatory bowel disease (IBD) and rheumatoid arthritis (RA) patients’ characteristics, temporal trends, sociodemographic characteristics, and predictors of major depressive disorders in the IBD-RA cohort. There is an increasing trend in the prevalence of IBD-RA, especially in younger patients. We believe this study will open the door for further research and educate the involved physicians to identify the early signs and symptoms of depression in patients with IBD or RA or IBD-RA combined and treat them or have them treated to have a better overall prognosis.
- Citation: Haider MB, Basida B, Kaur J. Major depressive disorders in patients with inflammatory bowel disease and rheumatoid arthritis. World J Clin Cases 2023; 11(4): 764-779
- URL: https://www.wjgnet.com/2307-8960/full/v11/i4/764.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i4.764
The coexistence between immune-mediated inflammatory diseases (IMID) with depression has long been studied[1,2]. Rheumatoid arthritis (RA) is a chronic systemic autoimmune disorder that primarily targets the synovial joints leading to inflammation (synovitis), joint erosion, and damage to the cartilage[3]. Inflammatory bowel disease (IBD) is broadly subdivided into ulcerative colitis and Crohn’s disease. Crohn’s disease is a relapsing systematic immune-mediated chronic IBD, and Ulcerative colitis is an idiopathic, chronic IBD with bloody diarrhea[4].
The global prevalence of RA is estimated at 460 per 100000 population, while its prevalence in the United States is 700 per 100000 population[5,6]. Both depression and RA contribute substantially to global disability, and the diseases often coexist. The global prevalence rate of IBD is 84.3 per 100000 population, while that in the United States is 464.5 per 100000 population[7].
Patients with RA suffer twice from depression than the general population[8], with 41%-66% reported lifetime prevalence[1,3]. The prevalence of major depressive disorders (MDDs) was 17% in patients with RA[3]. IBD patients have more than 25% lifetime diagnosis of depression[9,10].
Not many studies have identified the impact of depression in patients with both RA and IBD combined. Our study is researched using the national inpatient sampling database sought to evaluate the co-existence of depression in patients with RA and IBD. The role of the Brain-Gut axis and Brain-Joint axis in the development of depression has been illustrated before[11,12], but there is not enough literature demonstrating the combined Brain-Gut-Joint axis. Our study discusses the clinical data on this combined axis and emphasizes potentially valuable strategies for managing these patients. Our primary aim was to identify a pooled prevalence level and temporal trends of depression in hospitalized IBD-RA patients. To have a multimorbidity approach, which is more patient-focused, we aimed to investigate various comorbidities as predictors of depression.
We analyzed the nationwide inpatient sample (NIS) database from January 2000 to December 2019. The NIS is the largest all-payer publicly available database of hospitalizations in the United States, designed to produce national and regional inpatient utilization, cost, quality, and outcomes[13]. We used the international classification of diseases (ICD)-9, diagnosis and procedures before October 1, 2015, and ICD-10 afterward[14]. In this study, we used the latest available datasets. The NIS dataset is very suitable for temporal trends, but the dataset has undergone several changes over time. We used the Healthcare Cost and Utilization Project (HCUP) recommendation to use trend weights before 2012 to make estimates comparable to the 2012 NIS design[15]. The dataset was de-identified to protect the privacy of individual patients, physicians, and hospitals. As the dataset lacks patient information, this study was exempted from Institutional Review Board-IRB approval from Wayne State University under the Health Insurance Portability and Accountability Act[16].
The study population included patients with a primary or secondary IBD-RA overlap disease using corresponding ICD-9 and ICD-10 codes. For IBD, we included Crohn’s disease and ulcerative colitis patients. We used the clinical classification software refined to determine the ICD codes of depressive disorders[17,18]. The study population was divided into IBD-RA without MDD (controls) and IBD-RA with MDD (cases).
The outcomes included temporal trends and predictors of MDDs in the IBD-RA cohort. In the first part, we performed a trend analysis of weighted prevalence, the percentage change in prevalence compared to the year 2000, mean age, gender and ethnicity, length of stay, and total hospital charges. Next, we compared baseline patient and hospital characteristics and comorbidities between cases (IBD-RA with MDD) and controls (IBD-RA without MDD). We also evaluated predictors for MDD in IBD-RA patients.
Patient demographics included gender, age, ethnicity, socioeconomic status, and primary health insurance. Socioeconomic status was defined as the median household income in the patient’s zip code and was divided into quartiles by year (2019): Less than $47999, $48000–$60999, $61000–$81999, $82000 or more). Hospitalization care costs were adjusted using the United States Bureau of Labor and Statistics’ consumer price index[19]. In addition, we used NIS characterization for hospital factors. The complete list of comorbidities is shown in Table 1.
| IBD-RA without depressive disorders, weighted, n (%) | IBD-RA with depressive disorders, weighted, n (%) | P value | |
| Liver disease, mild | 6400 (5.97) | 2130 (8.14) | < 0.0001a |
| Liver disease, moderate to severe | 786 (0.73) | 230 (0.88) | 0.2871a |
| Peptic ulcer disease | 1709 (1.59) | 632 (2.41) | < 0.0001a |
| Esophageal disorders | 30712 (28.65) | 10569 (40.40) | < 0.0001a |
| Gastritis | 3781 (3.52) | 1124 (4.30) | 0.0095a |
| Diabetes mellitus with chronic complications | 6367 (5.94) | 2005 (7.66) | < 0.0001a |
| Diabetes mellitus without chronic complications | 14014 (13.07) | 3533 (13.50) | 0.4244a |
| Hypertension | 40782 (38.05) | 10493 (40.12) | 0.0080a |
| Heart Failure | 12798 (11.94) | 2908 (11.11) | 0.1176a |
| Coronary artery disease | 16644 (15.53) | 3549 (13.57) | 0.0007a |
| Cardiac dysrhythmias | 16114 (15.04) | 3299 (12.61) | < 0.0001a |
| Myocardial infarction | 1316 (1.23) | 224 (0.86) | 0.0237a |
| Stroke | 1530 (1.42) | 230 (0.88) | 0.0018a |
| Iron Deficiency Anemia | 9298 (8.68) | 2296 (8.78) | 0.8167a |
| Nutritional deficiencies | 4062 (3.79) | 1494 (5.71) | < 0.0001a |
| Lipid Metabolism disorders | 24990 (23.32) | 7036 (26.90) | < 0.0001a |
| Thyroid disorders | 18029 (16.82) | 5531 (21.15) | < 0.0001a |
| Chronic Pancreatitis | 1165 (1.09) | 507 (1.94) | < 0.0001a |
| Renal failure, moderate | 8563 (7.99) | 2132 (8.15) | 0.7113a |
| Renal failure, severe | 2965 (2.77) | 767 (2.93) | 0.5434a |
| Menopausal disorders | 536 (0.50) | 222 (0.84) | 0.0033a |
| Osteoporosis | 11752 (10.97) | 3544 (13.55) | < 0.0001a |
| Osteoarthritis | 15084 (14.07) | 4562 (17.44) | < 0.0001a |
| Obesity | 11706 (10.92) | 4062 (15.53) | <.00011 |
| Weight Loss | 1201 (1.12) | 365 (1.39) | 0.1101a |
| Protein-calorie malnutrition | 8479 (7.91) | 2370 (9.06) | 0.0085a |
| Leukemia | 558 (0.52) | 134 (0.51) | 0.9401a |
| Lymphoma | 838 (0.78) | 181 (0.69) | 0.5132a |
| Metastatic cancer | 1381 (1.29) | 329 (1.26) | 0.8524a |
| Solid tumor without metastasis, malignant | 3785 (2.84) | 850 (0.64) | 0.8524a |
| Paralysis | 1498 (1.40) | 495 (1.89) | 0.0114a |
| Dementia | 2291 (2.14) | 886 (3.39) | < 0.0001a |
| AIDS | 212 (0.19) | 45 (0.17) | 0.6818a |
| Smoking | 32336 (30.17) | 9893 (37.82) | < 0.0001a |
| Alcohol | 1747 (1.63) | 950 (3.63) | < 0.0001a |
| Opioids | 3180 (2.97) | 1797 (6.87) | < 0.0001a |
The statistical analyses are performed in SAS software (SAS Institute Inc., Cary, NC, United States). The HCUP NIS database was redesigned beginning in 2012 to improve national estimates. We deployed HCUP recommendations for trend analysis and used survey sampling and analysis procedures for weighted analysis. Continuous variables such as age, total charges, and length of stay are represented with a mean ± SD, and categorical variables are presented with frequency and computed percentages. For group comparison between MDD vs no MDD, we used Student's t-test for continuous variables and Rao-Scott Chi-square tests for categorical variables. We used the Cochran-Armitage trend test for categorical variables and poison regression with a log link for continuous variables for temporal trends analysis. The univariate analysis is performed with logistic regression. The multivariate analysis is achieved with a weighted multi-level mixed-effects model using the Glimmix procedure with maximum likelihood estimation and Gauss-Hermite quadrature likelihood approximation. We categorized age into five sub-categories per HCUP standard categories (< 18, 18-44, 45-64, 65-84, and ≥ 85) for group-level comparison. The missing values are categorized as either missing or unknown. All hypothesis testing is performed with two-tailed significance level of 0.05 (P < 0.05 was considered significant).
The baseline characteristics of the study population with and without MDD are described in Table 2. From January 2000 to December 2019, we found 133315 IBD-RA overlap patients in weighted settings, of which 26155 patients (19.62%) had MDD. Among the IBD-RA patients, those who had MDD were younger [mean age of 56 years (SD ± 15) as compared to mean age of 59 years (SD ± 17)] in IBD-RA without MDD patients with P < 0.0001, more females (80% among cases vs 73% among controls) than males (20% for cases vs 17% for controls) with P < 0.0001, frequent in the white race (79% among cases vs 73% among controls) than black race (7% for cases vs 10% for controls) and Hispanic (4.00% among cases vs 4.63% among controls) with P < 0.0001, and more frequent in patients with Medicaid (12% among cases vs 9% among controls) than patients with private insurance (28% among cases vs 31% among controls) P < 0.0001. A comparison of comorbidities of the study population with and without MDD is described in Table 1.
| IBD-RA without MDD, weighted, n (%) | IBD-RA with MDD, weighted, n (%) | P value | |
| Weighted total, n (%) | 107160 (80.38) | 26155 (19.62) | |
| Sex | < 0.0001a | ||
| Female | 78088 (72.87) | 21052 (80.49) | |
| Male | 29073 (27.13) | 5103 (19.51) | |
| Age (yr), mean ± SD | 58.70 ± 16.89 | 56.36 ± 15.47 | < 0.0001b |
| Age groups (yr) | < 0.0001c | ||
| < 18 | 319 (0.29) | 25 (0.10) | |
| 18-44 | 22215 (20.73) | 5887 (22.50) | |
| 45-64 | 40363 (37.67) | 11544 (44.14) | |
| 65-84 | 39111 (36.50) | 7902 (30.21) | |
| ≥ 85 | 5152 (4.81) | 798 (3.05) | |
| Ethnicity | < 0.0001c | ||
| White | 77818 (72.62) | 20612 (78.80) | |
| Black | 10543 (9.83) | 1850 (7.07) | |
| Hispanic | 4963 (4.63) | 1045 (3.99) | |
| Asian or Pacific Islander | 741 (0.69) | 94 (0.36) | |
| Native American | 500 (0.47) | 91 (0.35) | |
| Other | 12596 (11.75) | 2464 (9.42) | |
| Primary payer status | < 0.0001c | ||
| Medicare | 59738 (55.75) | 14522 (55.52) | |
| Medicaid | 10128 (9.45) | 3257 (12.45) | |
| Private | 32808 (30.62) | 7239 (27.68) | |
| Self-Pay | 2034 (1.89) | 505 (1.93) | |
| No charge | 186 (0.17) | 40 (0.15) | |
| Other | 2266 (2.11) | 592 (2.26) | |
| Median socioeconomic status by national quartiles | 0.0008c | ||
| 0-25 | 24484 (22.85) | 6111 (23.37) | |
| 25-50 | 26531 (24.75) | 6690 (25.58) | |
| 50-75 | 27857 (26.00) | 7171 (27.42) | |
| 75-100 | 26431 (24.66) | 5876 (22.46) | |
| Other | 1857 (1.73) | 307 (1.17) | |
| Hospital bed size | 0.0147c | ||
| Small | 16789 (15.67) | 4496 (17.19) | |
| Medium | 28359 (26.46) | 7067 (27.02) | |
| Large | 62012 (57.87) | 14592 (55.79) | |
| Location/teaching status of the hospital | < 0.0001c | ||
| Rural | 10398 (9.70) | 2299 (8.79) | |
| Urban nonteaching | 36576 (34.13) | 7801 (29.83) | |
| Urban teaching | 60186 (56.17) | 16055 (61.38) | |
| Hospital region | < 0.0001c | ||
| Northeast | 21819 (20.36) | 4601 (17.59) | |
| Midwest or North Central | 27596 (25.75) | 7636 (29.19) | |
| South | 40204 (37.52) | 9583 (36.64) | |
| West | 17541 (16.36) | 4335 (16.57) | |
| Discharge outcomes | |||
| Routine Discharge | 69143 (64.52) | 16289 (62.28) | < 0.0031a |
| Transfer to Short-term Hospital | 2240 (2.09) | 475 (1.82) | 0.2013a |
| Transfer to other facilities | 16349 (15.26) | 4612 (17.63) | < 0.0001a |
| HHC | 17635 (16.45) | 4536 (17.34) | 0.1373a |
| In-hospital mortality | 1792 (1.67%) | 243 (0.93%) | < 0.0001a |
| Length of stay (days), mean ± SD | 5.49 ± 5.75 | 5.65 ± 5.79 | 0.0738a |
| Total charges (USD), mean ± SD | 43881 ± 61300 | 45157 ± 60859 | 0.1762a |
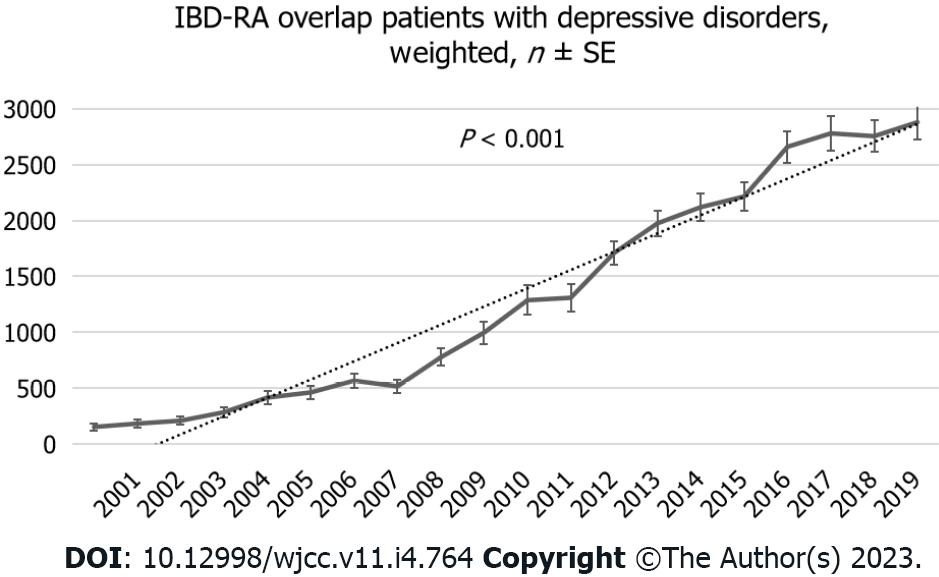
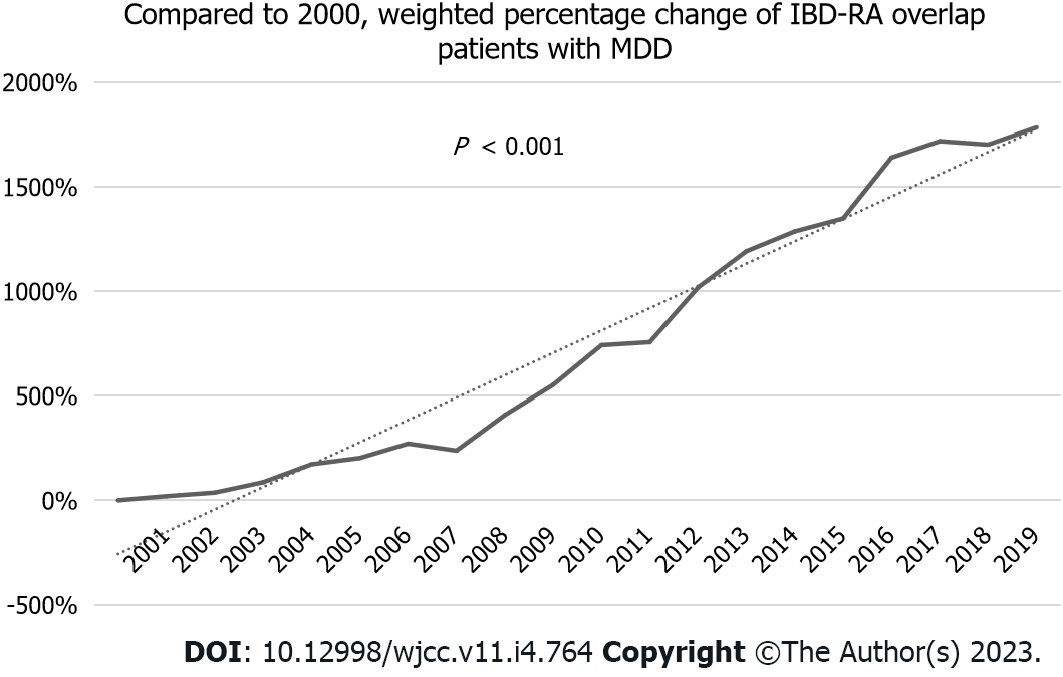
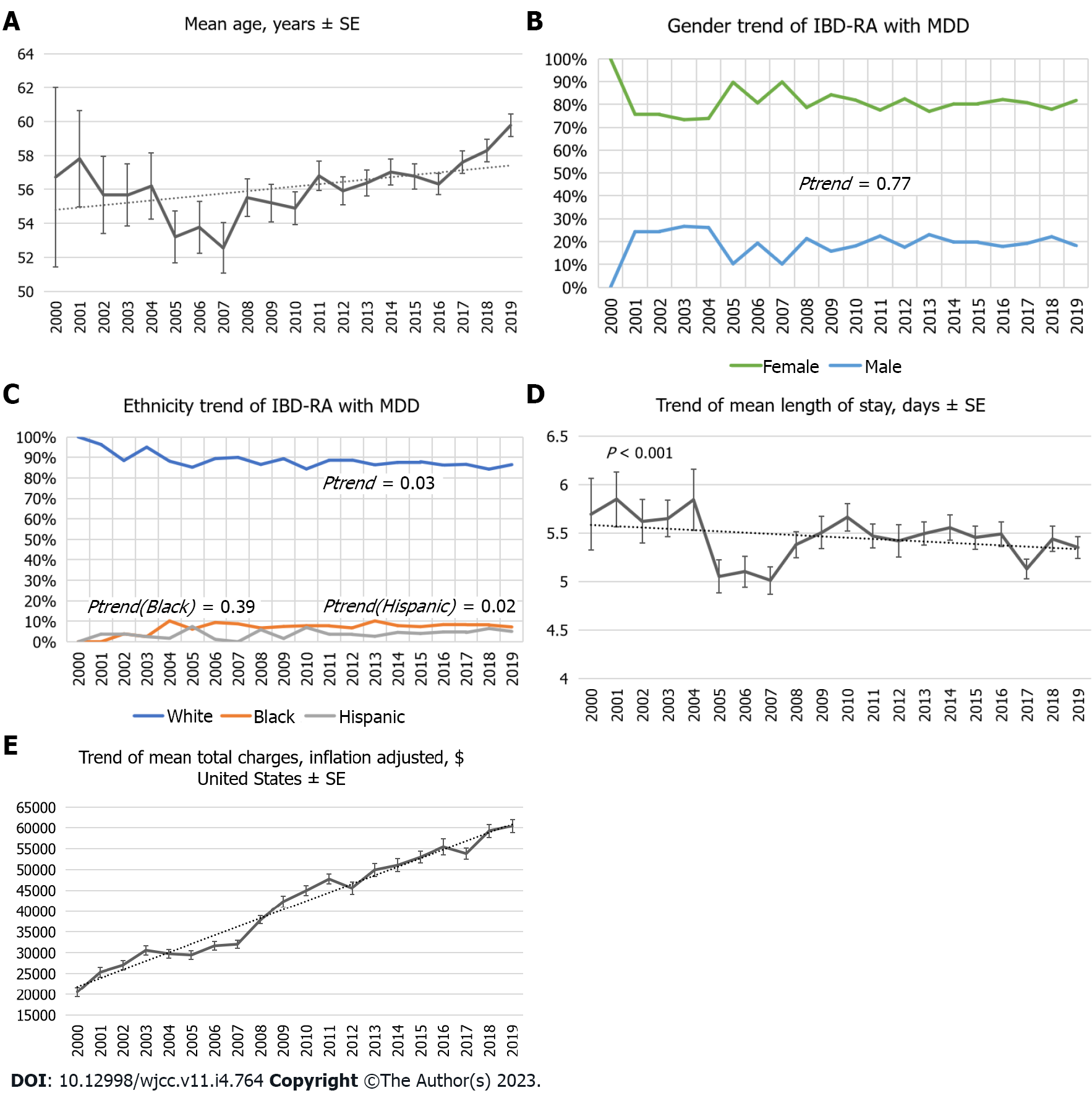
Over the 19 years, the number of patients with MDD in IBD-RA increased from 153 (the year 2000) to 2880 (the year 2019) in weighted NIS (Figure 1), representing a 1782% increase (Figure 2) compared to the year 2000 with P < 0.001. The mean age of IBD-RA in MDD patients was 56.36 years in 2000 compared with 59.8 years in 2019, with a P < 0.001 (Figure 3A). There was no significant change in the male vs female trend of MDD in the IBD-RA cohort (Figure 3B). Overall, the white race among IBD-RA with MDD is 78.80%. We observed a decreasing trend in the white race with P = 0.03. The black race is 7.07%, and no change is followed over time. The Hispanic ethnicity is 3.99%, and we noted an increasing trend with P = 0.02 (Figure 3C). In terms of hospital burden, the mean length of stay of IBD-RA with MDD patients decreased from 5.69 d in 2000 to 5.35 d in 2019 with a P < 0.001 (Figure 3D), and the mean total cost of care (inflation-adjusted) increased from $20564 in 2000 to $60428 in 2019 with P < 0.001 (Figure 3E).
The univariate and multivariate analyses for IBD-RA patients with MDD are shown in Table 3. The multivariate analyses showed that demographic factors associated with higher MDD included female gender [adjusted odds ratios (aOR): 1.52; 95%CI: 1.46-1.58; P < 0.0001], patients aged 18-44 (aOR: 1.89; 95%CI: 1.79-1.99; P < 0.0001) and patients aged 45-64 (aOR: 1.69; 95%CI: 1.63-1.76; P < 0.0001) as compared to the reference group (patients aged 65-84), white (aOR: 1.83; 95%CI: 1.73-1.94; P < 0.0001) and Hispanic race (aOR: 1.39; 95%CI: 1.27-1.52; P < 0.0001) as compared to the black race, and median socioeconomic status in the second quartile (aOR: 1.07; 95%CI: 1.03-1.12; P = 0.002) as compared to quartile 1.
| Variables | Univariate analysis | Multivariate analysis | ||
| Unadjusted odds ratio1 (95%CI) | P value | Adjusted odds ratio2 (95%CI) | P value | |
| Gender, Female vs Male | 1.533 (1.424–1.651) | < 0.0001 | 1.518 (1.463-1.575) | < 0.0001 |
| Age groups (yr) | ||||
| < 18 | 0.380 (0.153–0.944) | 0.0371 | 0.793 (0.523-1.203) | 0.2842 |
| 18-44 | 1.320 (1.214-1.434) | < 0.0001 | 1.888 (1.790-1.991) | < 0.0001 |
| 45-64 | 1.418 (1.321-1.521) | < 0.0001 | 1.693 (1.625-1.764) | < 0.0001 |
| 65-84 | Reference | NA | Reference | NA |
| ≥ 85 | 0.773 (0.649-0.920) | 0.0037 | 0.749 (0.688-0.814) | < 0.0001 |
| Race | ||||
| White | 1.500 (1.338-1.682) | < 0.0001 | 1.830 (1.728-1.939) | < 0.0001 |
| Black | Reference | NA | Reference | NA |
| Hispanic | 1.193 (0.993-1.434) | 0.0598 | 1.391 (1.272-1.521) | < 0.0001 |
| Asian or Pacific Islander | 0.720 (0.441-1.174) | 0.1876 | 0.856 (0.679-1.078) | 0.1876 |
| Native American | 1.029 (0.616-1.720) | 0.9120 | 0.937 (0.733-1.198) | 0.6120 |
| Other | 1.109 (0.959-1.282) | 0.1627 | 1.456 (1.351-1.568) | < 0.0001 |
| Primary payer status | ||||
| Medicare | Reference | NA | Reference | NA |
| Medicaid | 1.327 (1.205-1.462) | < 0.0001 | 1.043 (0.99-1.10) | 0.1148 |
| Private | 0.911 (0.850-0.977) | 0.0085 | 0.774 (0.744-0.805) | < 0.0001 |
| Self-Pay | 1.035 (0.831-1.289) | 0.7616 | 0.892 (0.800-0.994) | 0.0382 |
| No charge | 0.853 (0.398-1.827) | 0.6825 | 0.698 (0.485-1.005) | 0.0555 |
| Other | 1.069 (0.871-1.311) | 0.5255 | 0.974 (0.88-1.077) | 0.5930 |
| Median socioeconomic status by national quartiles | ||||
| 0-25 | Reference | NA | Reference | NA |
| 25-50 | 1.006 (0.923-1.097) | 0.8836 | 1.031 (0.988-1.075) | 0.1595 |
| 50-75 | 1.026 (0.943-1.117) | 0.5455 | 1.071 (1.025-1.118) | 0.0019 |
| 75-100 | 0.890 (0.814-0.972) | 0.0095 | 0.998 (0.952-1.045) | 0.9323 |
| Other | 0.664 (0.505-0.873) | 0.0034 | 0.697 (0.61-0.795) | < 0.0001 |
| Hospital bed size | ||||
| Small | Reference | NA | Reference | NA |
| Medium | 1.061 (0.967-1.164) | 0.2109 | 0.956 (0.913-1.002) | 0.0585 |
| Large | 0.936 (0.873-1.005) | 0.0669 | 0.926 (0.888-0.966) | 0.0004 |
| Location/teaching status of the hospital | ||||
| Rural | Reference | NA | Reference | NA |
| Urban nonteaching | 1.028 (0.917-1.152) | 0.6376 | 0.994 (0.938-1.054) | 0.8450 |
| Urban teaching | 1.253 (1.172-1.339) | < 0.0001 | 1.235 (1.168-1.306) | < 0.0001 |
| Hospital region | ||||
| Northeast | Reference | NA | Reference | NA |
| Midwest or North Central | 1.312 (1.199-1.437) | < 0.0001 | 1.223 (1.162-1.286) | < 0.0001 |
| South | 1.124 (1.031-1.225) | 0.0078 | 1.083 (1.032-1.137) | 0.0015 |
| West | 1.179 (1.065-1.306) | 0.0015 | 1.148 (1.085-1.213) | < 0.0001 |
| Comorbidities | ||||
| Liver disease, mild | 1.401 (1.251-1.569) | < 0.0001 | 1.156 (1.091-1.224) | < 0.0001 |
| Liver disease, moderate to severe | 1.206 (0.867-1.677) | 0.2655 | 0.976 (0.827-1.152) | 0.7708 |
| Peptic ulcer disease | 1.532 (1.247-1.881) | < 0.0001 | 1.254 (1.133-1.387) | < 0.0001 |
| Esophageal disorders | 1.684 (1.582-1.792) | < 0.0001 | 1.528 (1.481-1.576) | < 0.0001 |
| Gastritis | 1.236 (1.063-1.436) | 0.0059 | 1.096 (1.018-1.18) | 0.0145 |
| Diabetes mellitus with chronic complications | 1.326 (1.181-1.489) | < 0.0001 | 1.201 (1.131-1.274) | < 0.0001 |
| Diabetes mellitus without chronic complications | 1.033 (0.946-1.128) | 0.4688 | 1.064 (1.018-1.113) | 0.0059 |
| Hypertension | 1.082 (1.018-1.151) | 0.0115 | 1.091 (1.055-1.128) | < 0.0001 |
| Heart Failure | 0.922 (0.839-1.014) | 0.0943 | 1.005 (0.955-1.058) | 0.8505 |
| Coronary artery disease | 0.858 (0.787-0.936) | 0.0005 | 0.901 (0.861-0.944) | < 0.0001 |
| Cardiac dysrhythmias | 0.811 (0.742-0.887 | < 0.0001 | 0.884 (0.844-0.925) | < 0.0001 |
| Myocardial infarction | 0.703 (0.512-0.965) | 0.0295 | 0.684 (0.587-0.797) | < 0.0001 |
| Stroke | 0.604 (0.443-0.825) | 0.0015 | 0.564 (0.484-0.656) | < 0.0001 |
| Iron Deficiency Anemia | 1.009 (0.908-1.122) | 0.8635 | 1.017 (0.966-1.071) | 0.5143 |
| Nutritional deficiencies | 1.539 (1.344-1.762) | < 0.0001 | 1.282 (1.199-1.37) | < 0.0001 |
| Lipid Metabolism disorders | 1.210 (1.130-1.296) | < 0.0001 | 1.192 (1.15-1.237) | < 0.0001 |
| Thyroid disorders | 1.327 (1.231-1.430) | < 0.0001 | 1.247 (1.201-1.295) | < 0.0001 |
| Chronic Pancreatitis | 1.788 (1.415-2.259) | < 0.0001 | 1.356 (1.207-1.524) | < 0.0001 |
| Renal failure, moderate | 1.026 (0.919-1.145) | 0.6530 | 1.04 (0.981-1.102) | 0.1839 |
| Renal failure, severe | 1.072 (0.897-1.282) | 0.4445 | 1.117 (1.02-1.223) | 0.0172 |
| Menopausal disorders | 1.716 (1.211-2.432) | 0.0024 | 1.433 (1.206-1.702) | < 0.0001 |
| Osteoporosis | 1.280 (1.171-1.399) | < 0.0001 | 1.21 (1.157-1.265) | < 0.0001 |
| Osteoarthritis | 1.287 (1.187-1.395) | < 0.0001 | 1.215 (1.167-1.264) | < 0.0001 |
| Obesity | 1.500 (1.377-1.635) | < 0.0001 | 1.228 (1.176-1.283) | < 0.0001 |
| Weight Loss | 1.253 (0.964-1.628 | 0.0913 | 1.212 (1.067-1.378) | 0.0032 |
| Protein-calorie malnutrition | 1.162 (1.045-1.291) | 0.0054 | 1.103 (1.047-1.162) | 0.0002 |
| Leukemia | 0.980 (0.643-1.492) | 0.9237 | 1.05 (0.856-1.288) | 0.6351 |
| Lymphoma | 0.875 (0.610-1.255) | 0.4685 | 1.014 (0.852-1.207) | 0.8744 |
| Metastatic cancer | 0.975 (0.744-1.277) | 0.8532 | 1.095 (0.948-1.265) | 0.2168 |
| Solid tumor without metastasis, malignant | 0.917 (0.775-1.085) | 0.3140 | 0.944 (0.863-1.034) | 0.2131 |
| Paralysis | 1.391 (1.110-1.744) | 0.0042 | 1.48 (1.32-1.659) | < 0.0001 |
| Dementia | 1.601 (1.344-1.908) | < 0.0001 | 2.082 (1.906-2.274) | < 0.0001 |
| AIDS | 0.866 (0.422-1.776) | 0.6938 | 0.826 (0.58-1.178) | 0.2847 |
| Smoking | 1.410 (1.325-1.502) | < 0.0001 | 1.231 (1.193-1.271) | < 0.0001 |
| Alcohol | 2.296 (1.922-2.743) | < 0.0001 | 2.109 (1.927-2.308) | < 0.0001 |
| Opioids | 2.430 (2.128-2.774) | < 0.0001 | 2.009 (1.882-2.145) | < 0.0001 |
Comorbidities associated with higher MDD in IBD-RA patients included alcohol (aOR: 2.11; 95%CI: 1.93–2.31; P < 0.0001), opioids (aOR: 2.00; 95%CI: 1.83–2.15; P < 0.0001), smoking (aOR: 1.23; 95%CI: 1.19–1.27; P < 0.0001), esophageal disorders (aOR: 1.53; 95%CI: 1.48-1.58; P < 0.0001), peptic ulcer disease (PUD) (aOR: 1.26; 95%CI: 1.13-1.39; P < 0.0001), chronic pancreatitis (aOR: 1.36; 95%CI: 1.21-1.53; P < 0.0001), mild liver disease (aOR: 1.16; 95%CI: 1.09-1.23; P < 0.0001), gastritis (aOR: 1.10; 95%CI: 1.09-1.18; P = 0.02), paralysis (aOR: 1.48; 95%CI: 1.32-1.66; P < 0.0001), dementia (aOR: 2.08; 95%CI: 1.91–2.27; P < 0.0001), menopausal disorders (aOR: 1.43; 95%CI: 1.21-1.70; P < 0.0001), obesity (aOR: 1.29; 95%CI: 1.18-1.28; P < 0.0001), nutritional deficiencies (aOR: 1.28; 95%CI: 1.20-1.37; P < 0.0001), weight loss (aOR: 1.21; 95%CI: 1.07-1.38; P < 0.0001), protein-calorie malnutrition (aOR: 1.10; 95%CI: 1.05-1.16; P = 0.0002),thyroid disorders (aOR: 1.25; 95%CI: 1.20-1.30; P < 0.0001), osteoarthritis (aOR: 1.22; 95%CI: 1.17-1.27; P < 0.0001), osteoporosis (aOR: 1.21; 95%CI: 1.16-1.27; P < 0.0001), diabetes mellitus with chronic complications (aOR: 1.20; 95%CI: 1.13-1.28; P < 0.0001), lipid metabolism disorders (aOR: 1.19; 95%CI: 1.15-1.24; P < 0.0001), and severe renal failure (aOR: 1.12; 95%CI: 1.02-1.23; P < 0.0001).
Various studies have been done in the past, which show a higher rate of depression in patients with IMIDs like RA and IBD than in the general population[20,21]. Patients with coexisting IMID and depression tend to have higher disease activity[1], more fatigue[22], distressing symptoms of pain[23], lower health-related quality of life, and increasing health care costs[24] than patients without depression[1]. Depression as a comorbidity negatively impacts IMID patients’ physical function, mental health, symptom severity, overall morbidity, and mortality[24].
RA is an autoimmune-mediated disease leading to joint destruction, directed mainly by the action of T cells and inflammatory cytokines[3,22]. There is a lack of complete understanding of the biological mechanism responsible for this association of depression with these autoimmune inflammatory disorders. It is mainly attributed to chronic inflammation caused by elevated cytokines like interleukin (IL)-1β, tumor necrosis factor-α, and IL-6. These cytokines decrease neurogenesis and influence the homeostasis of the neurotransmission axis, like glutamate-dependent pathways, monoaminergic pathways, and the hypothalamic-pituitary-adrenal (HPA) axis[8]. Hence, these factors contribute to the development of depression by the impaired physiological responses to stress, resulting in increased pain, fever, fatigue, lack of interest, and thus poor long-term outcomes[3].
Due to the bidirectional communication via the gut-brain axis, reduced social functioning, and impaired quality of life, patients with IBD have increased rates of psychiatric disorders as compared to the general population. Walker et al[10] demonstrated that individuals with IBD have more than double the lifetime prevalence of MDD compared to the general population[10]. Marrie et al[25] showed that the incidence and prevalence of depression, anxiety, and bipolar disorders are elevated in RA patients compared to a matched population[25]. The prevalence of depression in patients with RA is approximately 19%, with conservative estimates, most common in females and younger age groups[5]. In our study, the prevalence of depression in the IBD-RA cohort is approximately 20%, females have 50% more odds of depression than males, and younger patients have higher odds of depression (patients aged 18-44 have 88% higher odds of depression than patients aged 65-84 years). We noted an increasing trend of MDD in the IBD-RA cohort (Figure 1) and an increasing trend in the mean age (Figure 3A). We also exhibited a growing trend in hospital charges (Figure 3E) with a decreasing trend in the length of stay (Figure 3D).
Pezzato et al[26] assessed 490 Italian patients with RA and found that depression was more frequent in females and unemployed patients[26]. In our study, patients with private insurance have lower odds of depression than patients with Medicare. Physicians should be aware that women and those of lower socioeconomic status are at increased risk of these disorders.
Multimorbidity, defined as the coexistence of multiple health conditions, is a growing public health challenge[27]. EULAR considers comorbidities like cardiovascular diseases, malignancy, osteoporosis, infection, and depression in rheumatic diseases[28]. Depression screening may be a valuable tool for interventions to improve health-related quality of life in individuals with IMIDs. In our study, various comorbidities are independently linked with depression.
GI diseases are closely linked with depression, possibly due to the gut-brain axis. A literature review showed a positive correlation between the severity of reflux esophagitis with depression[29]. In our study, patients with esophageal disorders are at 1.5 times increased risk of MDDs. Kim et al[30] demonstrated that patients with PUD have 1.47 times higher odds of depression. Alkhayyat et al[31] showed that chronic pancreatitis patients are at an increased risk of depression than those without it. Our study showed a 1.3 times increase in risk with chronic pancreatitis and 1.2 times with PUD. Kim et al[30] mentioned the bidirectional relationship between PUD and depression, as depression causes consistent activation of the HPA axis, leading to immune dysfunction and elevating the risk of PUD. Conversely, PUD increases the risk of depression by increasing neuropeptide expression of substance P and its receptors.
Among the elderly, malnourished subjects were 31% more likely to have symptoms of depression than those with normal nutritional status[32,33]. Our results showed that nutritional deficiencies are 1.3 times higher in the depression cohort. There is growing evidence of the crucial necessity of neuron membrane cholesterol in the organization and function of the 5-HT1A serotonin receptor. Hence, low cholesterol level is associated with depression and suicidality[34]. Our study also found lipid metabolism disorders as an independent predictor of depression.
In our study, severe renal failure is an independent predictor of depression in IBD-RA subjects. The literature review showed that about one-quarter of dialysis patients suffer from a MDD. Medications reduced physical function, and dietary restrictions are the main contributing factors[35]. The prevalence of depression in chronic kidney disease (CKD) stage 5 was 39 times higher than in CKD stages 1-5[36].
Depression has also been associated with elevated pain and enhanced functional disability in patients with osteoarthritis. Depression affects the HPA axis by altering the release of hypothalamic corticotropin-releasing hormone, increasing its levels in the cerebrospinal fluid and changing the set point threshold for negative feedback, which results in hypercortisolism and thus increased bone loss[37]. Stubbs et al[38] meta-analysis showed that patients with depression had lower bone mass than controls[38]. Our study showed 1.2 times higher odds of having depression with both osteoporosis and osteoarthritis. Our study results agree with Stubbs et al[38] findings that showed a 1.17% relative risk of depression in osteoarthritis patients compared with the non-osteoarthritis group[38].
Obesity at baseline increased the risk of the onset of depression at follow-up. However, depression increased the odds of developing obesity (OR: 1.58; 95%CI: 1.33-1.87; P < 0.001)[39]. Our study also exhibited increased odds of depression with obesity (aOR: 1.29; 95%CI: 1.18-1.28; P < 0.0001). Interestingly, our study also increased the odds of depression with weight loss (aOR: 1.21; 95%CI: 1.07-1.38; P < 0.0001). Dietary effects on mental health can be explained by the anti-inflammatory effect (i.e., omega-3 polyunsaturated fatty acids), antioxidant effect (anthocyanins, etc.), or functional modulation (group B vitamins, L-ornithine, tryptophan amino acids, glycine, etc.)[40,41]. A National Health and Nutrition Examination Survey study reported that using even a single unhealthy weight-loss strategy was significantly linked with depression[42]. In our study, the odds of depression are 28% higher in patients with nutritional deficiencies.
Depression can be both a risk factor and a prodrome for dementia. Amongst all the proposed mechanisms, trophic, inflammatory, and cerebrovascular factors may contribute, along-with monoamine deficiency and severity of plaques and tangle pathology[43]. In a meta-analysis that included 5897 subjects with dementia from 20 studies, the prevalence rates of depression in mild, moderate, and severe dementia were 38%, 41%, and 37%, respectively[44]. In our study, patients with dementia have two times increased likelihood of depression.
Nearly one-third of patients with MDD also have substance use disorders. It yields a higher risk of suicide and greater social and personal impairment[45]. Alcohol use disorder is notable in our society (lifetime prevalence of 29.1%) and is associated with MDD[46]. We also found alcohol an independent predictor of depression in patients with RA-IBD. Other Substance abuses include nicotine; its withdrawal is known to cause a marked increase in negative affect among smokers in the general population[47]. A group of cross-sectional studies showed that current smokers were more depressed than never-smokers and former smokers[48]. In our study, smoking is independently associated with 1.2 times higher odds of depression. Similarly, the literature review showed that opioids were associated with higher odds of new-onset major depressive symptoms without baseline symptoms of MDD[49]. These findings correspond with our results that showed opioid use doubled the risk of depression in the IBD-RA group.
Another vital study finding includes thyroid disorders as an independent predictor of depression. A literature search showed depression is associated with a functional interruption of the hypothalamus, causing dysregulation of the hippocampal inhibitory glucocorticoid feedback pathway to the hypothalamus. This results in increased cortisol levels and impaired dexamethasone suppression. A similar situation exists with the thyroid axis. An increased T4 and blunted thyrotropin response to exogenous thyrotropin-releasing hormone (TRH) in depression. This is due to the glucocorticoid activation of the TRH neuron that increases TRH secretion and down-regulates the TRH receptor on the thyrotrope[50,51]. In our study, patients with lipid disorders have an increased likelihood of depression. It correlates with a meta-analysis Wei et al[52] that showed that hyperlipidemia patients have 1.7 times more odds of MDD than the general population[52].
To our best knowledge, this is the first large study performed in the United States, analyzing twenty years of inpatient sample data (n = 26155) of patients with depression affected by RA and IBD. This study's biggest strength is identifying the prevalence of depression among patients with dual autoimmune diseases and a motivation to incorporate such values of depression screening in outpatient clinics. Our analysis also recommends the possibility of a Rheumatology-Gastroenterology Comorbidity clinic initiative, which could identify various comorbidities like depression and substance use disorder among IMID patients. This is an area that requires more attention. Most rheumatologists and gastroenterologists do not routinely screen their RA patients for depression which was evident during the verbal surveys taken at the various conferences. It could be related to time limitations, inadequate psychiatry referral services, lack of training, or even lack of confidence in managing mental health problems. A simple Patient Health Questionnaire (PHQ-2) or PHQ-9 questionnaire can be incorporated into the clinic outflow, especially at the onset of the diagnosis. Such efforts are pivotal in identifying depression in our patients, ensuring patient-mental health support system interrelation, and hence normalizing the discussion around their mental health. This study's biggest strength is identifying the prevalence of depression among patients with dual autoimmune diseases and a motivation to incorporate such values in our outpatient clinics. This is pivotal for healthcare delivery systems and economies in the global context of treating chronic diseases like RA and IBD, especially in the era of the coronavirus disease 2019 pandemic as we understand that the standard care for these conditions, particularly immunomodulators, is extremely costly.
This study has several limitations using the NIS dataset, including the inability to access laboratory values, treatment options, and testing conditions, including colonoscopy findings and severity of IBD based on histology. We intended to investigate the prevalence of IMIDs and depression; it’s challenging to know the clinical correlates of depression regarding RA/IBD disease activity and disability. This study is performed on the inpatient population. However, IBD/RA and MDD are outpatient diagnoses except for IBD flare-ups. Individuals with fibromyalgia, connective tissue diseases (Sjogren, sclerodermas, dermatomyositis, polymyositis), vasculitis, gout, infective arthritis, polymyalgia rheumatica, or other IBDs were excluded or not chosen as comorbidity which could also be a reason for depressive symptoms. However, these ailments were not selected as they have been known from previous studies, and other co-morbidities mentioned in our research could be highlighted. NIS entry is equivalent to one hospitalization. If a patient is admitted more than once, one patient may contribute multiple entries. Finally, inherent database limitations include a lack of disease process-specific variables and coding errors without formal validation.
This study offers the opportunity to increase our knowledge of the various comorbidities by investigating depression and its clinical correlates as part of the routine clinical monitoring in the outpatient clinic setting and, hence, a multimorbidity approach that is more patient-focused. There is an inevitable rise in the prevalence of depression in younger patients with IBD-RA combined autoimmune disease compared to their counterparts. These patients are also at higher risk of the increased cost of care, disability, and poor treatment adherence. It is crucial to educate the involved physicians to identify the early signs and symptoms of depression in patients with IBD or RA or IBD-RA combined and treat them or have them treated to have a better overall prognosis. As physicians, we can play an important role as part of social determinants of health by giving good quality of care. Timely recognition of depression in these patients is critical to preventing disability.
Inflammatory bowel disease (IBD) and rheumatoid arthritis (RA), are found to have a substantial societal burden, increased healthcare costs, and progressive disability. Studies suggest that patients with vs without depression have a more significant disability, a lower likelihood of remission, and reduced adherence to therapy.
The role of the Brain-Gut axis and Brain-Joint axis in the development of depression has been discussed, but there is not enough literature demonstrating the combined Brain-Gut-Joint axis.
Our primary aim is to identify a pooled prevalence level and temporal trends of depression in hospitalized IBD-RA patients. We aimed to investigate clinical factors associated with depression in these patients.
All adult hospitalized patients from January 2000 to December 2019 in the nationwide inpatient sample were captured. The study population included all patients with a primary or secondary IBD-RA overlap disease using corresponding international classification of diseases (ICD)-9 and ICD-10 codes.
Other factors associated with higher major depressive disorder included younger age, female gender, white race, alcohol, opioids, esophageal disorders, peptic ulcer disease, chronic pancreatitis, paralysis, dementia, menopausal disorders, obesity, nutritional deficiencies, diabetes mellitus with chronic complications, and osteoarthritis.
There is an inevitable rise in the prevalence of depression in younger patients with IBD-RA combined autoimmune diseases. As physicians, we can play an important role in social determinants of health by giving good quality care. Timely recognition of depression in these patients is critical to preventing disability.
Our study discusses the clinical data on this combined axis and emphasizes potentially valuable strategies for managing these patients. This study will open the door for further research and educate the involved physicians to identify the early signs and symptoms of depression in patients with IBD or RA or IBD-RA combined and treat them or have them treated to have a better overall prognosis.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Liu XQ, China; Xiao J, China S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Enns MW, Bernstein CN, Kroeker K, Graff L, Walker JR, Lix LM, Hitchon CA, El-Gabalawy R, Fisk JD, Marrie RA; CIHR Team in Defining the Burden and Managing the Effects of Psychiatric Comorbidity in Chronic Immunoinflammatory Disease. The association of fatigue, pain, depression and anxiety with work and activity impairment in immune mediated inflammatory diseases. PLoS One. 2018;13:e0198975. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 60] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 2. | Englbrecht M, Alten R, Aringer M, Baerwald CG, Burkhardt H, Eby N, Flacke JP, Fliedner G, Henkemeier U, Hofmann MW, Kleinert S, Kneitz C, Krüger K, Pohl C, Schett G, Schmalzing M, Tausche AK, Tony HP, Wendler J. New insights into the prevalence of depressive symptoms and depression in rheumatoid arthritis - Implications from the prospective multicenter VADERA II study. PLoS One. 2019;14:e0217412. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 3. | Lwin MN, Serhal L, Holroyd C, Edwards CJ. Rheumatoid Arthritis: The Impact of Mental Health on Disease: A Narrative Review. Rheumatol Ther. 2020;7:457-471. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 92] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 4. | Ordás I, Eckmann L, Talamini M, Baumgart DC, Sandborn WJ. Ulcerative colitis. Lancet. 2012;380:1606-1619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1151] [Cited by in RCA: 1547] [Article Influence: 119.0] [Reference Citation Analysis (5)] |
| 5. | Almutairi K, Nossent J, Preen D, Keen H, Inderjeeth C. The global prevalence of rheumatoid arthritis: a meta-analysis based on a systematic review. Rheumatol Int. 2021;41:863-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 373] [Article Influence: 74.6] [Reference Citation Analysis (0)] |
| 6. | Pryce CR, Fontana A. Depression in Autoimmune Diseases. Curr Top Behav Neurosci. 2017;31:139-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 7. | GBD 2017 Inflammatory Bowel Disease Collaborators. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020;5:17-30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1466] [Cited by in RCA: 1455] [Article Influence: 291.0] [Reference Citation Analysis (0)] |
| 8. | Isnardi CA, Capelusnik D, Schneeberger EE, Bazzarelli M, Berloco L, Blanco E, Benítez CA, Luján Benavidez F, Scarafia S, Lázaro MA, Pérez Alamino R, Colombres F, Kohan MP, Sosa J, Gonzalez Lucero L, Barbaglia AL, Maldonado Ficco H, Citera G. Depression Is a Major Determinant of Functional Capacity in Rheumatoid Arthritis. J Clin Rheumatol. 2021;27:S180-S185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Barberio B, Zamani M, Black CJ, Savarino EV, Ford AC. Prevalence of symptoms of anxiety and depression in patients with inflammatory bowel disease: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2021;6:359-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 398] [Article Influence: 99.5] [Reference Citation Analysis (0)] |
| 10. | Walker JR, Ediger JP, Graff LA, Greenfeld JM, Clara I, Lix L, Rawsthorne P, Miller N, Rogala L, McPhail CM, Bernstein CN. The Manitoba IBD cohort study: a population-based study of the prevalence of lifetime and 12-month anxiety and mood disorders. Am J Gastroenterol. 2008;103:1989-1997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 334] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 11. | Peppas S, Pansieri C, Piovani D, Danese S, Peyrin-Biroulet L, Tsantes AG, Brunetta E, Tsantes AE, Bonovas S. The Brain-Gut Axis: Psychological Functioning and Inflammatory Bowel Diseases. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 59] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 12. | Süß P, Rothe T, Hoffmann A, Schlachetzki JCM, Winkler J. The Joint-Brain Axis: Insights From Rheumatoid Arthritis on the Crosstalk Between Chronic Peripheral Inflammation and the Brain. Front Immunol. 2020;11:612104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 13. | Introduction to the NIS. Healthcare Cost and Utilization Project (HCUP). February 2018. Agency for Healthcare Research and Quality, Rockville, MD. [cited 10 August 2022]. Available from: www.hcup-us.ahrq.gov/db/nation/nis/NIS_Introduction_2015.jsp. |
| 14. | HCUP National Inpatient Sample (NIS). Healthcare Cost and Utilization Project (HCUP). 2012. Agency for Healthcare Research and Quality, Rockville, MD. [cited 3 February 2022]. Available from: www.hcup-us.ahrq.gov/nisoverview.jsp. |
| 15. | HCUP NIS Trend Weights. Healthcare Cost and Utilization Project (HCUP). October 2021. Agency for Healthcare Research and Quality, Rockville, MD. [cited 3 February 2022]. Available from: www.hcup-us.ahrq.gov/db/nation/nis/trendwghts.jsp.. |
| 16. | DUA Training - Accessible Version. Healthcare Cost and Utilization Project (HCUP). April 2020. Agency for Healthcare Research and Quality, Rockville, MD. [cited 3 February 2022]. Available from: www.hcup-us.ahrq.gov/DUA/dua_508/DUA508version.jsp. |
| 17. | HCUP Clinical Classifications Software Refined (CCSR) for ICD-10-CM diagnoses, v2021. 2. Healthcare Cost and Utilization Project (HCUP). Agency for Healthcare Research and Quality, Rockville, MD. [cited 4 April 2022]. Available from: www.hcup-us.ahrq.gov/toolssoftware/ccsr/dxccsr.jsp. |
| 18. | HCUP Clinical Classifications Software Refined (CCSR) for ICD-10-PCS procedures, v2021. 1. Healthcare Cost and Utilization Project (HCUP). Agency for Healthcare Research and Quality, Rockville, MD. [cited 15 January 2022]. Available from: www.hcup-us.ahrq.gov/toolssoftware/ccsr/prccsr.jsp. |
| 19. | US Bureau of Labor Statistics. Consumer price index for all urban consumers: Hospital services. [cited 15 January 2022]. Available from: https://data.bls.gov/timeseries/CUUR0000SA0. |
| 20. | Matcham F, Rayner L, Steer S, Hotopf M. The prevalence of depression in rheumatoid arthritis: a systematic review and meta-analysis. Rheumatology (Oxford). 2013;52:2136-2148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 637] [Cited by in RCA: 585] [Article Influence: 48.8] [Reference Citation Analysis (0)] |
| 21. | Neuendorf R, Harding A, Stello N, Hanes D, Wahbeh H. Depression and anxiety in patients with Inflammatory Bowel Disease: A systematic review. J Psychosom Res. 2016;87:70-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 448] [Cited by in RCA: 403] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 22. | van Hoogmoed D, Fransen J, Bleijenberg G, van Riel P. Physical and psychosocial correlates of severe fatigue in rheumatoid arthritis. Rheumatology (Oxford). 2010;49:1294-1302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 162] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 23. | Anderson KO, Bradley LA, McDaniel LK, Young LD, Turner RA, Agudelo CA, Keefe FJ, Pisko EJ, Snyder RM, Semble EL. The assessment of pain in rheumatoid arthritis. Validity of a behavioral observation method. Arthritis Rheum. 1987;30:36-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 43] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | Li N, Chan E, Peterson S. The economic burden of depression among adults with rheumatoid arthritis in the United States. J Med Econ. 2019;22:372-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 25. | Marrie RA, Hitchon CA, Walld R, Patten SB, Bolton JM, Sareen J, Walker JR, Singer A, Lix LM, El-Gabalawy R, Katz A, Fisk JD, Bernstein CN; Canadian Institutes of Health Research Team in Defining the Burden and Managing the Effects of Psychiatric Comorbidity in Chronic Immunoinflammatory Disease. Increased Burden of Psychiatric Disorders in Rheumatoid Arthritis. Arthritis Care Res (Hoboken). 2018;70:970-978. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 96] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 26. | Pezzato S, Bonetto C, Caimmi C, Tomassi S, Montanari I, Gnatta MG, Fracassi E, Cristofalo D, Rossini M, Carletto A, Tosato S. Depression is associated with increased disease activity and higher disability in a large Italian cohort of patients with rheumatoid arthritis. Adv Rheumatol. 2021;61:57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 27. | Johnston MC, Crilly M, Black C, Prescott GJ, Mercer SW. Defining and measuring multimorbidity: a systematic review of systematic reviews. Eur J Public Health. 2019;29:182-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 253] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 28. | Baillet A, Gossec L, Carmona L, Wit Md, van Eijk-Hustings Y, Bertheussen H, Alison K, Toft M, Kouloumas M, Ferreira RJ, Oliver S, Rubbert-Roth A, van Assen S, Dixon WG, Finckh A, Zink A, Kremer J, Kvien TK, Nurmohamed M, van der Heijde D, Dougados M. Points to consider for reporting, screening for and preventing selected comorbidities in chronic inflammatory rheumatic diseases in daily practice: a EULAR initiative. Ann Rheum Dis. 2016;75:965-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 150] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 29. | Wang R, Wang J, Hu S. Study on the relationship of depression, anxiety, lifestyle and eating habits with the severity of reflux esophagitis. BMC Gastroenterol. 2021;21:127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 30. | Kim SY, Min C, Oh DJ, Choi HG. Reciprocal association between depression and peptic ulcers: Two longitudinal follow-up studies using a national sample cohort. Sci Rep. 2020;10:1749. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 31. | Alkhayyat M, Abou Saleh M, Coronado W, Abureesh M, Al-Otoom O, Qapaja T, Mansoor E, Simons-Linares CR, Stevens T, Chahal P. Increasing Prevalence of Anxiety and Depression Disorders After Diagnosis of Chronic Pancreatitis: A 5-Year Population-Based Study. Pancreas. 2021;50:153-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 32. | Wei J, Fan L, Zhang Y, Li S, Partridge J, Claytor L, Sulo S. Association Between Malnutrition and Depression Among Community-Dwelling Older Chinese Adults. Asia Pac J Public Health. 2018;30:107-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 33. | García-Montero C, Ortega MA, Alvarez-Mon MA, Fraile-Martinez O, Romero-Bazán A, Lahera G, Montes-Rodríguez JM, Molina-Ruiz RM, Mora F, Rodriguez-Jimenez R, Quintero J, Álvarez-Mon M. The Problem of Malnutrition Associated with Major Depressive Disorder from a Sex-Gender Perspective. Nutrients. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 34. | Segoviano-Mendoza M, Cárdenas-de la Cruz M, Salas-Pacheco J, Vázquez-Alaniz F, La Llave-León O, Castellanos-Juárez F, Méndez-Hernández J, Barraza-Salas M, Miranda-Morales E, Arias-Carrión O, Méndez-Hernández E. Hypocholesterolemia is an independent risk factor for depression disorder and suicide attempt in Northern Mexican population. BMC Psychiatry. 2018;18:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 58] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 35. | Natale P, Palmer SC, Ruospo M, Saglimbene VM, Rabindranath KS, Strippoli GF. Psychosocial interventions for preventing and treating depression in dialysis patients. Cochrane Database Syst Rev. 2019;12:CD004542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 36. | Palmer S, Vecchio M, Craig JC, Tonelli M, Johnson DW, Nicolucci A, Pellegrini F, Saglimbene V, Logroscino G, Fishbane S, Strippoli GF. Prevalence of depression in chronic kidney disease: systematic review and meta-analysis of observational studies. Kidney Int. 2013;84:179-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 409] [Cited by in RCA: 546] [Article Influence: 45.5] [Reference Citation Analysis (0)] |
| 37. | Cizza G, Primma S, Coyle M, Gourgiotis L, Csako G. Depression and osteoporosis: a research synthesis with meta-analysis. Horm Metab Res. 2010;42:467-482. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 107] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 38. | Stubbs B, Aluko Y, Myint PK, Smith TO. Prevalence of depressive symptoms and anxiety in osteoarthritis: a systematic review and meta-analysis. Age Ageing. 2016;45:228-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 192] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 39. | Luppino FS, de Wit LM, Bouvy PF, Stijnen T, Cuijpers P, Penninx BW, Zitman FG. Overweight, obesity, and depression: a systematic review and meta-analysis of longitudinal studies. Arch Gen Psychiatry. 2010;67:220-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3235] [Cited by in RCA: 2850] [Article Influence: 190.0] [Reference Citation Analysis (0)] |
| 40. | Grosso G. Nutritional Psychiatry: How Diet Affects Brain through Gut Microbiota. Nutrients. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 41. | Chaitoff A, Swetlik C, Ituarte C, Pfoh E, Lee LL, Heinberg LJ, Rothberg MB. Associations Between Unhealthy Weight-Loss Strategies and Depressive Symptoms. Am J Prev Med. 2019;56:241-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 42. | Enache D, Winblad B, Aarsland D. Depression in dementia: epidemiology, mechanisms, and treatment. Curr Opin Psychiatry. 2011;24:461-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 222] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 43. | Leung DKY, Chan WC, Spector A, Wong GHY. Prevalence of depression, anxiety, and apathy symptoms across dementia stages: A systematic review and meta-analysis. Int J Geriatr Psychiatry. 2021;36:1330-1344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 136] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 44. | Davis L, Uezato A, Newell JM, Frazier E. Major depression and comorbid substance use disorders. Curr Opin Psychiatry. 2008;21:14-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 332] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 45. | Grant BF, Goldstein RB, Saha TD, Chou SP, Jung J, Zhang H, Pickering RP, Ruan WJ, Smith SM, Huang B, Hasin DS. Epidemiology of DSM-5 Alcohol Use Disorder: Results From the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015;72:757-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1532] [Cited by in RCA: 1921] [Article Influence: 192.1] [Reference Citation Analysis (0)] |
| 46. | Mathew AR, Hogarth L, Leventhal AM, Cook JW, Hitsman B. Cigarette smoking and depression comorbidity: systematic review and proposed theoretical model. Addiction. 2017;112:401-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 190] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 47. | Luger TM, Suls J, Vander Weg MW. How robust is the association between smoking and depression in adults? Addict Behav. 2014;39:1418-1429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 169] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 48. | Li X, Fu Q, Scherrer JF, Humphrey D, Leigh I. A temporal relationship between nonmedical opioid Use and major depression in the U.S.: A Prospective study from the National Epidemiological Survey on Alcohol and Related Conditions. J Affect Disord. 2020;273:298-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 49. | Jackson IM. The thyroid axis and depression. Thyroid. 1998;8:951-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 135] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 50. | Williams MD, Harris R, Dayan CM, Evans J, Gallacher J, Ben-Shlomo Y. Thyroid function and the natural history of depression: findings from the Caerphilly Prospective Study (CaPS) and a meta-analysis. Clin Endocrinol (Oxf). 2009;70:484-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 69] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 51. | Chien IC, Lin CH, Chou YJ, Chou P. Increased risk of hyperlipidemia in patients with major depressive disorder: a population-based study. J Psychosom Res. 2013;75:270-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 52. | Wei YG, Cai DB, Liu J, Liu RX, Wang SB, Tang YQ, Zheng W, Wang F. Cholesterol and triglyceride levels in first-episode patients with major depressive disorder: A meta-analysis of case-control studies. J Affect Disord. 2020;266:465-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 68] [Article Influence: 13.6] [Reference Citation Analysis (0)] |