Published online Feb 6, 2023. doi: 10.12998/wjcc.v11.i4.756
Peer-review started: September 1, 2022
First decision: November 22, 2022
Revised: November 29, 2022
Accepted: January 12, 2023
Article in press: January 12, 2023
Published online: February 6, 2023
Processing time: 157 Days and 21.2 Hours
The Omicron variant of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) mainly infects the upper respiratory tract. This study aimed to determine whether the probability of pulmonary infection and the cycle threshold (Ct) measured using the fluorescent polymerase chain reaction (PCR) method were related to pulmonary infections diagnosed via computed tomography (CT).
To analyze the chest CT signs of SARS-CoV-2 Omicron variant infections with different Ct values, as determined via PCR.
The chest CT images and PCR Ct values of 331 patients with SARS-CoV-2 Omicron variant infections were retrospectively collected and categorized into low (< 25), medium (25.00-34.99), and high (≥ 35) Ct groups. The characteristics of chest CT images in each group were statistically analyzed.
The PCR Ct values ranged from 13.36 to 39.81, with 99 patients in the low, 155 in the medium, and 77 in the high Ct groups. Six abnormal chest CT signs were detected, namely, focal infection, patchy consolidation shadows, patchy ground-glass shadows, mixed consolidation ground-glass shadows, subpleural interstitial changes, and pleural changes. Focal infections were less frequent in the low Ct group than in the medium and high Ct groups; these infections were the most common sign in the medium and high Ct groups. Patchy consolidation shadows and pleural changes were more frequent in the low Ct group than in the other two groups. The number of patients with two or more signs was greater in the low Ct group than in the medium and high Ct groups.
The chest CT signs of patients with pulmonary infection caused by the Omicron variants of SARS-CoV-2 varied depending on the Ct values. Identification of the characteristics of Omicron variant infection can help subsequent planning of clinical treatment.
Core Tip: Pulmonary infections caused by the Omicron variant of severe acute respiratory syndrome coronavirus 2 were highly correlated with cycle threshold (Ct) values. Lower Ct values were associated with a higher incidence and degree of pulmonary damage.
- Citation: Ying WF, Chen Q, Jiang ZK, Hao DG, Zhang Y, Han Q. Chest computed tomography findings of the Omicron variants of SARS-CoV-2 with different cycle threshold values. World J Clin Cases 2023; 11(4): 756-763
- URL: https://www.wjgnet.com/2307-8960/full/v11/i4/756.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i4.756
Since the emergence of the Omicron variant of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) on November 24, 2021[1], it has spread in most countries and caused infection in numerous individuals worldwide[2]. Although the virulence of Omicron appears to be weaker than that of previous SARS-CoV-2 variants, the large-scale use of vaccines against SARS-CoV-2, particularly with enhanced needles, has reduced the mortality rate associated with SARS-CoV-2[3]. Omicron is more infectious and transmissible than other variants[4,5] and causes damage to the lungs of some patients to different degrees[6]. Therefore, determining the degree of pulmonary damage caused by different Omicron viral load levels is key to understanding the characteristics of Omicron variant infection and its inhibition[7].
Fluorescent polymerase chain reaction (PCR) is the gold standard for diagnosing SARS-CoV-2 infection, and its cycle threshold (Ct) values can help achieve a reliable assessment and comparison of viral loads in patients[8-10]. Ct diagnosis has an extremely high diagnostic efficiency for determining the degree of pulmonary damage in patients with SARS-CoV-2 infections[11,12]. So far, only a few studies have attempted to correlate Ct values and pulmonary damage evidenced on chest computed tomography (CT) images obtained from patients with Omicron variant infection. The author’s hospital is a designated treatment facility for symptomatic patients with Omicron variant infection. This study aimed to assess the chest CT signs of patients with Omicron variant infection with different Ct values for determining the severity of the infection and providing guidance for subsequent clinical treatments.
Chest CT scans of patients with Omicron variant infection admitted to Shanghai Xuhui Dahua Hospital were collected from April to May 2022. The inclusion criteria were patients with PCR-positive results (Ct value of < 40) and those with viral infection signs on chest CT within 48 h after PCR. The exclusion criteria were patients with bacterial infections, as determined using laboratory indexes, or those with a clinical or CT diagnosis of other basic pulmonary infectious diseases. This study complied with the ethical standards and was approved by the Ethics Committee of Shanghai Xuhui Dahua Hospital (approval No. 20220804).
All CT scans were obtained using a Siemens 64-slice spiral CT scanner (SOMATOM sensation) from the pulmonary apex to the pulmonary bottom using the following scanning parameters: Detector collimation, 64.0 mm × 0.6 mm; tube voltage, 120 KV; tube current, automatic milliampere; slice thickness, 5 mm; reconstructed slice thickness, 1.5 mm; reconstructed slice spacing, 1.5 mm; and matrix, 512 × 512.
The lower Ct value between the ORF and N genes was selected as the PCR Ct value. The patients were divided into three groups based on their Ct values: Low (< 25), medium (25.00-34.99), and high (≥ 35) Ct groups. Double-blind analysis was conducted using the CT data of each group by two physicians with > 10 years of experience in radiological diagnosis. In cases of disagreement, consensus was achieved after mutual consultation. When patients exhibited pulmonary infection foci with a long diameter (≤ 20 mm), it was considered as a focal infection. Other infection signs, including patchy consolidation shadows, patchy ground-glass density shadows, subpleural interstitial changes, mixed consolidation ground-glass shadows, and pleural changes, were judged based on their characteristics.
Statistical analysis of the data was performed using SPSS 23.0. Normally distributed data are expressed as mean ± SD, whereas enumeration data are expressed as case numbers or percentages. Within- and between-group comparisons of CT signs were performed using χ2 test. A P value of < 0.05 was considered to indicate statistical significance.
Chest CT images of 331 patients [143 men and 188 women; age: 76 ± 12 (range: 25-102) years] with Omicron variant infection were collected. All patients showed respiratory symptoms of varying degrees, mainly including fever (n = 247, 74.62%), cough (n = 203, 61.33%), and chest tightness (n = 49, 14.8%). Among them, 187 (56.5%) patients were vaccinated thrice against SARS-CoV-2, 74 (22.36%) were vaccinated twice, 12 (3.63%) were vaccinated once, and 58 (17.52%) were not vaccinated. Additionally, 236 (71.3%) patients had a history of close or secondary contact with patients with SARS-CoV-2 infection, 65 (19.64%) had a definite history of gathering in public places, and 30 (9.06%) had no definite history of close contact with patients with SARS-CoV-2 infection.
The PCR Ct values ranged from 13.36 to 39.81 (average, 28.85 ± 6.68), with 99 (29.91%) patients in the low, 155 (46.83%) in the medium, and 77 (23.26%) in the high Ct groups.
Among all patients, the most common CT sign was focal infection (n = 178, 45.18%), followed by subpleural interstitial changes (n = 81, 20.56%), patchy ground-glass density shadows (n = 76, 19.29%), patchy consolidation shadows (n = 27, 6.85%), pleural changes (n = 20, 5.08%), and mixed consolidation ground-glass shadows (n = 12, 3.04%).
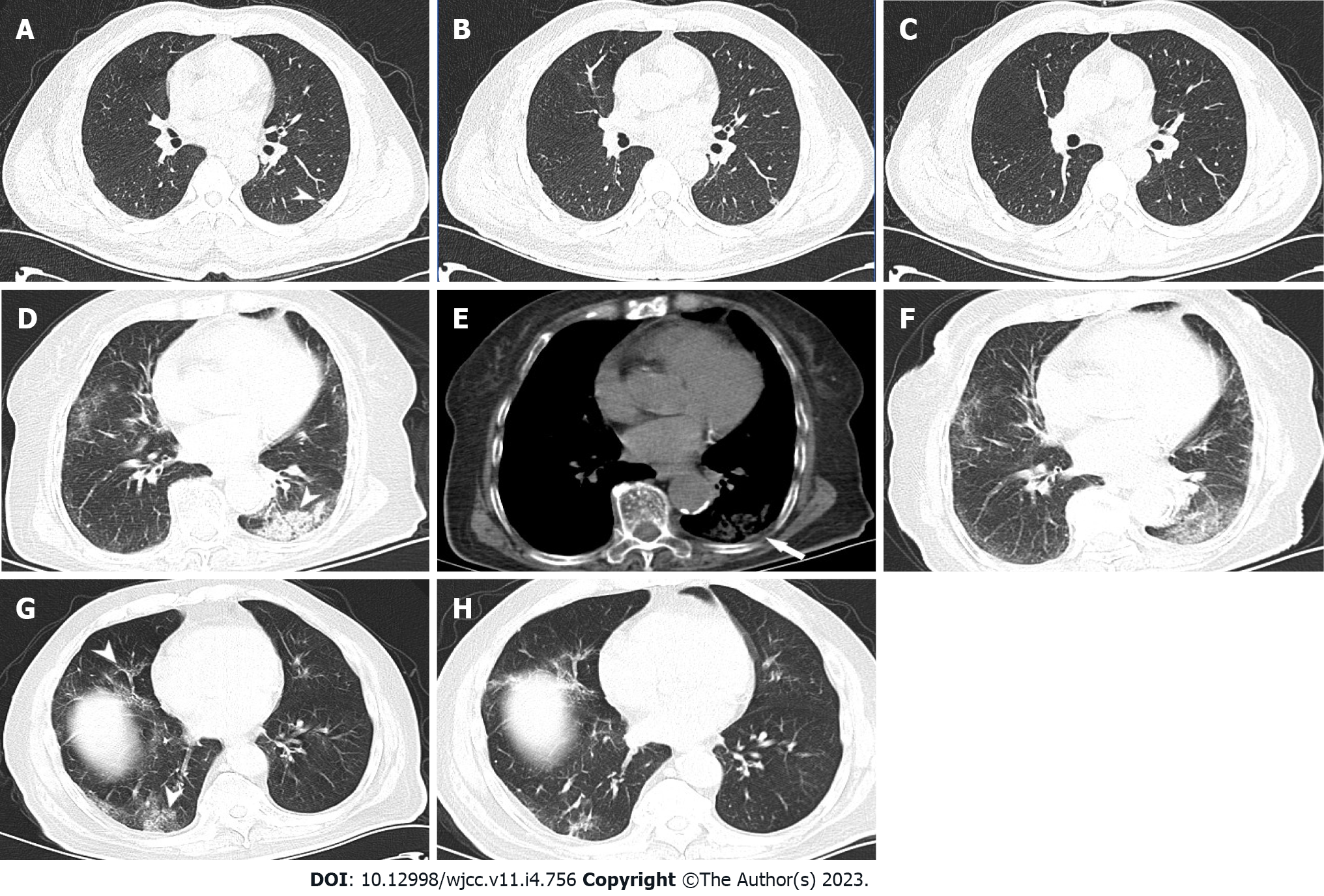
Focal infections were less frequent in the low Ct group (30%) than in the medium (52.27%, χ2 = 10.004, P = 0.002) and high (53.41%, χ2 = 10.895, P = 0.002) Ct groups. Focal infection was the most common sign in the medium and high Ct groups (compared with the second most common sign in the groups: The medium Ct group, χ2 = 23.780, P < 0.001 and the high Ct group, χ2 = 19.100, P < 0.001), with statistically significant differences (Figure 1A-C).
The frequency of patchy consolidation shadows (Figure 1D-F) was the highest in the low Ct group (14.62%), less in the medium Ct group (3.98%; χ2= 7.037, P = 0.014), and the lowest in the high Ct group (1.14%), with statistically significant between-group differences (χ2 = 13.315, P < 0.001).
There were no statistically significant differences in the frequency of patchy ground-glass density shadows (Figure 1G and H) among the low (16.92%), medium (19.32%), and high (22.73%) Ct groups (χ2 = 0.136, χ2 = 1.125, and χ2 = 0.482; P = 0.854, P = 0.377, and P = 0.603, respectively).
Furthermore, no statistically significant differences were observed in subpleural interstitial changes among the low (25.38%), medium (17.61%), and high (19.32%) Ct groups (χ2 = 1.452, χ2 = 1.049, and χ2 = 0.033; P = 0.302, P = 0.394, and P = 1.000, respectively).
In addition, mixed consolidation ground-glass shadows showed no statistically significant differences among the low (2.31%), medium (3.98%), and high (2.27%) Ct groups (χ2 = 0.687, χ2= 0.000, and χ2 = 0.687; P = 0.683, P = 1.000, and P = 0.683, respectively).
The frequency of pleural changes (Figure 1D-F) was the highest in the low Ct group (10.77%), less frequent in the medium Ct group (2.84%; χ2 = 4.916, P = 0.049), and the lowest in the high Ct group (1.13%; χ2 = 8.865, P < 0.005), with statistically significant between-group differences.
Among the three groups, the number of patients with two or more abnormal chest CT signs was the highest in the low Ct group (30.3%, 30/99), less in the medium Ct group [12.26% (19/155), χ2 = 9.765, P = 0.003], and the lowest in the high Ct group [12.99% (10/77), χ2 = 8.562, P = 0.005], with statistically significant between-group differences (Table 1).
| Group | CT sign | ||||||
| Focal infection | Patchy consolidation shadows | Patchy ground-glass density shadows | Mixed consolidation ground-glass shadows | Subpleural interstitial changes | Pleural changes | Total | |
| Low Ct group | 391 | 192 | 22 | 3 | 33 | 142 | 130 |
| Medium Ct group (25.00-34.99) | 921 | 7 | 34 | 7 | 31 | 5 | 176 |
| High Ct group (≥ 35) | 471 | 1 | 20 | 2 | 17 | 1 | 88 |
| Total | 178 | 27 | 76 | 12 | 81 | 20 | 394 |
SARS-CoV-2 is constantly mutating. A recent report in Lancet confirmed that the viral genomes of the current round of local viral epidemics in Shanghai (present since late February) consist of the SARS-CoV-2 BA.2.2 variant, which is a subpopulation of the SARS-CoV-2 Omicron variant (B.1.1.159)[13]. Although its virulence is weaker than that of previous variants (including the Delta variant), it exhibits higher infectivity and stronger ability to escape the immune system, resulting in large-scale infections, high Ct values among symptomatic patients, and high mortality rates, which have an impact on the society. Therefore, it is particularly important to understand the clinical manifestations and imaging characteristics of patients with Omicron variant infection.
Currently, the autopsy reports of patients with Omicron variant infection are rarely reported; therefore, the pathological mechanism of this infection remains unclear. According to the autopsy reports of other SARS-CoV-2 subtypes abroad[14-16], SARS-CoV-2 directly infects target cells, including bronchial and alveolar epithelial cells, vascular epithelial and endothelial cells, and immune cells. The formation of early vasculitis leads to vascular wall and perivascular inflammation, immune cell infiltration, vascular stenosis, thrombosis, and secondary bleeding. In the later stage of SARS-CoV-2 infection, interstitial fibrosis and diffuse alveolar injury of the perivascular lung parenchyma can often occur secondary to infection with various bacteria and mucor, consolidation, and complications, such as mucus plugs formed by airway mucus secretion and peripheral pleural changes. Viruses can travel through the blood and induce pathological changes in other parts of the body.
PCR Ct values indicate the number of amplifications required for detecting SARS-CoV-2. The lower the Ct value, the higher the viral load in a nucleic acid sample and vice versa. A previous study reported a linear correlation between Ct values and viral loads. Accordingly, Ct values can reflect the viral levels in patients to a certain extent[8,10]. Some studies have considered Ct values of < 25 to indicate high viral loads; these patients required a longer viral clearance time[17]. We included patients with Ct values of < 25 in the low Ct group. According to the Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (Trial Version 9) of China, the standard for dissolution in our region was a Ct value of ≥ 35. Moreover, another study reported that the virus can no longer be isolated from samples at Ct values ≥ 35[18]. Thus, we included patients with Ct values ≥ 35 and < 25 in the high and low Ct groups, respectively. Patients with Ct values of 25.00-34.99 were included in the medium Ct group. Along with chest CT features, Ct values can help improve our understanding of the pathological characteristics of patients with Omicron variant infection.
In this study, among all groups, the most common abnormal chest CT sign was focal infection (30.00%-53.41%, all P < 0.05), which was observed as blurred shadows, consolidation shadows, ground-glass density shadows with a long diameter (≤ 2 cm), and small nodule-like shadows with fuzzy edges, most of which were located under the pleura. These findings differ from the most common abnormal sign (patchy ground-glass density shadows) observed in patients with other previously identified SARS-CoV-2 subtype infection[19]. This suggests that the virulence of the Omicron variant is weaker than that of other SARS-CoV-2 variants. In addition, the large-scale use of vaccines leads to the most infection-caused vasculitis being replaced by peripheral vasculitis, more limited secondary peripheral pathological changes, and more common focal infections in the medium and high Ct groups. These findings indicate that lower viral loads in patients with Omicron variant infection result in fewer pulmonary infection foci and improved prognosis.
Among all Ct groups, patchy ground-glass density shadows (16.92%-22.73%) and subpleural interstitial changes (17.61%-25.38%) were also common (but not the most common) chest CT signs. Mixed consolidation ground-glass shadows were less common (2.27%-3.98%). In addition, the above abnormal signs were not significantly different among the three groups (all P > 0.05). These abnormal signs were consistent with the chest CT findings of pneumonia caused by other SARS-CoV-2 variants[20-22], however, their proportions were significantly decreased, indicating that the virulence of Omicron is weaker than that of previously identified variants.
The number of patients with patchy consolidation shadows (14.62%), pleural changes (10.77%), and two or more abnormal signs (30.3%) was higher in the low Ct group than in the other two groups (all P < 0.05). Overall, Omicron infection showed fewer signs of pulmonary consolidation than other SARS-CoV-2 variant infections[23]. Consolidation shadows may result from vasculitis, hemorrhage, and peripheral serous exudation caused by high viral loads attacking blood vessels, secondary or direct alveolar inflammation, massive immune cell filling, and small airway mucus plugs. In addition, exudative and inflammatory stimulation of some lesions induces changes such as pleural thickening and pleural effusion. These results suggest that high viral loads can lead to severe and complex pulmonary injuries in some patients.
We used a Ct value of ≥ 35 as an inclusion criterion for patients in the high Ct group. Ct values of ≥ 35 in two consecutive tests is a criterion for patient discharge from mobile cabin hospitals, according to the Diagnosis and Treatment Plan for SARS-CoV-2 Pneumonia (trial version 9) issued by the National Health Commission of the People’s Republic of China. Although such patients are noninfectious, their lungs might show abnormal signs to different degrees. Therefore, follow-up observations and treatment of such patients is important.
The sample size of this study was reasonable. Although future studies should be conducted with larger sample sizes, considering the urgent epidemic situation, the sample size of this study was adequate. It is expected that studies with a large sample size will further validate our findings.
In this study, patients with SARS-CoV-2 Omicron variant infection were grouped based on their Ct values. We found that the chest CT signs of patients with different viral loads varied to a certain extent. Focal infection was the most common abnormal chest CT sign in the medium and high Ct groups. Patients in the high Ct group more commonly presented with patchy consolidation shadows, pleural changes, and two or more abnormal CT signs than those in the other two groups. The results of this study can effectively enhance our understanding of the characteristics of Omicron variant infections and provide guidance for subsequent clinical treatments of patients with such infections.
The Omicron variant of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) mainly infects the upper respiratory tract. Chest computed tomography (CT) can reveal the presence of pulmonary infection. The measured cycle threshold (Ct) was related to pulmonary infections diagnosed via CT.
To explore the relationship between chest CT characteristics and Ct value using the fluorescent polymerase chain reaction (PCR) method.
Pulmonary infections caused by the Omicron variant of severe acute respiratory syndrome coronavirus 2 were highly correlated with Ct values. Lower Ct values were associated with a higher incidence and degree of pulmonary damage.
The chest CT images and PCR Ct values of 331 patients with Omicron variant infections were retrospectively collected; categorized into low (< 25), moderate (25.00-34.99), and high (≥ 35) Ct groups; and analyzed statistically.
Focal infections were less frequent in the low Ct group than in the medium and high Ct groups. Patchy consolidation shadows and pleural changes were more common in the low Ct group than in the other two groups. The number of patients with two or more signs was greater in the low Ct group than in the medium and high Ct groups.
Pulmonary infection and the Ct measured using the fluorescent PCR method were related to pulmonary infections diagnosed via CT.
Future studies with large sample sizes and multiple centers will further validate our findings.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: Member of tumor imaging special committee of Shanghai anticancer association Member of Shanghai radiologist Association.
Specialty type: Infectious diseases
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Shahid M, Pakistan S-Editor: Chen YL L-Editor: Filipodia P-Editor: Chen YL
| 1. | Meo SA, Meo AS, Al-Jassir FF, Klonoff DC. Omicron SARS-CoV-2 new variant: global prevalence and biological and clinical characteristics. Eur Rev Med Pharmacol Sci. 2021;25:8012-8018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 90] [Reference Citation Analysis (0)] |
| 2. | Nyberg T, Ferguson NM, Nash SG, Webster HH, Flaxman S, Andrews N, Hinsley W, Bernal JL, Kall M, Bhatt S, Blomquist P, Zaidi A, Volz E, Aziz NA, Harman K, Funk S, Abbott S; COVID-19 Genomics UK (COG-UK) consortium, Hope R, Charlett A, Chand M, Ghani AC, Seaman SR, Dabrera G, De Angelis D, Presanis AM, Thelwall S. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: a cohort study. Lancet. 2022;399:1303-1312. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 835] [Cited by in RCA: 842] [Article Influence: 280.7] [Reference Citation Analysis (0)] |
| 3. | Pérez-Then E, Lucas C, Monteiro VS, Miric M, Brache V, Cochon L, Vogels CBF, Malik AA, De la Cruz E, Jorge A, De Los Santos M, Leon P, Breban MI, Billig K, Yildirim I, Pearson C, Downing R, Gagnon E, Muyombwe A, Razeq J, Campbell M, Ko AI, Omer SB, Grubaugh ND, Vermund SH, Iwasaki A. Neutralizing antibodies against the SARS-CoV-2 Delta and Omicron variants following heterologous CoronaVac plus BNT162b2 booster vaccination. Nat Med. 2022;28:481-485. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 293] [Cited by in RCA: 298] [Article Influence: 99.3] [Reference Citation Analysis (0)] |
| 4. | Garcia-Beltran WF, St Denis KJ, Hoelzemer A, Lam EC, Nitido AD, Sheehan ML, Berrios C, Ofoman O, Chang CC, Hauser BM, Feldman J, Roederer AL, Gregory DJ, Poznansky MC, Schmidt AG, Iafrate AJ, Naranbhai V, Balazs AB. mRNA-based COVID-19 vaccine boosters induce neutralizing immunity against SARS-CoV-2 Omicron variant. Cell. 2022;185:457-466.e4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 832] [Cited by in RCA: 760] [Article Influence: 253.3] [Reference Citation Analysis (0)] |
| 5. | Chen J, Wang R, Gilby NB, Wei GW. Omicron Variant (B.1.1.529): Infectivity, Vaccine Breakthrough, and Antibody Resistance. J Chem Inf Model. 2022;62:412-422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 491] [Cited by in RCA: 443] [Article Influence: 147.7] [Reference Citation Analysis (0)] |
| 6. | Kozlov M. Omicron's feeble attack on the lungs could make it less dangerous. Nature. 2022;601:177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 54] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 7. | Ren SY, Wang WB, Gao RD, Zhou AM. Omicron variant (B.1.1.529) of SARS-CoV-2: Mutation, infectivity, transmission, and vaccine resistance. World J Clin Cases. 2022;10:1-11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 289] [Cited by in RCA: 225] [Article Influence: 75.0] [Reference Citation Analysis (13)] |
| 8. | Garrett N, Tapley A, Andriesen J, Seocharan I, Fisher LH, Bunts L, Espy N, Wallis CL, Randhawa AK, Ketter N, Yacovone M, Goga A, Bekker LG, Gray GE, Corey L. High Rate of Asymptomatic Carriage Associated with Variant Strain Omicron. medRxiv. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 64] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 9. | Osterman A, Badell I, Basara E, Stern M, Kriesel F, Eletreby M, Öztan GN, Huber M, Autenrieth H, Knabe R, Späth PM, Muenchhoff M, Graf A, Krebs S, Blum H, Durner J, Czibere L, Dächert C, Kaderali L, Baldauf HM, Keppler OT. Impaired detection of omicron by SARS-CoV-2 rapid antigen tests. Med Microbiol Immunol. 2022;211:105-117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 79] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 10. | Juanola-Falgarona M, Peñarrubia L, Jiménez-Guzmán S, Porco R, Congost-Teixidor C, Varo-Velázquez M, Rao SN, Pueyo G, Manissero D, Pareja J. Ct values as a diagnostic tool for monitoring SARS-CoV-2 viral load using the QIAstat-Dx® Respiratory SARS-CoV-2 Panel. Int J Infect Dis. 2022;122:930-935. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Tsakok MT, Watson RA, Saujani SJ, Kong M, Xie C, Peschl H, Wing L, MacLeod FK, Shine B, Talbot NP, Benamore RE, Eyre DW, Gleeson F. Reduction in Chest CT Severity and Improved Hospital Outcomes in SARS-CoV-2 Omicron Compared with Delta Variant Infection. Radiology. 2023;306:261-269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 60] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 12. | Yoon SH, Lee JH, Kim BN. Chest CT Findings in Hospitalized Patients with SARS-CoV-2: Delta versus Omicron Variants. Radiology. 2023;306:252-260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 40] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 13. | Zhang X, Zhang W, Chen S. Shanghai's life-saving efforts against the current omicron wave of the COVID-19 pandemic. Lancet. 2022;399:2011-2012. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 224] [Article Influence: 74.7] [Reference Citation Analysis (0)] |
| 14. | Goussard P, Schubert P, Parker N, Myburgh C, Rabie H, van der Zalm MM, Van Zyl GU, Preiser W, Maponga TG, Verster J, Gie AG, Andronikou S. Fatal SARS-CoV-2 Omicron variant in a young infant: Autopsy findings. Pediatr Pulmonol. 2022;57:1363-1365. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 15. | Martín-Martín J, Martín-Cazorla F, Suárez J, Rubio L, Martín-de-Las-Heras S. Comorbidities and autopsy findings of COVID-19 deaths and their association with time to death: a systematic review and meta-analysis. Curr Med Res Opin. 2022;38:785-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Calabrese F, Pezzuto F, Fortarezza F, Hofman P, Kern I, Panizo A, von der Thüsen J, Timofeev S, Gorkiewicz G, Lunardi F. Pulmonary pathology and COVID-19: lessons from autopsy. The experience of European Pulmonary Pathologists. Virchows Arch. 2020;477:359-372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 213] [Cited by in RCA: 199] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 17. | Aranha C, Patel V, Bhor V, Gogoi D. Cycle threshold values in RT-PCR to determine dynamics of SARS-CoV-2 viral load: An approach to reduce the isolation period for COVID-19 patients. J Med Virol. 2021;93:6794-6797. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 67] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 18. | Ke R, Martinez PP, Smith RL, Gibson LL, Mirza A, Conte M, Gallagher N, Luo CH, Jarrett J, Zhou R, Conte A, Liu T, Farjo M, Walden KKO, Rendon G, Fields CJ, Wang L, Fredrickson R, Edmonson DC, Baughman ME, Chiu KK, Choi H, Scardina KR, Bradley S, Gloss SL, Reinhart C, Yedetore J, Quicksall J, Owens AN, Broach J, Barton B, Lazar P, Heetderks WJ, Robinson ML, Mostafa HH, Manabe YC, Pekosz A, McManus DD, Brooke CB. Daily longitudinal sampling of SARS-CoV-2 infection reveals substantial heterogeneity in infectiousness. Nat Microbiol. 2022;7:640-652. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 103] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 19. | Solomon JJ, Heyman B, Ko JP, Condos R, Lynch DA. CT of Post-Acute Lung Complications of COVID-19. Radiology. 2021;301:E383-E395. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 115] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 20. | Chinese Research Hospital Association; Respiratory Council. [Expert recommendations for the diagnosis and treatment of interstitial lung disease caused by novel coronavirus pneumonia]. Zhonghua Jie He He Hu Xi Za Zhi. 2020;43:827-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 21. | Hu Q, Guan H, Sun Z, Huang L, Chen C, Ai T, Pan Y, Xia L. Early CT features and temporal lung changes in COVID-19 pneumonia in Wuhan, China. Eur J Radiol. 2020;128:109017. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 75] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 22. | Yan S, Chen H, Xie RM, Guan CS, Xue M, Lv ZB, Wei LG, Bai Y, Chen BD. Chest CT Evaluation of 11 Persistent Asymptomatic Patients with SARS-CoV-2 Infection. Jpn J Infect Dis. 2021;74:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 23. | Granata V, Fusco R, Villanacci A, Magliocchetti S, Urraro F, Tetaj N, Marchioni L, Albarello F, Campioni P, Cristofaro M, Di Stefano F, Fusco N, Petrone A, Schininà V, Grassi F, Girardi E, Ianniello S. Imaging Severity COVID-19 Assessment in Vaccinated and Unvaccinated Patients: Comparison of the Different Variants in a High Volume Italian Reference Center. J Pers Med. 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Reference Citation Analysis (0)] |