Published online Feb 6, 2023. doi: 10.12998/wjcc.v11.i4.725
Peer-review started: November 6, 2022
First decision: December 11, 2022
Revised: December 24, 2022
Accepted: January 12, 2023
Article in press: January 12, 2023
Published online: February 6, 2023
Processing time: 91 Days and 8.6 Hours
Portal vein aneurysm (PVA) is a rare vascular abnormality, representing 3% of all venous aneurysms in the human body, and is not well understood. It can be congenital or acquired, located mainly at the level of confluence, main trunk, branches and bifurcation. A PVA as an abnormality of the portal venous system was first reported in 1956 by Barzilai and Kleckner. A review from 2015 entitled “Portal vein aneurysm: What to know” considered fewer than 200 cases. In the last seven years, there has been an increase in the number of PVAs diagnosed thanks to routine abdominal imaging. The aim of this review is to provide a comprehensive update of PVA, including aetiology, epidemiology, and clinical assessment, along with an evaluation of advanced multimodal imaging features of aneurysm and management approaches.
Core Tip: The number of reported portal vein aneurysms (PVAs) across the world with this review stands at about 280. In relation to a new acquired aetiology of PVA, the following conditions are noted: Budd-Chiari syndrome, splenomegaly in thalassaemia major, giant splenic artery aneurysm and a long-term cholelithiasis. Percentage of 30 to 50 of patients experienced non-specific abdominal pain, the most frequent complications of PVA are thrombosis and biliopathy. Recently, endoscopic ultrasound and intraductal ultrasonography, as an additional tool have also been used for assessment of PVA in more detail. With this review we have highlighted treatment of PVA with comorbidities based on the transjugular intrahepatic portosystemic shunt, percutaneous approach, and endoscopic approach.
- Citation: Kurtcehajic A, Zerem E, Alibegovic E, Kunosic S, Hujdurovic A, Fejzic JA. Portal vein aneurysm-etiology, multimodal imaging and current management. World J Clin Cases 2023; 11(4): 725-737
- URL: https://www.wjgnet.com/2307-8960/full/v11/i4/725.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i4.725
A portal vein aneurysm (PVA) is the abnormal focal saccular or fusiform dilatation of the portal venous system, and it is defined as a PV diameter exceeding 19 mm in cirrhotic patients and 15 mm in a normal liver. It is a rare vascular abnormality, representing 3% of all venous aneurysms in the human body, and is not well understood[1-7].
Douglass et al[8] studied 92 autopsies and reported that the diameter of the PV was between 0.64 mm and 12.1 mm in patients without cirrhosis and those without portal hypertension. In 1976, Doust et al[9] conducted a vascular study of 53 patients to assess the size of the PV and underlying liver status through abdominal ultrasound, and they detected that the maximum calibre of the PV was 19 mm in cirrhotic patients and 15 mm in patients with normal livers. Hence, a portal vein diameter of > 20 mm is universally regarded as the threshold for diagnosis of a PVA.
In a retrospective study by Koc et al[10], involving 4186 patients who had undergone routine abdominal contrast-enhanced computed tomography (CT), the prevalence of PVAs was 0.43%. The location of a PVA can be extrahepatic or intrahepatic. Extrahepatic PVAs often occur in the main trunk of the PV, the splenomesenteric confluence, at the level of the PV bifurcation, the main branches of the PV, the splenic vein (SV) and the superior mesenteric vein (SMV). A study by Doust et al[9] characterized intrahepatic PVAs as having a diameter measuring more than 7 mm in normal patients and 8.5 mm in cirrhotic patients. PVA as an abnormality of portal venous system firstly was reported 1956 by Barzilai and Kleckner[11]. A review from 2015 entitled “Portal vein aneurysm: What to know” considered 96 reports and included 190 patients[1].
Aiming to clarify novelty as regards this visceral vascular abnormality, we performed a literature search of the PubMed database for all articles relating to PVA between January 2015 and July 2022[12-68]. We collected 57 reports, involving 62 patients with a PVA[3-7,12-16,19,21-25,27,29-68]; we also found one retrospective study with 18 PVA patients[2], and three cases of PV pseudoaneurysm[69-71].
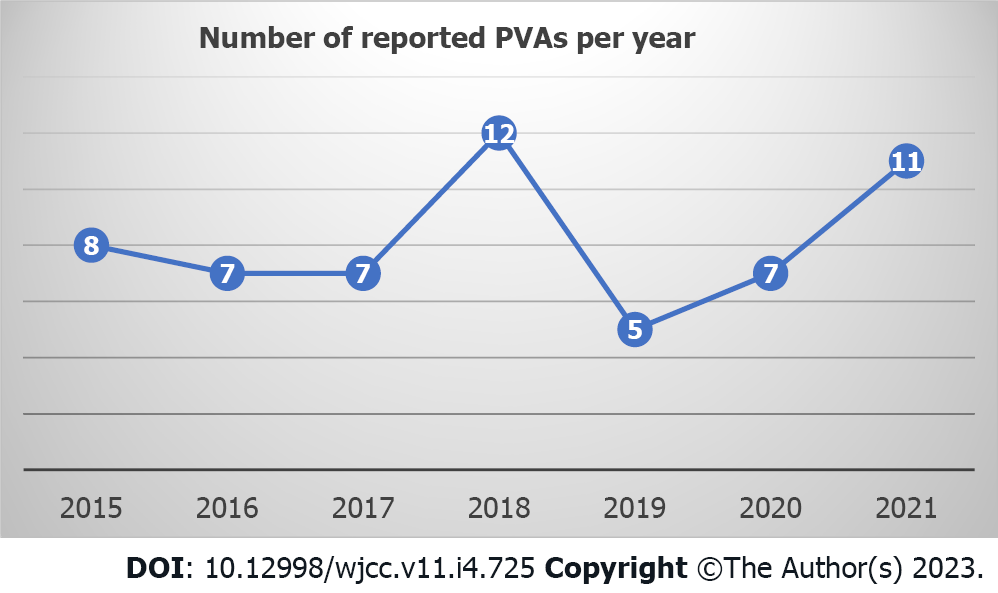
Of the 62 patients in the review, 33 (53%) were male; the patients were between 1 (youngest) and 95 (oldest) years of age, and the mean patient age at diagnosis was 54.85 years (± 21.72). A number of reported PVA cases per year is shown in Figure 1.
In terms of aetiology, the frequency of congenital PVAs was 29 (46.7%), and it was 17 (27.4%) for acquired PVAs. In 16 (25.8%) patients, the aetiology of the PVAs was unclear. Regarding the location of PVAs, 27.41% were at the level of the splenomesenteric confluence; 19.35% were at the main trunk; 17.74% were at branches; 6.45% were at the PV bifurcation; 6.45% were at the SV; and 4.83% were at the SMV; 14.51% were classified as intrahepatic PVAs. A retrospective study by Ahmed et al[2] included 18 patients [13 of whom were female (72.2%)], aged between 20 years and 101 years, with an average age of 56 years. Our review also covered three patients (all male) with a PV pseudoaneurysm resulting from trauma.
The aetiology of PVA is not clear. Postulated origins include both congenital and acquired causes. It is well known that the main cause of acquired PVA is chronic liver disease (cirrhosis and fibrosis) with portal hypertension. Long-standing portal hypertension causes intimal thickening with compensatory medial hypertrophy of the PV. Over time, medial hypertrophy is replaced by fibrous tissue, leading to weakening of the vein wall, thus making it susceptible to aneurysmal dilatation[12,13]. However, the incidence of portal hypertension and PVA is disproportionate, suggesting the existence of other contributory factors.
Acquired PVA can also be part of severe acute pancreatitis, likely to be due to leakage of digestive enzymes, causing localized inflammation of the PV. Malignancy was also noted as a cause of acquired PVA[1].
In several reports[69-71] a pseudoaneurysm of the PV is defined as post-traumatic (surgical pancreatic procedure, liver transplantation, or other rare clinical situations) uncommon finding (dilation) of the portal venous system. It is a serious condition followed life-threatening complications requiring an interventional approach.
In relation to a new acquired aetiology of PVA, the following conditions were noted: Budd-Chiari syndrome[14], splenomegaly in thalassaemia major[15] and giant splenic artery aneurysm[16]. Long-term cholelithiasis was also considered as a possible cause of PVA[17].
Some PVAs are congenital. During gestation, three pairs of veins are developed: The cardinal veins, umbilical veins and vitelline veins. The PV, hepatic veins and part of the inferior cava vein (ICV) come from umbilical veins and vitelline veins. Generally, cranial segments of the left vitelline vein and caudal segments of the right vitelline vein regress during the foetal period, and the SV and SMV are derived from the left vitelline vein[18].
Evidence supporting a congenital cause includes reported cases of in utero diagnosis of PVA, evidence of PVA in patients with histologically proven normal livers (particularly in children and young adults), normal portal venous pressure in the presence of a PVA, and the frequent stability of aneurysms at follow-up imaging. Theories for a congenital cause involve inherent weakness in the vessel wall or incomplete regression of the distal right primitive vitelline vein, leading to a vascular diverticulum that ultimately develops into an aneurysm. Congenital PVAs are usually incidentally diagnosed later in life (not in neonatal or paediatric age groups) when an abdominal ultrasound is carried out because of some other indication[4,6,19]. Burdall et al[20] evaluated the relation between trisomy 21 (Down’s syndrome) and congenital vascular malformation of the liver in a study of 45 children, seven of whom had vascular malformation and two of whom had evidence of a PVA.
The clinical presentation of PVA is controversial and poorly understood. According to the review article by Laurenzi et al[1], 30% of patients with a PVA were asymptomatic, and 50% experienced non-specific abdominal pain. In our review, we found that up to 25% of patients were asymptomatic; for 15% of patients, the authors did not provide clear presenting symptoms relating to the PVA, and approximately 30% of patients experienced non-specific abdominal pain. In patients with a PVA, the nature of non-specific abdominal pain should be clarified. The main question is whether PVA low-pressure truly the source of the pain; gastritis, duodenitis and cholecystitis, etc., should be ruled out. A retrospective study by Ahmed et al[2] showed that in eight (44.4%) patients with abdominal pain, a PVA was actually the source of the pain in only one patient.
Up to 10% of cases involve portal hypertension, gastrointestinal bleeding (varices) or presenting symptoms related to compression of adjacent organs (abdominal swelling or jaundice)[1]. With a PVA, presenting symptoms or complications such as portal hypertension and bleeding are discussible. One thing that should be clarified is whether a PVA is a consequence of portal hypertension or whether the PVA is causing portal hypertension. Khan et al[16] found coexistence of a giant splenic artery aneurysm, portal hypertension without liver cirrhosis and a PVA at the level of bifurcation. In this case, the PVA and portal hypertension were presumed to be secondary to the pressure effect from the splenic artery aneurysm. Güngör et al[21] presented an 11-mo-old girl with a congenital PVA, and oesophageal and fundal varices with bleeding. This was the only case in our review where PVA caused portal hypertension complications.
Clinical presentation has a close relation with morphology, size and location of the PVA. When it grows, there can be contact with the biliary tract, the ICV and duodenum, etc., and complications can arise from compression of these organs. Six patients in our review (9.67%) had compression complications, including four biliopathies[22-25], one thrombosis in the ICV[7] and one intestinal obstruction[19].
Laurenzi et al[1] reported PVA complications such as thrombosis (which happened in 20% of cases) and a rupture (which occurred twice). A recent retrospective study by Ahmed et al[2] reported 18 patients with a PVA; 22.22% of patients had thrombosis, and no ruptures were reported. PVA with a complication of thrombosis is reported in the literature as nearly always being symptomatic, with 91% of patients reporting abdominal pain, 53% reporting fever and 38% presenting with ascites[26].
In our review, thrombosis occurred in 12 (19.35%) patients (six of whom were female), with a median age of 38.33 years. Abdominal pain was reported in 10 of 12 patients; in a one-year-old girl, the symptoms manifested as haematemesis and melena[21]; a 69-year-old female with a congenital PVA followed by thrombosis did not experience any symptoms[5]. In five patients, treatment was based on anticoagulation medication; seven patients underwent open surgery or invasive radiology procedures. In our review, a rupture as a complication of a PVA was not reported.
Patients with a PVA have a normal laboratory results, including complete blood count, inflammatory parameters, basic metabolic profile and liver function tests[1].
Increased use of abdominal cross-sectional imaging in recent years has led to a growing number of cases describing PVA, and as such, proper handling of this lesion is increasingly relevant to both diagnostic and interventional radiologists. Evaluation of PVA by multiple imaging modalities is important because a PVA can mimic solid, cystic or hypervascular abdominal masses[1-7].
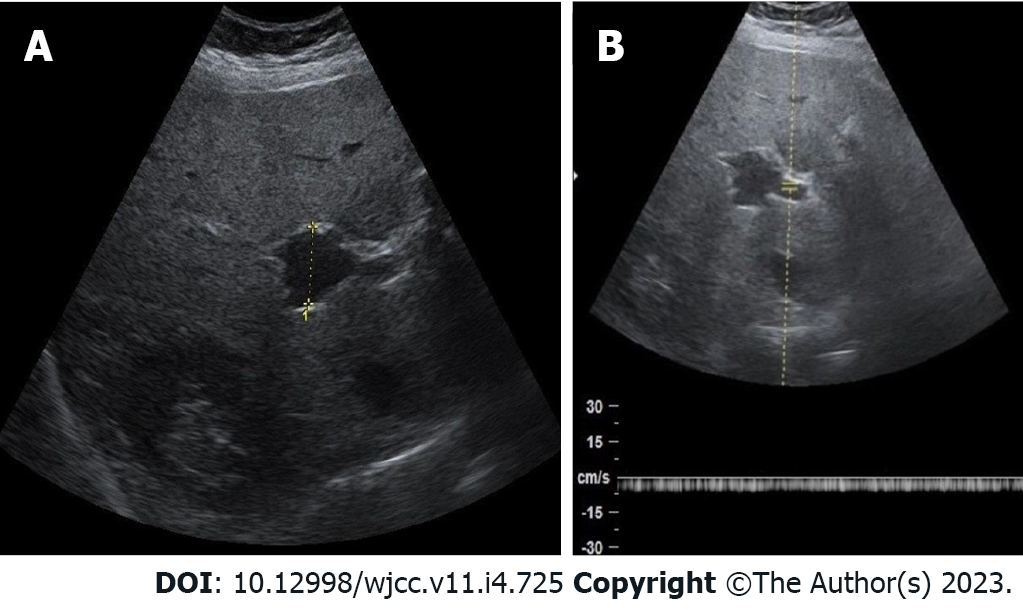
Sonography assessment can be performed for differential diagnosis to determine whether anechoic area or cyst at porta hepatis are PVA, hepatic artery aneurysm or choledochal cyst. Abdominal ultrasound based on the greyscale of the PVA produces an anechoic structure with a “smoke effect” within, which simulates a natural contrast agent, determined by slowed venous flow (Figure 2A). Spectral Doppler sonography reveals the presence of a monophasic, non-pulsatile venous flow pattern inside the aneurysm (Figure 2B). With colour Doppler sonography of a PVA, anechoic areas will be completely filled, looking like the Korean flag or a “yin-yang” sign. Hepatic artery aneurysms show a colour flow with arterial waveform, but choledochal cysts do not show such colour flow and are connected to biliary channels[6,15].
Contrast-enhanced CT with angiography shows the filling of PVA. On a CT and magnetic resonance imaging (MRI) scan, a PVA will appear as a well-defined contrast-enhanced focal saccular anomaly or fusiform dilatation of the portal venous system during the portal venous phase[4,27].
In one case, CT angiography facilitated better assessment of the portal venous system, which contained some thin calcifications in the aneurysmal wall and the main portal trunk[13]. Iimuro et al[28] presented “computational fluid dynamics software”, analyzing the haemodynamics of the portal venous system, including congenital saccular PVA at the level of confluence. Turbulent flow was obvious in PVA, and the wall shear stress against the upper-posterior part of the aneurysm wall was greater than in other parts of the aneurysm. In order to prevent the PVA from growing and avoid thrombosis or a rupture, an aneurysmectomy of the PVA was performed.
The diagnostic role of endoscopic ultrasound (EUS) was highlighted in congenital PVA at the level of the splenomesenteric confluence. EUS confirmed the presence of anechoic lesions adjacent to the neck of the pancreas[29]. EUS as a diagnostic tool was also used for assessment of an intrahepatic aneurysmal portosystemic venous shunt[30].
“Intraductal ultrasonography” (IDU) was used for the first time to identify an adjacent PVA as the cause of a common hepatic duct stricture, showing a lobulated hypoechoic mass containing a mobile echogenic substance, outside of the biliary tract, highly suggestive of a vascular lesion[24].
Because of their rarity, the natural history of PVA remains unclear, and the optimal strategy for management is controversial. Following diagnosis of a PVA, treatment will depend on the size, presenting symptoms and location of the PVA, and comorbidities.
If the PVA is asymptomatic (as in 30% of cases), it does not require any active treatment, and monitoring (a policy of “wait and see”) should be adopted[1]. While asymptomatic aneurysms smaller than 30 mm can be clinically observed, surgical intervention may be necessary in large asymptomatic aneurysms (> 30 mm)[1,10]. The origin, morphology and symptomatology of a PVA, along with comorbidities and conservative treatment, are shown in Table 1.
| Year | Ref. | Gender | Age | Etiology | Location | Morphology/size | Symptomatology | Complications | Comorbidity | Imaging | Treatment | Follow-up |
| 2015 | Prabhakar et al[48] | Male | 47 | None | Intrahepatic, left br | 18 mm | Shunt | CT | Surveillance | Alive | ||
| 2015 | Srikanth et al[49] | Female | 12 | Congenital | Right branch | Saccular | US, CT | Surveillance | ||||
| 2015 | Starikov et al[50] | Male | 27 | None | SMV aneurysm | 86 mm | Abdominal pain | Thrombosis CTPV | Acute pancreatitis | CT, MRI | Anticoagulation | 24 mo |
| 2016 | Gaba et al[3] | Female | 61 | None | Confluence | 50 mm | Ca colon; surgery | US, CT | Surveillance | |||
| 2016 | Hanafiah et al[12] | Male | 70 | Acquired | Right branch | Fusiform, 22 mm | HBV; cirrhosis, HCC | US, CT | No treatment | Died | ||
| 2016 | Nayman et al[51] | Female | 48 | Congenital | Intrahepatic, two br | Right 43, left 13 mm | Abdominal pain | US, CT | Surveillance | |||
| 2016 | Kurtcehajic et al[22] | Female | 75 | Congenital | Bifurcation | Saccular | Jundice | Biliopathy | US, CT, MRI | Usodeoxolic acid | Alive | |
| 2016 | Khairallah et al[13] | Female | 68 | Acquired | Bifurcation | Fusiform, 40 mm | Bleeding | Cirrhosis; portal Hyp | US, CT | No treatment | ||
| 2017 | Jaiswal et al[52] | Female | 59 | Congenital | Confluence | 53.7 mm | Abdominal pain | Cholelithias | US, CT | Surveillance | 18 mo | |
| 2017 | Guilbaud et al[53] | Male | 88 | Congenital | Main trunk | Saccular, 59 mm | Cholecystectomy | CT | Surveillance | |||
| 2018 | Maia et al[54] | Female | 67 | Congenital | Main trunk | 35 mm | Abdominal pain | Diabetes, arterial Hyp | US, CT | Surveillance | 12 mo | |
| 2018 | Ashmore et al[55] | Female | 62 | None | Confluence | Abdominal pain | CT | Surveillance | ||||
| 2018 | Martínez et al[56] | Female | 29 | Congenital | Left branch | Saccular, 28 mm | US, CT | Surveillance | ||||
| 2018 | Hirji et al[57] | Male | 62 | Congenital | Confluence | 50 mm | Diabetes, Parkinson | CT | Surveillance | 6 mo | ||
| 2018 | Alur et al[58] | Female | 45 | Congenital | Left branch | 21.5 mm | US, MRI | Surveillance | ||||
| 2018 | Chaubard et al[59] | Male | 66 | Congenital | Confluence | Saccular, 40 mm | Abdominal pain | T-cell hemopathy | US, CT | Surveillance | ||
| 2019 | Ramamoorthy et al[60] | Female | 42 | Congenital | Confluence | Fusiform, 26 mm | Abdominal pain | US, MRI | Surveillance | Alive | ||
| 2019 | De Vloo et al[4] | Male | 67 | None | Confluence | Fusiform, 55 mm | Abdominal pain | Thrombosis CTPV | Neuroendocrine tm | US, CT | Anticoagulation | |
| 2020 | Kabir et al[61] | Male | 77 | Congenital | Intrahepatic, left br | 28 mm | Abdominal pain | Abd. aortic aneurysm | US, MRI | Surveillance | 3 mo | |
| 2020 | Rana et al[29] | Female | 51 | Congenital | Confluence | 38 mm | Abdominal pain | US, EUS, CT | Surveillance | 30 mo | ||
| 2020 | Shams et al[62] | Male | 67 | None | SMV aneurysm | Abdominal pain | Thrombosis | Anticoagulation | 12 mo | |||
| 2020 | Watanabe et al[5] | Female | 69 | Congenital | Right branch | 35 mm | Thrombosis | US, CT | Surveillance | 120 mo | ||
| 2020 | Schilardi et al[6] | Male | 86 | Congenital | Right branch | 55 mm | Heart failure; COPD | US, CT | Surveillance | 24 mo | ||
| 2021 | Hernando et al[63] | Female | 51 | Congenital | Right branch | 25 mm | Abd. pain, jundice | Choledocholithiasis | US, MRI | Surveillance | ||
| 2021 | Priadko et al[32] | Male | 81 | Acquired | 48 mm | HBV, cirrhosis | US | Surveillance | 36 mo | |||
| Female | 52 | None | Saccular, 42.3 mm | US, CT, MRI | Surveillance | 60 mo | ||||||
| Male | 73 | None | Intrahepatic, right br | 27 mm | US | Surveillance | 12 mo | |||||
| 2021 | Tan et al[64] | Male | Congenital | Main trunk | 26 mm | CT | Surveillance | Alive | ||||
| 2021 | López et al[65] | Female | 41 | None | SMV aneurysm | 43 mm | Abdominal pain | Splenorenal shunt | CT | Surveillance | ||
| 2022 | Tri et al[31] | Male | 10 | Congenital | Main trunk | 36 mm | Abdominal pain | Thrombosis | CT | Anticoagulation | 6 mo | |
| 2022 | Villani et al[66] | Male | 73 | Acquired | Main trunk | Saccular, 40 mm | Cirrhosis, ascites | US, CT | Surveillance | |||
| 2022 | Mortazavi et al[67] | Male | 49 | Congenital | Main trunk | 21 mm | Abdominal pain | Cholelithias | CT | Surveillance | ||
| 2022 | Mohamadnejad et al[30] | Female | 76 | None | Intrahepatic, left br | 25 mm | Aneurysm, shunt | CT, EUS | Surveillance | |||
| 2022 | Kanamalla et al[7] | Male | 95 | Congenital | Confluence | Saccular, 35 mm | Thrombosis of ICV | Diabetes, arterial Hyp | CT | Anticoagulation |
Where there is thrombosis due to a PVA, anticoagulation treatment should be considered. In a recently published case, a 10-year-old boy with PVA thrombosis was treated with enoxaparin. The thrombosis disappeared completely after 6 mo[31]. In a case involving biliopathy, where the PVA comprised hepatic ducts, ursodeoxycholic acid was used to decrease the level of conjugated bilirubin[22].
While, in some studies, a CT scan every 12 mo was the preferred monitoring strategy, most published studies indicate that sonography is the preferred imaging technique for monitoring PVA growth, as it is relatively inexpensive and does not involve radiation exposure[32].
Open surgery approach: If the PVA is growing and constricting adjacent organs, thrombosis occurs, aiming to prevent potential rupture, open surgery methods should be considered. An aneurysmectomy for fusiform aneurysms (aneurysm resection, followed by insertion of a synthetic or cadaveric graft as a replacement conduit) and an aneurysmorrhaphy for saccular aneurysms (restores the normal diameter of the portal vein, if the remaining venous wall is of good quality) are considered for symptomatic aneurysms and to prevent a negative PVA prognosis.
The origin, morphology, PVA symptomatology and comorbidities as regards invasive treatment are shown in Table 2.
| Year | Ref. | Gender | Age | Etiology | Location | Morphology /size | Symptomatology | Complications | Comorbidity | Imaging | Treatment | Follow-up |
| 2015 | Fleming et al[33] | Female | 70 | None | Confluence, SMV | From 30 mm to 50 mm | CT | Aneurysmectomy | 85 mo | |||
| Female | 47 | None | Confluence, SMV | From 35 mm to 60 mm | Abdominal pain | HCV | US, CT | Aneurysmectomy | 65 mo | |||
| Female | 29 | None | 40 mm | Abdominal pain | Thrombosis/CTPV | CT | Aneurysmectomy | 144 mo | ||||
| Female | 49 | None | Confluence, SMV | 40 mm | Abdominal pain | Aneurysmorrhaphy | 17 mo | |||||
| 2015 | Tsauo et al[14] | Female | 65 | Acquired | Right branch | Saccular, 32 mm | Abdominal pain | Budd Chiari Sy | CT | TIPS | 12 mo | |
| 2016 | Khan et al[16] | Female | 40 | Acquired | Bifurcation | 34 mm | Splenic artery aneurysm | US, CT | Aneurysmectomy | Alive | ||
| 2016 | Ierardi et al[69] | Male | 42 | Car accident | Confluence | PSA, 23 mm | Abdominal pain | Liver trauma | CT | Expanding stent graft | 13 d | |
| 2016 | Shukla et al[39] | Male | 55 | None | Intrahepatic, right br | Saccular, 30 mm | Sigmoid hemicolectomy | CT | Percut embolisation | Alive | ||
| 2017 | Shrivastava et al[27] | Male | 55 | Acquired | Intrahepatic, right br | 62 mm | Portal Hyp, pancreatitis | US, CT | Percut embolisation | 12 mo | ||
| 2017 | Ding et al[45] | Male | 48 | Acquired | Bifurcation | 70 mm | HBV, cirrhosis | CT | TIPS | 72 mo | ||
| 2017 | Kim et al[34] | Female | 34 | Congenital | Main trunk | Fusiform, from 57 mm to 62 mm | Abdominal pain | Thrombosis | Celiac artery dissection | US, CT | Aneurysmectomy | 6 mo |
| 2017 | Das et al[15] | Female | 18 | None | Confluence | Fusiform, 30 mm | Thalassemia major | US, CT | Splenectomy | |||
| 2017 | Ding et al[23] | Male | 80 | Acquired | Left branch | Fusiform | Abd. pain, jaundice | Biliopathy | Cirrhosis, portal Hyp. | US, CT, MRI | ERCP, biliary stent | 3 mo |
| 2018 | Walton et al[70] | Male | 42 | Percut Biopsy | Main trunk | PSA, 13 mm | Haemobilia | Lymphomatosus of pancreas | Percut, covered stent | |||
| 2018 | Kimura et al[44] | Male | 62 | Acquired | Branch | 40 mm | HCV, HCC, art portal fistula | MRI | Selective embolisation | |||
| 2018 | Sun et al[24] | Male | 85 | Congenital | Main trunk | 32 mm | Abdominal pain | Cholangitis | Diabetes, ulcer disease | IDUS, CT | ERCP, biliary stent | 42 mo |
| 2018 | Chandran et al[19] | Male | 1 | Congenital | Colonic obstruction | Ligation of PVA | 3 mo | |||||
| 2018 | Chadha et al[35] | Male | 66 | Acquired | SV aneurysm | 39 mm | HCV, cirrhos | External Sundt carotid shunt | 6 mo | |||
| 2018 | Güngör et al[21] | Female | 1 | Congenital | Intrahepatic, right br | Fusiform | Hematemesis, melena | Portal Hyp, thrombosis | US, CT | Sugiura oper | ||
| 2018 | Ktenidis et al[36] | Male | 43 | Acquired | SV aneurysm | 98 mm | Splenectomy, AV shunt | CT | Open surgery | Alive | ||
| 2019 | Cleveland et al[71] | Male | 68 | Vehicle collision | PSA | Abdominal pain | Shock bowel | CT | Dead | |||
| 2019 | Juscafresa et al[40] | Female | 77 | Acquired | SV aneurysm | 45 mm | Pancreatitis | CT | Percut, Viabahn stent | 12 mo | ||
| 2019 | Bremer et al[42] | Male | 65 | Acquired | Intrahepatic, right br | 64 mm | Abdominal pain | Cirrhosis, transplantation | TAE, stent graft exclusion | 6 mo | ||
| 2019 | Oguslu et al[43] | Female | 58 | Acquired | Left branch | Saccular, 130 mm | Abdominal pain | Thrombosis | Art. portal fistula, portal Hyp | US, CT | Percut embolisation | 9 mo |
| 2020 | Field et al[25] | Male | 25 | Congenital | Main trunk | 55 mm | Abdominal pain | Biliopathy, thrombosis | CT, MRI | Thrombolysis, thrombectomy | 12 mo | |
| 2021 | Sura et al[38] | Male | 80 | Congenital | Main trunk | Saccular, 37 mm | Diaphragmatic hernia | CT | Open surgery | 6 mo | ||
| 2021 | Marmor et al[41] | Male | 67 | Congenital | SV aneurysm | Saccular, 40 mm | Ca bladder | CT | Balloon expandable stent | 12 mo | ||
| 2021 | Matsumoto et al[68] | Male | 75 | Acquired | Main trunk | 42 mm | Abdominal pain | Ca pancreas | CT | Open surgery, omental graft | 3 mo | |
| 2021 | Gorolay et al[37] | Female | 36 | Congenital | Confluence | Saccular, from 45 mm to 65 mm | Abdominal pain | Thrombosis | US, CT | Hybrid operative repair | 36 mo | |
| 2021 | Dunlap et al[46] | Male | 32 | Acquired | Confluence | From 52 mm to 57 mm | Cirrhosis, portal Hyp | TIPS | 6 mo | |||
| 2022 | Kohlbrenner et al[47] | Male | 37 | Congenital | Confluence | Fusiform, 51 mm | Abdominal pain | Thrombosis, ischemia | CT | Thrombolysis, TIPS | 24 mo |
Fleming et al[33] demonstrated the efficacy of open surgery (aneurysmectomy) in three cases (two patients with an autograft and one with ePTFE). The two women with an autogenous graft remained asymptomatic at 85 mo and 65 mo, respectively; the third woman with ePTFE got thrombosis during pregnancy. The same report also included an aneurysmorrhaphy as the chosen treatment in one woman with a PVA.
Kim et al[34] presented a case with a PVA at the level of the main trunk, growing and with thrombosis complications. An aneurysm excision with an interposition bypass was successfully performed. The patient’s postoperative recovery was rapid and uneventful, with normal portal flow revealed by colour Doppler ultrasonography and a contrast-enhanced CT scan.
Khan et al[16] presented a case where splenomegaly, a giant splenic artery aneurysm and a PVA were found to coexist. The patient underwent a splenectomy and excision of the splenic artery aneurysm. It was determined that her PVA shrank considerably. Das et al[15] presented a case with thalassaemia major, splenomegaly and a PVA. After a splenectomy (necessitated by the existence of hypersplenism), the PVA significantly reduced.
Chadha et al[35] reported the case of a 66-year-old male with an acquired SV aneurysm and described novel use of a “Sundt external carotid endarterectomy shunt” as a temporary portacaval shunt to control portomesenteric hypertension, before transplantation of the liver. A giant SV aneurysm 98 mm in size developed as a consequence of a splenectomy, an arteriovenous fistula and portal hypertension; this aneurysm was treated successfully with open surgery[36].
Male and female patients, both of whom had a congenital PVA and subsequent thrombosis complications, were treated with a hybrid operative repair involving a transhepatic catheter thrombectomy, and their aneurysms were operated on in open surgery[25,37].
The limited number of PVAs that have been reported means that there are no clear indications for open surgery on PVA. Koc et al[10] studied the size of PVA and concluded that aneurysms larger than 30 mm should be surgically treated with the aim of preventing thrombosis or rupture. On the other hand, a recently reported case of a patient with a congenital PVA 35 mm in size, with subsequent thrombosis complications, showed spontaneous resolution after 10 years[5].
Sura et al[38] reported the case of an 80-year-old man who had open surgery on a 37-mm PVA at the level of the main trunk. The reasons for PVA surgery were not postulated, but given the congenital origin, advanced age of the patient and absence of symptoms or thrombosis, it is our view that surgery was not the best treatment choice.
Interventional radiology procedures: In cases where a PVA is a consequence of portal hypertension and/or coexists with life-threatening conditions (injuries), the high risk associated with open surgery methods means that interventional radiology procedures via a percutaneous approach, endovascular approach and even more endoscopic approach should be considered[1,2].
Percutaneous approach: Shukla et al[39] successfully demonstrated percutaneous embolization of a saccular intrahepatic PVA, which prevented further growth or other clinical sequelae. Shrivastava et al[27] presented the largest intrahepatic PVA and the first case where the endovascular technique was used for treatment of the same. Under sonography and fluoroscopy guidance, the PVA was directly punctured with an 18G needle and embolized with a Lipiodol-Glue combination.
Juscafresa et al[40] reported the case of an elderly female treated for an acquired SV aneurysm 45 mm in size, through a transhepatic percutaneous approach, using a Viabahn covered stent. Marmor et al[41] presented the case of a patient with a congenital SV aneurysm 40 mm in size. Because the aneurysm was getting larger, it was treated with an expandable stent via a transhepatic approach.
In one case, after liver transplantation necessitated by HCV cirrhosis, the patient subsequently developed an arterioportal fistula with an intrahepatic PVA. The first step of the treatment was transarterial embolization, and the second step was stent graft exclusion of the PVA. As there was leakage, the patient underwent liver re-transplantation[42]. Oguslu et al[43] demonstrated two techniques for treatment of an arterioportal fistula with a giant saccular PVA at the level of the left branch. After failure of an endovascular approach due to tortuosity and angulation of the celiac artery, access to the hepatic artery was obtained directly via a percutaneous transhepatic route, and the fistula site was embolized with an Amplatzer Vascular Plug II and coils.
Treatment of portal vein pseudoaneurysm: Our review covered three patients (all males) with a PV pseudoaneurysm, all of which were a consequence of abdominal trauma or injury. In a patient with a traumatic pseudoaneurysm at the level of the splenomesenteric confluence, Ierardi et al[69] dem
A patient with a PV pseudoaneurysm at the level of the main trunk, resulting from invasive medical procedures [e.g., a percutaneous biopsy or endoscopic retrograde cholangiopancreatography (ERCP)] to address lymphomatosus infiltration of the pancreatic head (with symptoms of haemobilia), was treated using percutaneous transhepatic covered stenting[70].
In the last case, involving a patient with a pseudoaneurysm of the portal venous system resulting from a motor vehicle collision, the patient was brought into the emergency department with diffuse abdominal pain and bowel shock. Unfortunately, the patient soon succumbed to his injuries[71].
Endovascular approach: Gaining access to the treatment zone can be challenging, and the target vessel may have tortuosity and elongation due to haemodynamic changes created by the hyperdynamic flow. Kimura et al[44] presented a case involving a hepatectomy (hepatocellular carcinoma), where the patient subsequently developed an arterioportal fistula with hepatofugal flow and a 40-mm-diameter PVA. After selective embolization of the anterior hepatic artery, the PVA disappeared, and portal flow was normalized.
An endovascular approach includes creation of a transjugular intrahepatic portosystemic shunt (TIPS). In patients with portal hypertension, an attempt may be made to decrease portal venous pressure in order to reduce the size of the aneurysm. Our review covered four patients with a PVA where the treatment of choice was a TIPS. Tsauo et al[14] presented a case involving a PVA resulting from portal hypertension associated with Budd-Chiari syndrome. For the first time, a TIPS was created without complications. The patient’s abdominal pain completely ceased within two days, and she remained asymptomatic during the one-year follow-up. Ding et al[45] presented a case with a PVA at the level of bifurcation, with comorbidities such as portal hypertension, liver cirrhosis and HBV chronica. A TIPS successfully decreased the patient’s portal hypertension and reduced the size of the PVA from 53 mm × 76 mm to 23 mm × 25 mm. Two years later, a CT scan and digital subtraction angiography revealed that the aneurysm had disappeared. The patient remained asymptomatic for 72 mo[45]. Dunlap et al[46] also used a TIPS successfully to treat a PVA resulting from portal hypertension and liver cirrhosis. Kohlbrenner et al[47] demonstrated transhepatic pharmacomechanical thrombolysis of a large thrombosed PVA. This was followed by insertion of a TIPS, along with an additional trans-TIPS thrombectomy to improve sluggish portal outflow and prevent re-thrombosis. Nine months later, an MRI showed complete resolution of the thrombosis.
Endoscopic approach via ERCP: In older patients with a PVA and complication of biliopathy and jaundice, ERCP with biliary stenting can be an appropriate treatment choice. In an 80-year-old male with liver cirrhosis and portal hypertension, an acquired PVA at the level of the left branch was found. The patient had developed biliopathy due to compression of the common bile duct; this complication was successfully treated endoscopically via ERCP with a biliary stent[23]. Sun et al[24] reported the case of an 85-year-old man with cholangitis complications from PVA-induced compression. Given the age of the patient, surgery was not considered, and instead an ERCP biliary stent was deployed several times.
PVA is a rare morphological abnormality of the portal venous system, accounting for 3% of all venous aneurysms in the human body. The number of reported PVAs across the world now stands at about 280: The 200 PVAs covered in the previous review published in 2015[1], the 18 cases in the retrospective study[2] and the 62 PVAs in our review covering the last seven years. PVA can be congenital or acquired, located mainly at the level of confluence, main trunk, branches and bifurcation. Up to 30% of patients can be asymptomatic, and non-specific abdominal pain should be investigated to exclude other pathological causes, such as cholecystitis or peptic ulcer disease, etc. Thrombosis complications occur in approximately 19%-23% of patients, and biliopathy occurs in approximately 4%-6% of patients. Other complications can also arise from compression due to a PVA, including thrombosis of the ICV and intestinal obstruction. Diagnosis of a PVA is based on spectral and colour Doppler sonography, and CT and MRI. EUS and IDU have also been used as a diagnostic tool. If a PVA is asymptomatic, it does not require any active treatment, and monitoring (a policy of “wait and see”) should be adopted. The first choice for treatment of PVA thrombosis is anticoagulation medication. If the PVA is getting larger and compressing adjacent organs, thrombosis will occur, so to prevent a potential rupture, open surgery methods such as an aneurysmectomy or an aneurysmorrhaphy should be considered. Given the risk associated with open surgery methods, interventional radiology procedures via a percutaneous approach, endovascular approach or, better still, an endoscopic approach should be considered for cases where a PVA is a consequence of portal hypertension and/or coexists with life-threatening conditions (injuries).
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Bosnia and Herzegovina
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Ding X, China; Sripongpun P, Thailand S-Editor: Chen YL L-Editor: A P-Editor: Chen YL
| 1. | Laurenzi A, Ettorre GM, Lionetti R, Meniconi RL, Colasanti M, Vennarecci G. Portal vein aneurysm: What to know. Dig Liver Dis. 2015;47:918-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 61] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 2. | Ahmed O, Ohman JW, Vachharajani N, Yano M, Sanford DE, Hammill C, Fields RC, Hawkins WG, Strasberg SM, Doyle MB, Chapman WC, Khan AS. Feasibility and safety of non-operative management of portal vein aneurysms: a thirty-five year experience. HPB (Oxford). 2021;23:127-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 3. | Gaba RC, Hardman JD, Bobra SJ. Extrahepatic Portal Vein Aneurysm. Radiol Case Rep. 2009;4:291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | De Vloo C, Matton T, Meersseman W, Maleux G, Houthoofd S, Op de Beeck K, Laleman W, Van Malenstein H, Nevens F, Verbeke L, Van der Merwe S, Verslype C. Thrombosis of a portal vein aneurysm: a case report with literature review. Acta Clin Belg. 2019;74:115-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Watanabe Y, Takase K, Okada K, Aikawa M, Okamoto K, Koyama I. Portal vein aneurysm with complete spontaneous regression after 10 years using conservative treatment. Clin J Gastroenterol. 2020;13:940-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Schilardi A, Ciavarella A, Carbone M, Antonica G, Berardi E, Sabbà C. A large asymptomatic portal vein aneurysm in an old man. Clin Case Rep. 2021;9:15-18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Kanamalla K, Alwakkaa H. Thrombosis of the inferior vena cava secondary to incidental portal vein aneurysm. Radiol Case Rep. 2022;17:1532-1535. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Douglass BE, Baggenstoss AH, Hollinshead WH. The anatomy of the portal vein and its tributaries. Surg Gynecol Obstet. 1950;91:562-576. [PubMed] |
| 9. | Doust BD, Pearce JD. Gray-scale ultrasonic properties of the normal and inflamed pancreas. Radiology. 1976;120:653-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 96] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Koc Z, Oguzkurt L, Ulusan S. Portal venous system aneurysms: imaging, clinical findings, and a possible new etiologic factor. AJR Am J Roentgenol. 2007;189:1023-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 88] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 11. | BARZILAI R, KLECKNER MS Jr. Hemocholecyst following ruptured aneurysm of portal vein; report of a case. AMA Arch Surg. 1956;72:725-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 116] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Hanafiah M, Johari B, Koshy M, Misni MN. Intrahepatic Portal Vein Aneurysm with Concurrent Hepatocellular Carcinoma. Sultan Qaboos Univ Med J. 2016;16:e115-e116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 13. | Khairallah S, Elmansouri A, Jalal H, Idrissi MO, Ganouni NC. Calcified wall portal venous aneurysm: a case report. Pan Afr Med J. 2016;25:93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 14. | Tsauo J, Li X. Portal vein aneurysm associated with Budd-Chiari syndrome treated with transjugular intrahepatic portosystemic shunt: a case report. World J Gastroenterol. 2015;21:2858-2861. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Das S, Dey M, Kumar V, Lal H. Portal vein aneurysm in thalassaemia. BMJ Case Rep. 2017;2017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 16. | Khan A, Ayub M, Haider I, Humayun M, Shah Z, Ajmal F. Coexisting giant splenic artery and portal vein aneurysms leading to non-cirrhotic portal hypertension: a case report. J Med Case Rep. 2016;10:270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Kurtcehajic A, Alibegovic E, Hujdurovic A, Vele E, Kurtcehajic D. Role of Cholelithiasis in Development of Portal Vein Aneurysm. Am J Med. 2018;131:e119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Kim SH, Yu HW, Kim HY, Jo HS. Neonatal vitelline vein aneurysm with thrombosis: prompt treatment should be needed. Ann Surg Treat Res. 2015;89:334-337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Chandran S, Kumar M, Jacob TJK, Mohamed F. Intestinal obstruction with a twist: a rare case of congenital portal vein aneurysm causing intestinal obstruction. BMJ Case Rep. 2018;2018. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 20. | Burdall OC, Grammatikopoulos T, Sellars M, Hadzic N, Davenport M. Congenital Vascular Malformations of the Liver: An Association With Trisomy 21. J Pediatr Gastroenterol Nutr. 2016;63:e141-e146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Güngör Ş, Varol Fİ, Kutlu R, Yılmaz S, Selimoğlu MA. An intrahepatic Portal Vein Aneurysm Presenting with Esophageal Variceal Bleeding in a Pediatric Patient: A Rare Clinical Entity. Balkan Med J. 2018;35:442-444. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 22. | Kurtcehajic A, Vele E, Hujdurovic A. Portal vein aneurysm and portal biliopathy. J Hepatobiliary Pancreat Sci. 2016;23:658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | Ding F, Li Q, Duan X, Ye J. Hepatobiliary and Pancreatic: Ruptured aneurysm of intra-hepatic portal vein causing obstructive jaundice. J Gastroenterol Hepatol. 2017;32:1666. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 24. | Sun J, Sun CK. Repeated Plastic Stentings of Common Hepatic Duct for Portal Vein Aneurysm Compression in a Patient Unsuitable for Surgery. Case Rep Gastroenterol. 2018;12:570-577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 25. | Field Z, Madruga M, Carlan SJ, Abdalla R, Carbono J, Al Salihi H. Portal vein aneurysm with acute portal vein thrombosis masquerading as a pancreatic mass. Hematol Oncol Stem Cell Ther. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 26. | Chawla YK, Bodh V. Portal vein thrombosis. J Clin Exp Hepatol. 2015;5:22-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 117] [Article Influence: 11.7] [Reference Citation Analysis (1)] |
| 27. | Shrivastava A, Rampal JS, Nageshwar Reddy D. Giant Intrahepatic Portal Vein Aneurysm: Leave it or Treat it? J Clin Exp Hepatol. 2017;7:71-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 28. | Iimuro Y, Suzumura K, Ohashi K, Tanaka H, Iijima H, Nishiguchi S, Hao H, Fujimoto J. Hemodynamic analysis and treatment of an enlarging extrahepatic portal aneurysm: report of a case. Surg Today. 2015;45:383-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 29. | Rana SS, Dhalaria L, Sharma R, Gupta R. Extra-hepatic portal vein aneurysm diagnosed by EUS. Endosc Ultrasound. 2020;9:270-271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 30. | Mohamadnejad M, Al-Haddad M. Intrahepatic aneurysmal portosystemic venous shunt diagnosed on EUS. VideoGIE. 2022;7:138-139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 31. | Tri TT, Duy HP, Trung BH, Thuan LA, Thach PN, Hien NX, Duc NM. A rare pediatric case of portal vein aneurysm thrombosis. Radiol Case Rep. 2022;17:286-289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 32. | Priadko K, Romano M, Vitale LM, Niosi M, De Sio I. Asymptomatic portal vein aneurysm: Three case reports. World J Hepatol. 2021;13:515-521. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 33. | Fleming MD, Lall P, Nagorney DM, Gloviczki P, Kalra M, Duncan A, Oderich G, Toomey B, Bower TC. Operative interventions for extrahepatic portomesenteric venous aneurysms and long-term outcomes. Ann Vasc Surg. 2015;29:654-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 34. | Kim HJ, Ha TY, Ko GY, Noh M, Kwon TW, Cho YP, Lee SG. A Case of Extrahepatic Portal Vein Aneurysm Complicated by Acute Thrombosis. Ann Vasc Surg. 2017;43:311.e9-311.e13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 35. | Chadha RM, Dougherty MK, Musto KR, Harnois DM, Nguyen JH. Temporary Portomesenteric Decompression for Splenic Vein Aneurysm During Orthotopic Liver Transplant. Liver Transpl. 2018;24:1485-1488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 36. | Ktenidis K, Manaki V, Kapoulas K, Kourtellari E, Gionis M. Giant Splenic Aneurysm with Arteriovenous (A-V) Shunt, Portal Hypertension, and Ascites. Am J Case Rep. 2018;19:1410-1415. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 37. | Gorolay V, Nguyen D, Samra J, Neale M. Asymptomatic thrombosis of extrahepatic portal vein aneurysm necessitating hybrid operative repair. Vascular. 2021;29:762-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 38. | Sura TA, Boutrous ML, Ruiz MI, Williams MS. Operative management of an incidental portal vein aneurysm in the setting of an incarcerated congenital diaphragmatic hernia. J Vasc Surg Cases Innov Tech. 2021;7:64-67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 39. | Shukla PA, Kolber MK, Kumar A, Patel RI. Percutaneous Embolization of an Intrahepatic Portal Vein Aneurysm. J Vasc Interv Radiol. 2016;27:1747-1749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 40. | Juscafresa LC, Alfaro MP, Grochowicz L, Lorenzo JIL, Jaureguizar JIB. Endovascular treatment of a splenic vein aneurysm through a transhepatic approach. Diagn Interv Radiol. 2019;25:166-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 41. | Marmor RA, Goodman S, Parsa P. Endovascular Repair of Splenic Vein Aneurysm with Balloon Expandable Stent Placement. Ann Vasc Surg. 2021;73:554-556. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 42. | Bremer WA, Lokken RP, Gaba RC, Bui JT. Arterial-portal fistula treated with hepatic arterial embolization and portal venous aneurysm stent-graft exclusion complicated by type 2 endoleak. Radiol Case Rep. 2019;14:1301-1305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 43. | Oguslu U, Uyanik SA, Gümüş B. Endovascular treatment of hepatic arterioportal fistula complicated with giant portal vein aneurysm via percutaneous transhepatic US guided hepatic artery access: a case report and review of the literature. CVIR Endovasc. 2019;2:39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 44. | Kimura Y, Hori T, Machimoto T, Ito T, Hata T, Kadokawa Y, Kato S, Yasukawa D, Aisu Y, Takamatsu Y, Kitano T, Yoshimura T. Portal vein aneurysm associated with arterioportal fistula after hepatic anterior segmentectomy: Thought-provoking complication after hepatectomy. Surg Case Rep. 2018;4:57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 45. | Ding PX, Han XW, Hua ZH. Extrahepatic Portal Vein Aneurysm at the Portal Bifurcation Treated with Transjugular Intrahepatic Portosystemic Shunt. J Vasc Interv Radiol. 2017;28:764-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 46. | Dunlap R, Golden S, Lyons GR. Portal Vein Aneurysm Treated With Trans-Jugular Intrahepatic Porto-Systemic Shunt. Vasc Endovascular Surg. 2021;55:885-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 47. | Kohlbrenner R, Schwertner AB, Vogel AR, Conrad M, Lokken RP. Large thrombosed portomesenteric venous aneurysm treated with pharmacomechanical thrombolysis combined with TIPS placement. CVIR Endovasc. 2022;5:11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 48. | Prabhakar N, Vyas S, Taneja S, Khandelwal N. Intrahepatic aneurysmal portohepatic venous shunt: what should be done? Ann Hepatol. 2015;14:118-120. [PubMed] |
| 49. | Srikanth KP, Thapa BR. Aneurysm of Right Branch of Portal Vein in a Child. Indian Pediatr. 2015;52:440. [PubMed] |
| 50. | Starikov A, Bartolotta RJ. Massive superior mesenteric venous aneurysm with portal venous thrombosis. Clin Imaging. 2015;39:908-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 51. | Nayman A, Guler I, Koplay M, Erdogan H, Cebeci H. Intrahepatic Portal Vein Aneurysm : An Unusual Entity. Acta Gastroenterol Belg. 2016;79:385. [PubMed] |
| 52. | Jaiswal P, Yap JE, Attar BM, Wang Y, Devani K, Jaiswal R, Basu A, Mishra S. Massive Asymptomatic Extrahepatic Portal Vein Aneurysm. Am J Med. 2017;130:e383-e386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 53. | Guilbaud T, Birnbaum DJ, Duconseil P, Soussan J, Moutardier V. Portal vein aneurysm incidentaloma. Surgery. 2017;162:1177-1178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 54. | Maia L, Castro-Poças FM, Pedroto I. Portal Vein Aneurysm Mimicking a Liver Nodule. GE Port J Gastroenterol. 2018;25:105-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 55. | Ashmore S, Stacy K. Aneurysm of the Splenomesenteric Portal Venous Confluence: A Case Report. S D Med. 2018;71:199-201. [PubMed] |
| 56. | Martínez D, Belmonte MT, Kosny P, Gómez MR, Hellìn D. Aneurysm of the Left Portal Branch. Eur J Case Rep Intern Med. 2018;5:000868. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 57. | Hirji SA, Robertson FC, Casillas S, McPhee JT, Gupta N, Martin MC, Raffetto JD. Asymptomatic portal vein aneurysms: To treat, or not to treat? Phlebology. 2018;33:513-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 58. | Alur İ, Us M. An incidental intrahepatic portal vein aneurysm. Turk Gogus Kalp Damar Cerrahisi Derg. 2018;26:681-682. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 59. | Chaubard S, Lacroix P, Kennel C, Jaccard A. [Aneurysm of the portal venous system: A rare and unknown pathology]. Rev Med Interne. 2018;39:946-949. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 60. | Ramamoorthy E, Kumar H, Gupta P. An Uncommon Portal Vein Abnormality. Clin Gastroenterol Hepatol. 2019;17:e19-e20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 61. | Kabir T, Choke ETC, Kam JH. Unusual discovery in the liver: intrahepatic portal vein aneurysm. ANZ J Surg. 2020;90:E28-E29. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 62. | Shams T, Hug M, Wolff T, Roduit J. [Superior mesenteric vein aneurysm, a rare case]. Rev Med Suisse. 2020;16:1652-1655. [PubMed] |
| 63. | Hernando Sanz A, Navarro-Aguilar V, López-Andújar R. Portal vein aneurysm, an update on the subject. A case report. Rev Esp Enferm Dig. 2021;113:77-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 64. | Tan RLW, Ng ZQ. Portal venous aneurysm. BMJ Case Rep. 2021;14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 65. | López-Fernández J, García Plaza G, García Quesada SM, Hernández Hernández JR. Superior mesenteric vein aneurysm. Rev Esp Enferm Dig. 2021;113:615-616. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 66. | Villani R, Lupo P, Angeletti AG, Sacco AF, Macarini L, Serviddio G. Asymptomatic saccular portal vein aneurysm: a case report and review of the literature. J Ultrasound. 2022;25:799-803. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 67. | Mortazavi S, Trinder M, Li R, Abdul Aziz F. Extrahepatic portal vein aneurysm identification during cholecystectomy. ANZ J Surg. 2022;92:921-922. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 68. | Matsumoto T, Aoki T, Kubota K. Pancreatic Head Adenocarcinoma Complicated by Portal Venous Aneurysm. J Gastrointest Surg. 2021;25:1628-1630. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 69. | Ierardi AM, Berselli M, Cuffari S, Castelli P, Cocozza E, Carrafiello G. Uncommon Case of a Post-Traumatic Portal Vein Pseudoaneurysm Treated with Percutaneous Transhepatic Stent Grafting. Cardiovasc Intervent Radiol. 2016;39:1506-1509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 70. | Walton H, Yu D, Imber C, Webster G. Portal vein pseudoaneurysm secondary to pancreatic lymphoma and biliary stent insertion: a rare cause of haemobilia. CVIR Endovasc. 2018;1:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 71. | Cleveland NC, Nguyen DN, Tran CD, Maheshwary RK, Hartman MS. Traumatic Injury to the Portal Vein With Shock Bowel. Curr Probl Diagn Radiol. 2019;48:97-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |