Published online Feb 6, 2023. doi: 10.12998/wjcc.v11.i4.719
Peer-review started: October 6, 2022
First decision: December 13, 2022
Revised: December 22, 2022
Accepted: January 12, 2023
Article in press: January 12, 2023
Published online: February 6, 2023
Processing time: 123 Days and 1.9 Hours
Cardioembolic stroke, referred to as cardiogenic stroke, is a clinical syndrome in which emboli from the heart pass through the circulatory system and cause cerebral artery embolism and corresponding brain dysfunction. Compared to other subtypes of ischemic stroke, cardiogenic stroke presents with more etiologies, greater severity, worse prognosis, and a higher recurrence rate. In this minireview, we provide new insights into the etiological classification, diagnostic methods, and interventions of cardiogenic stroke.
Core Tip: There are many reviews focused on the diagnosis and treatment strategies of cardiogenic stroke. However, in clinical practice, there are still many problems such as non-standard diagnosis and large differences in treatment measures. In this minireview, we introduce the latest Chinese expert consensus on cardiogenic stroke-based diagnostic criteria and provide some new insights into the etiological classification and inter
- Citation: Fan ZX, Liu RX, Liu GZ. Development and refinement of diagnostic and therapeutic strategies for managing patients with cardiogenic stroke: An arduous journey. World J Clin Cases 2023; 11(4): 719-724
- URL: https://www.wjgnet.com/2307-8960/full/v11/i4/719.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i4.719
Stroke is a disease that can seriously endanger human health. The 2019 Global Burden of Disease study showed that stroke is the second leading cause of death worldwide after ischemic heart disease (11.6% of total deaths), and the third leading cause of death and disability combined (5.7% of total disability-adjusted life years) with ischemic strokes accounting for the majority of strokes (62.4%)[1]. Cardiogenic stroke, also known as cardioembolic stroke, constitutes 20% to 30% of all ischemic strokes. It is a clinical syndrome in which emboli from the heart pass through the circulatory system and cause cerebral artery embolism and corresponding brain dysfunction[2,3]. With the strengthening of community medical management, atherosclerosis risk factors were significantly reduced (e.g., low-density lipoprotein-cholesterol and blood pressure levels were better controlled than before). As a result, stroke/transient ischemic attacks caused by large-artery atherosclerosis (LAA) and small-vessel occlusion were remarkably decreased; conversely, cardiogenic stroke/transient ischemic attack increased significantly[4]. Compared to other subtypes of ischemic stroke, cardiogenic stroke presents with more etiologies, greater severity, worse prognosis, and a higher recurrence rate[5,6]. Although the diagnosis and treatment of cardiogenic stroke has substantially improved worldwide in recent years, there are still plenty of shortcomings, such as insufficient understanding of this disease and significant differences in treatment strategies[3,4]. Therefore, further strengthening the understanding of the etiological classification, diagnostic methods, and intervention measures of cardiogenic stroke, and uniformly improving the diagnosis and treatment of cardiogenic stroke, have become the top priority in the neurology community.
According to the definite (or potential) cause of cardiogenic stroke in the A-S-C-O (phenotype) classification and its epidemiological characteristics, the relatively common causes are divided into atrial fibrillation (AF), heart failure, acute coronary syndromes, patent foramen ovale (PFO), rheumatic heart disease, artificial heart valve, infectious endocarditis (IE), dilated cardiomyopathy, and cardiac myxoma. In most cases, the intracardiac wall thrombus, tumor surface thrombus/debris, shedding of vegetations on the valve intima or aortic arch plaque, or paradoxical embolism (derived from veins), underlie the pathogenesis, thereby contributing to the obstruction of cerebral blood vessels[7].
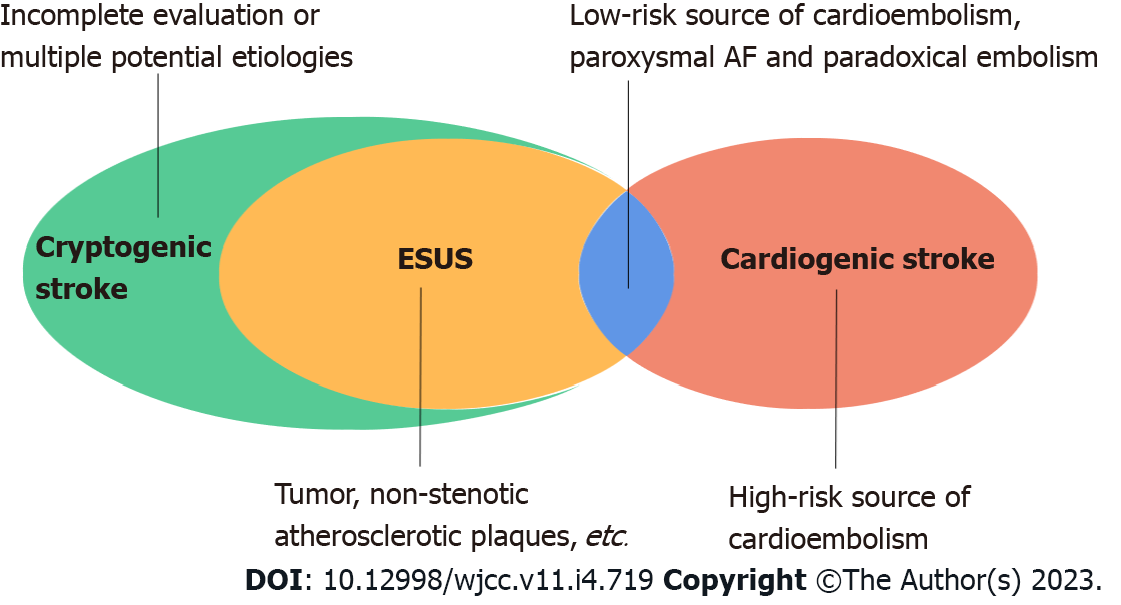
To date, the etiological attribution of cardiogenic stroke remains elusive. Firstly, the boundary between cardiogenic stroke and cryptogenic stroke, especially embolic stroke of undetermined source (ESUS), is blurred. Strokes that do not clearly meet the diagnostic criteria of the known ischemic stroke subtypes are classified as cryptogenic strokes[8,9], and cryptogenic stroke is further defined as ESUS when the clinical and neuroimaging features suggest a distant thrombus origin, the absence of lacunar infarcts, a high-risk source of cardiac embolism, or high-degree stenosis of the responsible vessel at the site of the infarction[10,11] (Figure 1). Regarding the etiology of embolism in ESUS, some cases may originate from arterial-arterial embolism caused by large atherosclerotic plaques in the brain, while other cases may result from some cardiac diseases (e.g., paroxysmal AF, PFO, atrial cardiomyopathy,
The diagnosis of cardiogenic stroke is frequently made based on its clinical and neuroimaging features, combined with other elements such as vascular and cardiac evaluation. With improvements in disease awareness and detection methods (e.g., long-term electrocardiogram monitoring, echocardiography), the detection rate of cardiogenic stroke has greatly increased in recent years when compared with other subtypes of ischemic stroke[15]. Nonetheless, several lines of clinical and radiological evidence to support cardiogenic stroke have been proposed in this regard since the 1990s[8,16,17], but there are no well-established diagnostic criteria. Additionally, there is also controversy over the specific examination protocol used to identify the etiology of cardiogenic stroke. Finally, there are many other problems, such as inconsistent diagnosis and treatment levels across different medical institutions, misdiagnosis, and mistreatment.
In an attempt to solve these problems, we first proposed new clinical diagnostic criteria for cardiogenic stroke[7]. According to the Chinese expert consensus on the diagnosis of cardiogenic stroke (2019) (Table 1), cardiogenic stroke is categorized into definite cardiogenic stroke, probable cardiogenic stroke, and possible cardiogenic stroke: Definite cardiogenic stroke = 2 of (A) + at least 1 of (B) + C; probable cardiogenic stroke = 2 of (A), or at least 1 of (A) + at least 1 of (B); possible cardiogenic stroke = at least 1 of (A). Clinical validation of the diagnostic criteria is currently underway.
| Criteria | Element |
| A: Essential criteria | Typical clinical manifestations |
| Characteristic neuroimaging (brain CT/MRI) findings | |
| B: Supportive criteria | Cardiogenic embolus on echocardiography1 |
| Arrhythmia on electrocardiogram, especially atrial fibrillation | |
| Characteristic vascular neuroimaging/cerebral angiography findings2 | |
| C: Exclusion of other diseases |
Even though the treatment principles of cardiogenic stroke are generally similar to other subtypes of ischemic stroke, more emphasis is placed on anticoagulation therapy. However, when to start or restart anticoagulation therapy and the choice of anticoagulants are mostly based on personal experience, mainly due to the lack of clinical evidence or clinical guidelines. Although the principle of using intravenous thrombolysis in the acute phase is similar of all subtypes, cardiogenic stroke also has its own specificity. In light of the above issues, the standardization of treating cardiogenic stroke has become an urgent issue which needs to be addressed.
For most stroke patients with AF, the guidelines for the early management of acute ischemic stroke from the 2018 AHA/ASA recommend that oral anticoagulation therapy should be initiated within 4 to 14 d after onset[17]. Nevertheless, in a recent multicenter real-world cohort study, initiation of oral anticoagulation within 4 to 14 d did not significantly reduce the incidence of ischemic and hemorrhagic stroke compared to that initiated within zero to three days[18]. Additionally, regarding the best time to restart oral anticoagulation after acute stroke, Hindricks et al[19] suggests that anticoagulation be restarted as soon as possible within 2 wk of onset under the guidance of a multi-disciplinary team (neurologist and cardiologist), in combination with the patient’s willingness to treat; however, so far there are no reliable data to support this viewpoint. Regarding the anticoagulant choice, results from four randomized controlled trials involving anticoagulation for stroke or systemic embolism in AF showed that, novel oral anticoagulants (NOACs) are noninferior to warfarin in reducing the risk of stroke or systemic embolism in patients with AF but are safer in terms of adverse reactions, such as risk of intracranial hemorrhage[20-23]. Due to this, we recommend that the risk of hemorrhagic transformation in cardiogenic stroke be taken into account, regardless of indications for anticoagulation (e.g., AF, valvular disease), and treatment should be started or restarted several days to several weeks after the onset of the disease, in consideration of the severity of the disease, the size of the acute cerebral infarction, and the risk of bleeding. It is also necessary to fully consider the faster effect and higher safety characteristics of NOACs compared with warfarin.
With regard to intravenous thrombolysis in cardiogenic stroke, to date, most studies were observational or small sample studies[24-26]; in addition, the consensus on efficacy and adverse reactions such as bleeding were different, mainly owing to differences in inclusion criteria and patient characteristics. It should be kept in mind that the use of intravenous thrombolysis is also limited or complicated under special circumstances, such as with prior anticoagulation therapy[27-32], recent valve surgery or percutaneous coronary intervention[33,34], and IE-related stroke[35]. Accordingly, a variety of guidelines and expert consensus have provided corresponding individualized treatment advice or recommendations[18,36-38]. Chinese neurologists have also recently reported a case of cardiogenic stroke successfully treated by intravenous thrombolysis with alteplase after reversal of dabigatran by idacilizumab[39]. Hence, for patients with acute cardiogenic stroke, especially those who received anticoagulation before disease onset, we recommend the treatment strategy should be individualized according to the specific situation, and intravenous thrombolytic therapy can be performed after multi-disciplinary consultation to improve patient outcomes.
In summary, three major issues are raised in this review: The etiology classification; the boundary between cardiogenic stroke, cryptogenic stroke and ESUS; as well as the attribution of AAA-related stroke all need to be clarified. Regarding the diagnosis, given the fact that currently no well-established diagnostic standard is available, we hence developed a new diagnostic system for cardiogenic stroke. Additionally, we recommend that anticoagulant therapy should be initiated or restarted several days to weeks after the onset of stroke, based on the patients’ specific situation, and treatment for acute phase and prevention of recurrent stroke should be actively carried out after multidisciplinary consultation. Despite substantial progress in the diagnosis and treatment of cardiogenic stroke worldwide, there is still a long way to go to address all issues. In particular, the development of a standard protocol for management of the acute phase and recovery phase should be determined as soon as possible. Thus, we look forward to seeing additional evidence-based research, real-world research, and health economics evidence in the future in order for clinicians to gain a more comprehensive understanding of cardiogenic stroke and precise prevention and treatment measures, thereby maximizing the clinical benefit and improving the prognosis of patients.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Alkhatib AJ, Jordan; Teramoto-Matsubara OT, Mexico S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | GBD 2019 Stroke Collaborators. Global, regional, and national burden of stroke and its risk factors, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021;20:795-820. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4299] [Cited by in RCA: 3596] [Article Influence: 899.0] [Reference Citation Analysis (0)] |
| 2. | Bogiatzi C, Hackam DG, McLeod AI, Spence JD. Secular trends in ischemic stroke subtypes and stroke risk factors. Stroke. 2014;45:3208-3213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 107] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 3. | Kamel H, Healey JS. Cardioembolic Stroke. Circ Res. 2017;120:514-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 291] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 4. | Spence JD. Cardioembolic stroke: everything has changed. Stroke Vasc Neurol. 2018;3:76-83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 5. | Lin HJ, Wolf PA, Kelly-Hayes M, Beiser AS, Kase CS, Benjamin EJ, D'Agostino RB. Stroke severity in atrial fibrillation. The Framingham Study. Stroke. 1996;27:1760-1764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 839] [Cited by in RCA: 920] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 6. | Bjerkreim AT, Khanevski AN, Thomassen L, Selvik HA, Waje-Andreassen U, Naess H, Logallo N. Five-year readmission and mortality differ by ischemic stroke subtype. J Neurol Sci. 2019;403:31-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 7. | Liu GZ, Hu R, Peng DT; Geriatric Neurology Group, Geriatric Branch of Chinese Medical Association; Writing Group of Chinese expert consensus on diagnosis of cardiogenic stroke. Chinese expert consensus on the diagnosis of cardiogenic stroke (2019). Chin Med J (Engl). 2021;134:505-507. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 8. | Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE 3rd. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7474] [Cited by in RCA: 8882] [Article Influence: 277.6] [Reference Citation Analysis (0)] |
| 9. | Ay H, Benner T, Arsava EM, Furie KL, Singhal AB, Jensen MB, Ayata C, Towfighi A, Smith EE, Chong JY, Koroshetz WJ, Sorensen AG. A computerized algorithm for etiologic classification of ischemic stroke: the Causative Classification of Stroke System. Stroke. 2007;38:2979-2984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 333] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 10. | Hart RG, Diener HC, Coutts SB, Easton JD, Granger CB, O'Donnell MJ, Sacco RL, Connolly SJ; Cryptogenic Stroke/ESUS International Working Group. Embolic strokes of undetermined source: the case for a new clinical construct. Lancet Neurol. 2014;13:429-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 980] [Cited by in RCA: 1183] [Article Influence: 107.5] [Reference Citation Analysis (0)] |
| 11. | Hart RG, Catanese L, Perera KS, Ntaios G, Connolly SJ. Embolic Stroke of Undetermined Source: A Systematic Review and Clinical Update. Stroke. 2017;48:867-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 409] [Article Influence: 51.1] [Reference Citation Analysis (0)] |
| 12. | Ntaios G. Embolic Stroke of Undetermined Source: JACC Review Topic of the Week. J Am Coll Cardiol. 2020;75:333-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 159] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 13. | Kleindorfer DO, Towfighi A, Chaturvedi S, Cockroft KM, Gutierrez J, Lombardi-Hill D, Kamel H, Kernan WN, Kittner SJ, Leira EC, Lennon O, Meschia JF, Nguyen TN, Pollak PM, Santangeli P, Sharrief AZ, Smith SC Jr, Turan TN, Williams LS. 2021 Guideline for the Prevention of Stroke in Patients With Stroke and Transient Ischemic Attack: A Guideline From the American Heart Association/American Stroke Association. Stroke. 2021;52:e364-e467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 519] [Cited by in RCA: 1638] [Article Influence: 409.5] [Reference Citation Analysis (0)] |
| 14. | Amarenco P, Davis S, Jones EF, Cohen AA, Heiss WD, Kaste M, Laouénan C, Young D, Macleod M, Donnan GA; Aortic Arch Related Cerebral Hazard Trial Investigators. Clopidogrel plus aspirin versus warfarin in patients with stroke and aortic arch plaques. Stroke. 2014;45:1248-1257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 135] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 15. | Pepi M, Evangelista A, Nihoyannopoulos P, Flachskampf FA, Athanassopoulos G, Colonna P, Habib G, Ringelstein EB, Sicari R, Zamorano JL, Sitges M, Caso P; European Association of Echocardiography. Recommendations for echocardiography use in the diagnosis and management of cardiac sources of embolism: European Association of Echocardiography (EAE) (a registered branch of the ESC). Eur J Echocardiogr. 2010;11:461-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 201] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 16. | Kelley RE, Kelley BP. Heart-Brain Relationship in Stroke. Biomedicines. 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 17. | Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk BM, Hoh B, Jauch EC, Kidwell CS, Leslie-Mazwi TM, Ovbiagele B, Scott PA, Sheth KN, Southerland AM, Summers DV, Tirschwell DL; American Heart Association Stroke Council. 2018 Guidelines for the Early Management of Patients With Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2018;49:e46-e110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2924] [Cited by in RCA: 3739] [Article Influence: 534.1] [Reference Citation Analysis (0)] |
| 18. | Yaghi S, Trivedi T, Henninger N, Giles J, Liu A, Nagy M, Kaushal A, Azher I, Mac Grory B, Fakhri H, Brown Espaillat K, Asad SD, Pasupuleti H, Martin H, Tan J, Veerasamy M, Liberman AL, Esenwa C, Cheng N, Moncrieffe K, Moeini-Naghani I, Siddu M, Scher E, Leon Guerrero CR, Khan M, Nouh A, Mistry E, Keyrouz S, Furie K. Anticoagulation Timing in Cardioembolic Stroke and Recurrent Event Risk. Ann Neurol. 2020;88:807-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 19. | Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, Boriani G, Castella M, Dan GA, Dilaveris PE, Fauchier L, Filippatos G, Kalman JM, La Meir M, Lane DA, Lebeau JP, Lettino M, Lip GYH, Pinto FJ, Thomas GN, Valgimigli M, Van Gelder IC, Van Putte BP, Watkins CL; ESC Scientific Document Group. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42:373-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3176] [Cited by in RCA: 6470] [Article Influence: 1617.5] [Reference Citation Analysis (0)] |
| 20. | Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J, Diaz R, Lewis BS, Darius H, Diener HC, Joyner CD, Wallentin L; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139-1151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7917] [Cited by in RCA: 8068] [Article Influence: 504.3] [Reference Citation Analysis (0)] |
| 21. | Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, Breithardt G, Halperin JL, Hankey GJ, Piccini JP, Becker RC, Nessel CC, Paolini JF, Berkowitz SD, Fox KA, Califf RM; ROCKET AF Investigators. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883-891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6519] [Cited by in RCA: 6921] [Article Influence: 494.4] [Reference Citation Analysis (2)] |
| 22. | Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, Al-Khalidi HR, Ansell J, Atar D, Avezum A, Bahit MC, Diaz R, Easton JD, Ezekowitz JA, Flaker G, Garcia D, Geraldes M, Gersh BJ, Golitsyn S, Goto S, Hermosillo AG, Hohnloser SH, Horowitz J, Mohan P, Jansky P, Lewis BS, Lopez-Sendon JL, Pais P, Parkhomenko A, Verheugt FW, Zhu J, Wallentin L; ARISTOTLE Committees and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6075] [Cited by in RCA: 6550] [Article Influence: 467.9] [Reference Citation Analysis (0)] |
| 23. | Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, Waldo AL, Ezekowitz MD, Weitz JI, Špinar J, Ruzyllo W, Ruda M, Koretsune Y, Betcher J, Shi M, Grip LT, Patel SP, Patel I, Hanyok JJ, Mercuri M, Antman EM; ENGAGE AF-TIMI 48 Investigators. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093-2104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4144] [Cited by in RCA: 3811] [Article Influence: 317.6] [Reference Citation Analysis (0)] |
| 24. | Vaclavik D, Vilionskis A, Jatuzis D, Karlinski MA, Gdovinova Z, Kõrv J, Tsivgoulis G, Mikulik R. Clinical outcome of cardioembolic stroke treated by intravenous thrombolysis. Acta Neurol Scand. 2018;137:347-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 25. | Anticoli S, Bravi MC, Perillo G, Siniscalchi A, Pozzessere C, Pezzella FR, Tanzi P, Gallelli L, Cartoni D. Effect of Cardioembolic Etiology on Intravenous Thrombolysis Efficacy for Acute Ischemic Stroke. Curr Neurovasc Res. 2016;13:193-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 26. | Wang XG, Zhang LQ, Liao XL, Pan YS, Shi YZ, Wang CJ, Wang YL, Liu LP, Zhao XQ, Wang YJ, Li D, Wang CX; Thrombolysis Implementation and Monitoring of acute ischemic Stroke in China (TIMS-China) Investigators. Unfavorable Outcome of Thrombolysis in Chinese Patients with Cardioembolic Stroke: a Prospective Cohort Study. CNS Neurosci Ther. 2015;21:657-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 27. | Xian Y, Liang L, Smith EE, Schwamm LH, Reeves MJ, Olson DM, Hernandez AF, Fonarow GC, Peterson ED. Risks of intracranial hemorrhage among patients with acute ischemic stroke receiving warfarin and treated with intravenous tissue plasminogen activator. JAMA. 2012;307:2600-2608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 120] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 28. | Toyoda K, Yamagami H, Koga M. Consensus Guides on Stroke Thrombolysis for Anticoagulated Patients from Japan: Application to Other Populations. J Stroke. 2018;20:321-331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 29. | Agosti S, Casalino L, Rocci E, Zaccone G, Rota E. Successful intravenous thrombolysis for ischemic stroke after reversal of dabigatran anticoagulation with idarucizumab: a case report. J Med Case Rep. 2017;11:224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 30. | Barber PA, Wu TY, Ranta A. Stroke reperfusion therapy following dabigatran reversal with idarucizumab in a national cohort. Neurology. 2020;94:e1968-e1972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 31. | Mazya MV, Lees KR, Markus R, Roine RO, Seet RC, Wahlgren N, Ahmed N; Safe Implementation of Thrombolysis in Stroke Investigators. Safety of intravenous thrombolysis for ischemic stroke in patients treated with warfarin. Ann Neurol. 2013;74:266-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 32. | Frank B, Grotta JC, Alexandrov AV, Bluhmki E, Lyden P, Meretoja A, Mishra NK, Shuaib A, Wahlgren NG, Weimar C, Lees KR; VISTA Collaborators. Thrombolysis in stroke despite contraindications or warnings? Stroke. 2013;44:727-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 96] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 33. | Manoukian SV, Feit F, Mehran R, Voeltz MD, Ebrahimi R, Hamon M, Dangas GD, Lincoff AM, White HD, Moses JW, King SB 3rd, Ohman EM, Stone GW. Impact of major bleeding on 30-day mortality and clinical outcomes in patients with acute coronary syndromes: an analysis from the ACUITY Trial. J Am Coll Cardiol. 2007;49:1362-1368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 641] [Cited by in RCA: 633] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 34. | Kamel H, Johnston SC, Kirkham JC, Turner CG, Kizer JR, Devereux RB, Iadecola C. Association between major perioperative hemorrhage and stroke or Q-wave myocardial infarction. Circulation. 2012;126:207-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 35. | Brownlee WJ, Anderson NE, Barber PA. Intravenous thrombolysis is unsafe in stroke due to infective endocarditis. Intern Med J. 2014;44:195-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 36. | Demaerschalk BM, Kleindorfer DO, Adeoye OM, Demchuk AM, Fugate JE, Grotta JC, Khalessi AA, Levy EI, Palesch YY, Prabhakaran S, Saposnik G, Saver JL, Smith EE; American Heart Association Stroke Council and Council on Epidemiology and Prevention. Scientific Rationale for the Inclusion and Exclusion Criteria for Intravenous Alteplase in Acute Ischemic Stroke: A Statement for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2016;47:581-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 482] [Article Influence: 53.6] [Reference Citation Analysis (0)] |
| 37. | Diener HC, Bernstein R, Butcher K, Campbell B, Cloud G, Davalos A, Davis S, Ferro JM, Grond M, Krieger D, Ntaios G, Slowik A, Touzé E. Thrombolysis and thrombectomy in patients treated with dabigatran with acute ischemic stroke: Expert opinion. Int J Stroke. 2017;12:9-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 38. | Berge E, Whiteley W, Audebert H, De Marchis GM, Fonseca AC, Padiglioni C, de la Ossa NP, Strbian D, Tsivgoulis G, Turc G. European Stroke Organisation (ESO) guidelines on intravenous thrombolysis for acute ischaemic stroke. Eur Stroke J. 2021;6:I-LXII. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 713] [Article Influence: 178.3] [Reference Citation Analysis (0)] |
| 39. | Xie D, Wang X, Li Y, Chen R, Zhao Y, Xu C, Zhang Q, Zhang Y. Intravenous Thrombolysis After Reversal of Dabigatran With Idarucizumab in Acute Ischemic Stroke: A Case Report. Front Aging Neurosci. 2021;13:765037. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |