Published online Dec 26, 2023. doi: 10.12998/wjcc.v11.i36.8542
Peer-review started: September 8, 2023
First decision: November 9, 2023
Revised: November 29, 2023
Accepted: December 12, 2023
Article in press: December 12, 2023
Published online: December 26, 2023
Processing time: 104 Days and 9.7 Hours
While stroke and lower extremity venous thromboemboli have been commonly reported following acute infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), spinal cord infarction or ischemia has been extremely rare. Findings of long coronavirus disease (COVID) in this select population have not been studied.
We present the case of a 70-year-old female with sudden onset of trunk and lower extremity sensorimotor loss due to spinal cord infarction, attributed to acute infection with SARS-CoV-2. Diagnostic work up confirmed a T3 complete (ASIA impairment Scale A) paraplegia resulting from a thrombotic infarct. Her reported myalgias, neuropathic pain, spasticity, bladder spasms, and urinary tract infections exceeded the frequency and severity of many spinal cord injury (SCI) individuals of similar age and degree of neurologic impairment. In her first year after contracting COVID-19, she underwent 2 separate inpatient rehabilitation courses, but also required acute hospitalization 6 additional times for subsequent infections or uncontrolled pain. Yet other complications of complete non-traumatic SCI (NTSCI), including neurogenic bowel and temperature hypersensitivity, were mild, and pressure injuries were absent. She has now transitioned from the acute to chronic phase of spinal cord injury care, with subsequent development of post-acute sequelae of SARS-CoV-2 infection (PASC).
This individual experienced significant challenges with the combined effects of acute T3 NTSCI and acute COVID-19, with subsequent progression to PASC.
Core Tip: Although stroke and venous thromboembolism have been frequently observed with acute coronavirus disease 2019 (COVID-19), spinal cord infarction leading to paraplegia has rarely been seen. We report a case of spinal cord infarction shortly following infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Consequently, this individual has experienced severe neurologic disability, with subsequent development of long COVID. Symptoms such as myalgias, neuropathic pain, muscle spasms, and frequent bacterial infections are present in post-acute sequelae of SARS-CoV-2 infection (PASC), independent of spinal cord injury (SCI). Over the past 3 years, the dual presence of PASC and recent SCI may have led to increased severity of symptoms shared by both conditions.
- Citation: Oleson CV, Olsen AC, Shermon S. Spinal cord infarction attributed to SARS-CoV-2, with post-acute sequelae of COVID-19: A case report. World J Clin Cases 2023; 11(36): 8542-8550
- URL: https://www.wjgnet.com/2307-8960/full/v11/i36/8542.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i36.8542
Among vascular events, spinal cord infarction is relatively rare, accounting for only 0.3%-1% of all strokes[1] and 5%-8% of acute myelopathies[2]. One cause of spinal cord infarction arises from a thrombotic event in vulnerable areas of the thoracic cord, particularly between T8-12, which is supplied by the artery of Adamkiewicz. While deep vein thrombosis, pulmonary embolism, and stroke are commonly observed complications of coronavirus disease 2019 (COVID-19), spinal cord infarction is comparatively infrequent[3-6]. The cytokine release following acute infection, which peaks 7 d after contracting the virus, may be responsible for the increase in thrombotic events associated with acute infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)[7,8].
This case discussed in this report differs from other published accounts describing spinal cord infarcts attributed to acute SARS-CoV-2, because we have followed this individual for nearly 3 years after contracting COVID-19, covering her difficulties with “long COVID,” which has now officially named post-acute sequelae of SARS-CoV-2 infection (PASC) by the World Health Organization[9]. The term PASC may be assigned to “individuals with a history of probable or confirmed SARS-CoV-2 infection, usually 3 mo from the onset of COVID-19 with symptoms that last for at least 2 mo and cannot be explained by an alternative diagnosis”. The definition further states that PASC generally impacts everyday functioning and that symptoms may be of new onset, follow initial recovery from an acute COVID-19 episode, or persist from the initial illness. Moreover, symptoms may also fluctuate or relapse over time[9].
Common complaints of PASC include fatigue, cough shortness of breath, cognitive deficits or “brain fog”. Reported features of PASC may also involve headache, heart palpitations, exercise intolerance, joint pain or swelling, myalgias, vertigo, peripheral neuropathy, altered taste or smell, disordered sleep, anxiety, depression, and thromboembolic events[10-13]. While a number of the above symptoms may occur subsequent to SCI, many would be unusual, such as persistent cough, fatigue months after SCI, changes in taste or smell, continued exercise intolerance, new onset cognitive deficits or “brain fog”, unrelated to any sedating medications or concomitant brain injury.
Our patient became symptomatic prior to COVID-19 vaccine availability and has given written consent to share her story for educational publication. This project was approved by the Institutional Review Board of the MetroHealth System.
A 71-year-old female with a past medical history of undifferentiated connective tissue disease (UCTD) presented to an acute care hospital in December, 2020 after experiencing sudden onset of lower extremity weakness over 8-10 min, sensory loss from the lower trunk down, urinary retention, and worsening hypotension.
This individual had recently been exposed to COVID-19 through a household member and subsequently tested positive, with COVID cycle thresholds suggestive of recent infection. She demonstrated cough and fever before hospitalization, but did not require supplemental oxygen beyond the first few hospital days. She was issued 5 d of IV methylprednisolone and remdesivir, followed by an oral prednisone taper of 5 additional days. Neurological exam in acute care found incomplete sensory deficits T3-8 but complete absence of sensation from T9 and below.
During inpatient rehabilitation, we did acquire some key historical information about her UCTD, which to date had never progressed to a defined connective tissue disorder such as mixed connective tissue disease (MCTD). This condition is characterized by the presence of certain antibodies, particularly presence of the U1 small nuclear ribonucleoprotein particles (snRNP). Notes received indicate a negative titer for U1snRNP in 2020 when she had acute COVID-19, similar to her level when last tested in 2016. No lab quantification of U1-anti RNP titer was listed among lab results, other than a note stating it was not present. She had no clinical features of MCTD other than presence of sclerodactyly and stated history of Raynaud’s, which was not active during rehabilitation. She had myalgias but no evidence of synovitis or myositis that would prompt us to request a muscle biopsy. At the time of her admission to rehabilitation, this individual’s discomfort and spasms, as well as pain were in the middle and upper trunk and mid-back. However, during past UCTD exacerbations, she had endured aching and often sharp pain specifically in the posterior cervical spine and shoulders and during more significant attacks, pain and swelling in her fingers. Except during practice with wheelchair transfers, she reported no shoulder, arm, or hand pain with physical or occupational therapies.
The possibility exists that some of the pain and spasms she was feeling was a different manifestation of her usual UCTD flare. In the past, such instances had always affected more proximal areas of the body, specifically neck, shoulders and hands. In her 2.5 years since discharge from her second inpatient rehabilitation stay, she has only experienced two significant UCTD exacerbations, both during an acute hospital admission for secondary complications of her NTSCI. The first occurred in the summer of 2022 when septic from a severe UTI. Her antibiotics for that condition included first intravenous cephalosporins and then ciprofloxacin. Both agents may have impaired absorption of hydroxychloroquine prescribed daily for chronic UCTD. In addition, several doses of this long-term medication were missed due to acute illness. During this hospitalization, she became weaker and had increased joint pain, neck pain, and hand swelling. However, the only serology that was abnormal among rheumatologic indices was an elevated ESR of 3 points beyond the upper limit of normal, which could have been outside the normal range simply due to the UTI. During this admission, the same labs as appeared in Table 1 were performed and no findings revealed a change in her degree of UCTD.
| Specific test | Individual result (normal range) | Test results in context of undifferentiated connective tissue disease[16] |
| Anti-Smith antibody | < 0.2 AI (≤ 0.9) | Highly sensitive for SLE |
| Anti-DS DNA | < 12 IU/mL (< 30) | Specific to SLE |
| SSA Ab (anti-Ro AB) | < 0.2 AI (≤ 0.9) | Seen as elevated in 90% of those with Sjogren’s and in 40%-50% of those with SLE |
| SSB AB (anti-La Ab) anti | < 0.2 AI (≤ 0.9) | |
| Scleroderma Ab IgG | < 0.2 AI (≤ 0.9) | Specific to scleroderma but can be found as positive in combined rheumatologic disorders |
| Jo-Ab | < 0.2 AI (≤ 0.9) | Positive in dermatomyositis and polymyositis and in other CTD |
| Ribosomal RNP | < 0.2 AI (≤ 0.9) | Nonspecific index seen in SLE and other rheumatologic disorders |
| Scleroderma AB IgG | < 0.2 AI (≤ 0.9) | Specific to scleroderma |
Upon arriving to rehabilitation, her exam demonstrated a C7 left, T3 right ASIA Impairment Scale A, with a zone of sensory preservation to T8 bilaterally and complete absence of sensory and motor function from T9-S5. Her first month of rehabilitation was marked by expected neurogenic bowel and bladder, moderate thoracic non-radiating back pain, and mild spasticity below T9. She also had a band-like tightness in the T4-5 dermatomes in the absence of imaging findings there. The pain continued to intensify during subsequent weeks in rehabilitation, progressively taking on more neuropathic features with relentless mid-back and chest tightness.
She was discharged home after 8 wk but continued to experience unrelenting truncal pain between the T3-T8 dermatomes, above and at the level of the infarct, estimated to be located at T8. Several additional acute care and rehabilitation admissions for pain and urinary tract infections ensued during the subsequent two months. Her exam in this time now showed T3 complete SCI with partial preservation to T8.
Table 2 gives additional studies undertaken in the diagnostic workup, including the presence of viruses other than SARS-CoV-2 (enterovirus, Varicella zoster, Herpes simplex, and West Nile), and markers of inflammatory, autoimmune, and neoplastic disorders. Specifically, there was no evidence of Neuromyelitis Optica, based on absent aquaporin-4, and no evidence of myelin oligodendrocyte glycoprotein antibody, which is characterized by immune mediated demyelination of the spinal cord and other regions of the central nervous system. Moreover, immunoglobulin G synthesis of the cerebral spinal fluid (CSF) index was also negative, suggesting other inflammatory processes were not present. No oligoclonal bands were detected in CSF, a finding commonly seen in multiple sclerosis (MS) and in neoplastic processes such as multiple myeloma. Myelin basic protein was elevated but is a nonspecific finding, present in autoimmune disorders such as MS and ischemic conditions. including stroke[14]. Lumbar puncture on presentation had serum and cerebrospinal fluid studies that were entirely unremarkable.
| Lab | Result |
| SARS-CoV-2 | Positive |
| CSF enterovirus | Negative |
| CSF VZV | Not detected |
| CSF WNV | Not detected |
| CSF HSV | Not detected |
| AQP4 | Not detected |
| Myelin basic protein | Elevated |
| MOG ab, IgG | Negative |
| Oligoclonal bands | Not detected |
| IgG synthesis CSF and serum | WNL |
Her managing team at the acute care hospital did not have access to her outpatient records about her rheumatologic condition from her community physicians. They did perform a comprehensive serologic workup during her acute COVID admission, but the specimens were sent to an outside lab. Her results were not finalized prior to acute hospital discharge and thus were never added to her inpatient record, nor subsequently forwarded to the rehabilitation team.
Several weeks into her first inpatient rehabilitation stay, partial rheumatologic history and the labs drawn while admitted for COVID-19 were eventually obtained from her outpatient physician’s office. This individual’s initial diagnosis of UCTD occurred in 1993, prior to acute COVID-19, and was classified as non-antinuclear antibody UCTD. Her condition was based on the presence of CREST syndrome, the pneumonic of which represents calcinosis, Raynaud’s syndrome, esophageal dysmotility, sclerodactyly, and telangiectasias[15]. This information was from her first available outpatient record dating back to 2004, nearly 20 years preceding this publication. In 1993, her predominant features were Raynaud’s affecting fingers and sclerodactyly. She began on disease-modifying medication hydroxychloroquine and a plan was made to arrange for oral prednisone as needed for any exacerbations. Outpatient records from 2004-2020 indicate patient reported feeling well, with a “stable UCTD presentation”, without changes in lab indices, recorded hospitalizations, or flares. Documents did note an exacerbation of symptoms of neck and shoulder pain in 2016 that was managed as an outpatient with a combination of hydroxychloroquine and nifedipine, a calcium-channel blocker. At that time a core panel of rheumatologic markers was drawn, identical to the panel drawn in December 2020 given in Table 1. No specific rheumatologic markers were concerning, with the exception of an elevated erythrocyte sedimentation rate that resolved using the above medications. Between 2016-2020, she continued with annual visits to rheumatology without a documented flare or change in medications. Although normal values vary from one lab to another, the purpose of each test and the ratio of positive to negative values is similar between institutions[16]. The labs were selected by the referring facility where the acute care team made the decisions in diagnostic workup.
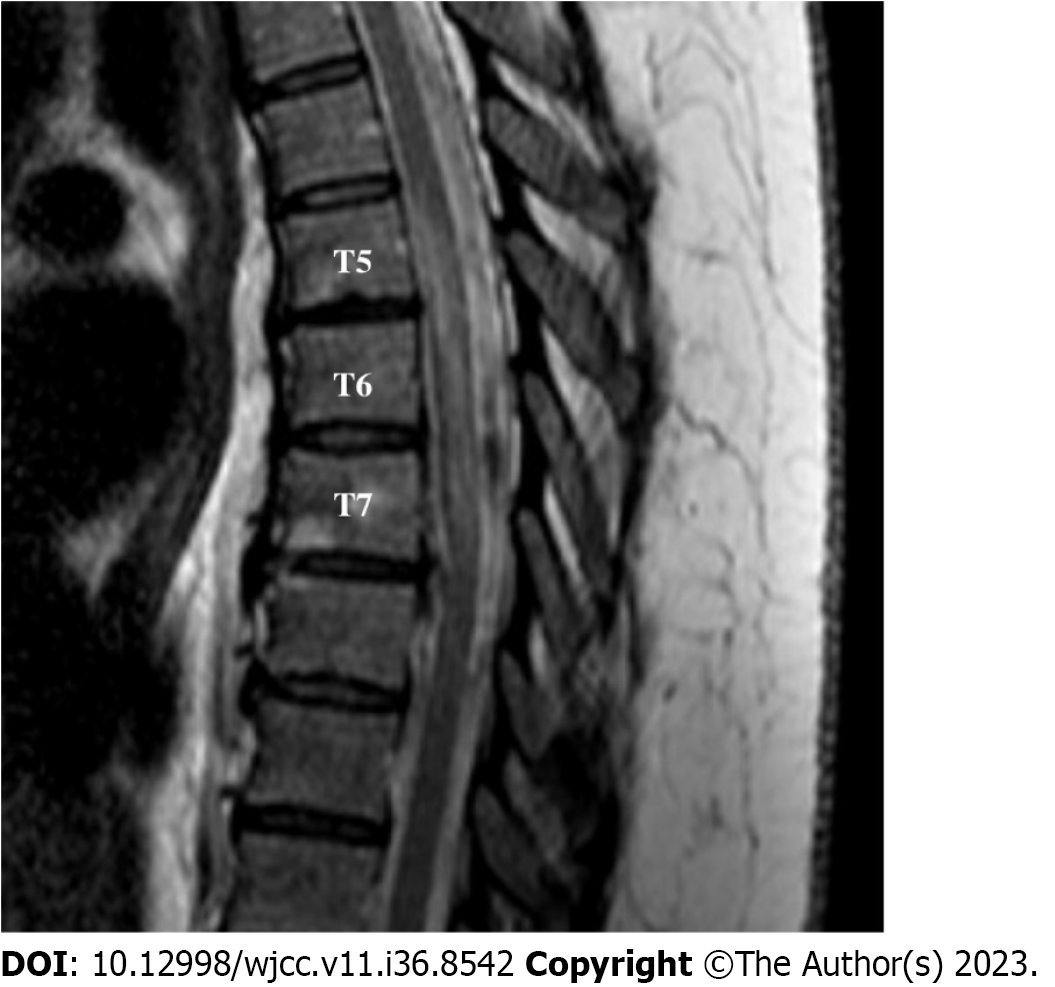
Initial workup included magnetic resonance imaging (MRI) of the cervical, thoracic, and lumbar spine, which showed acute cord ischemia T9 to the conus medullaris. Thoracic cord expansion and increased intramedullary signal extending many vertebral segments were compatible with a spinal cord infarct, particularly in light of the CSF findings and her acute onset of weakness. The above helped to differentiate an infarct from transverse myelitis. The brain MRI was negative for optic neuritis or lesions suggestive of MS, features needed to diagnose those conditions.
Figure 1 demonstrates a lengthy region of T2 hyperintensity from T9 to the conus, yet absent imaging findings above T9, despite observed sensory abnormalities for many segments rostral to T9. She was diagnosed with a T8 spinal cord thrombotic stroke. Her infarct occurred approximately 7 d after acute infection with COVID-19, consistent with the timing reported by Zhang et al[7] in relation to COVID cytokine storm.
Our final diagnosis is new-onset spinal cord infarction attributed to acute infection with SARS-CoV-2. Table 3 lists common symptoms of PASC and which of these were present in our patient[12,13]. Among the traditional symptoms of SCI, neuropathic pain, muscle spasms, and neurogenic bladder were severe. Other common conditions seen in complete SCI such as temperature dysregulation, pressure injuries, significant problem with neurogenic bowel, were noticeably absent or very well controlled with non-pharmacologic measures of positioning, diet, and environmental adaptations.
| Symptoms | Sneller et al[12] | Davis et al[13] | Present in case described |
| Fatigue | 26% | 98.3% | Yes |
| Cough | 5% | 66.2% | Yes |
| Concentration Deficit | 12% | 85.1% | Yes |
| Dyspnea | 19% | 77.4% | Yes |
| Anosmia/parosmia | 14% | 35.9% | No |
| Headache | 12% | 77% | Yes |
| Insomnia | 9% | 60% | No |
| Chest pain/discomfort | 8% | 53.1% | No |
| Anxiety | 6% | 57.9% | Yes |
| Myalgia | 6% | 69.1% | Yes |
| Tinnitus | 6% | 26.2% | No |
| Palpitations | 5% | 67.4% | No |
| Arthralgia | 3% | 52.2% | Yes |
| Taste disorder | 5% | 33.7% | Yes |
| Depression | 3% | 47.3% | Yes |
| Alopecia | 4% | N/A | No |
| Dizziness | 4% | 67.3% | No |
| Paresthesias | 1% | 35.4% | Yes |
| Visual Impairment | 1% | 10.4% | No |
Although her pain had been manageable in the first month after contracting COVID-19, the next 16 mo were marked by both bony and neuropathic pain of an unremitting nature along with common symptoms of PASC, including fatigue, headache, mental exhaustion (“brain fog”), and myalgias. Her neuropathic pain was largely unresponsive to anticonvulsant medications (gabapentin, pregabalin); serotonin-based agents (duloxetine); opiate medications; a thoracic paravertebral selective nerve root block; a spinal cord stimulator trial, and most recently, an intrathecal pain pump that is set at very low rates to minimize hypotension. She did not tolerate baclofen orally so no intrathecal administration of that agent was attempted. Muscle spasms in the region of T3-8 were similarly intense, limiting the number of hours she could sit and forcing her to lie supine due to painful muscle cramping. Yet no spasms occurred below the area of the infarct.
Detrusor areflexia was managed through frequent intermittent catheterization, yet still resulted in monthly UTIs. She experienced 8 urinary tract infections over a period of 9 mo, but the causative organism differed from month to month
Regarding flares of this individual’s UCTD, her second exacerbation came in April 2023 when she was hospitalized for severe constipation and a suspected small bowel obstruction. This situation arose following an increase in the morphine dose of her intrathecal pain pump. She again reported severe shoulder and neck pain but no discomfort in the regions of her back, pelvis or legs. She was unable to process any oral medications. Despite being given intravenous methylprednisolone as a replacement for hydroxychloroquine, her symptoms remained severe. During each of the aggravations of her UCTD that transpired while in the stage of PASC, pain and swelling remained localized to the same areas as prior to COVID-19 and did not affect the areas of her body impacted by PASC. From both this episode and her earlier exacerbation, it is apparent that neurogenic bowel and bladder complications, including UTIs, may precipitate a flare of UCTD. Because these are among the most common complications of any patient with SCI, increased clinical oversight and management of such patients is warranted from physicians in both rehabilitation and rheumatology.
Symptoms of PASC reported by this patient varied as her condition progressed. Although her spasms did improve after implantation of the morphine pump, she continued to endorse fatigue, post-exertional malaise, altered taste with poor appetite, and widespread muscle aches and pains. This patient’s complaints of myalgias and neuropathic pain above and below the neurologic level of injury are atypical of an SCI condition, in which such symptoms are traditionally at or below the infarct level. Unlike many with spinal infarcts, she regained no dorsal column function. There can be some inflammation surrounding the original infarct that makes the sensory examination altered in dermatomes rostral to the lesion. We believe this temporary finding could have occurred in this case. The best assessment of localized edema after an infarct would be MRI, and specifically the diffusion-weighted imaging (DWI) sequences. However in her case, DWI sequences were not performed[17]. One year after original diagnosis with COVID-19, a follow up examination demonstrated the neurologic level of injury was T6, rather than T3 complete SCI. There were present but impaired findings at dermatomes T7 and 8, with no sensory of motor function from T9 to the sacrum.
The case described in this narrative is characterized by some unusual features in her diagnosis and by the unpredictability of the clinical course. The patient’s sensory function was impaired but not completely absent between T3-8, yet it was in this region and not below T8, where the lancinating neuropathic pain and muscle spasms arose. Neurology and infectious disease researchers at the referring institution attributed the sensory loss in T3-T8 to a COVID-induced direct viral neurotoxicity, in a similar manner to HIV-1[18,19]. We suspect the infarct, which is clinically apparent at T9, occurred from a thrombus in the artery of Adamkiewicz, yet no sensory function was preserved. Similar cases of arterial involvement of this vessel would result in an anterior cord syndrome, sparing dorsal column function. However, this individual had no dorsal column function of proprioception, position sense or vibration. Despite clean intermittent catheterization technique, her monthly infections in the bladder far exceeded the average number of 2.6 infections per year found in those SCI individuals choosing clean technique intermittent catheterization[20].
This case is also notable for the heroic measures that have been undertaken subsequent to acute COVID infection to manage both neuropathic pain and intense back spasms localized to the mid-thoracic region. Patients with PASC frequently report myalgias, “pins and needles”[21,22] and spasms[22,23]. These struggles are similarly echoed by many of those with PASC who endure daily challenges that severely impact their quality of life and participation in the community[24]. In the case of our patient, an exacerbation of any aspect of her bowel or bladder function may lead to interventions that trigger a UCTD flare, further creating challenges for the patient and the physicians who manage her complex care.
In this case report of a NTSCI directly attributed to COVID-19, the most persistent complaints were myalgia, neuropathic pain, muscle spasms, and bladder dysfunction in the forms of bladder hyperreflexia and urinary tract infections[25]. While myalgias and neuropathic pain are commonly reported[11,13], the urinary symptoms of PASC, apart from renal impairment, have only recently been recognized. Lamb and colleagues have published a study on COVID-19 associated cystitis among those with PASC[26]. Their group has also linked COVID-19 inflammation to an increase in urine cytokines and to bladder hyperreflexia, nocturia, and urge incontinence[27]. In a person with SCI, such factors could certainly contribute to frequent urinary tract infections.
The severity and persistence of her clinical complaints represent a combination of severe SCI sequelae and those of PASC, the sum of which has greatly diminished her quality of life and participation in her community.
For the individual discussed in this report, the neurological sequelae of COVID-19 may be contributing to the secondary effects of NTSCI. In the chronic care setting, physicians may have difficulty separating which symptoms are due to a NTSCI and which are a direct consequence of PASC. Regardless, appropriate rehabilitation interventions for each condition encountered must be developed regardless of the cause. In the case of this particular patient, the plan must also encompass measures to minimize exacerbations of a chronic CTD. Only through such a comprehensive approach can we hope to optimize an individual’s quality of life. Over the next decade, many of those living with SCI could face a new disability. It will be our role to provide life-long care for their COVID-19 concerns, as well as their chronic spinal cord injury needs.
The authors acknowledge efforts of Samuel Onusko DO in creating a secure database for entry of demographic and clinical data.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: American Spinal Injury Association; American Society of Bone and Mineral Research.
Specialty type: Medicine, research and experimental
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Masyeni S, Indonesia S-Editor: Fan JR L-Editor: A P-Editor: Zhang XD
| 1. | Romi F, Naess H. Spinal Cord Infarction in Clinical Neurology: A Review of Characteristics and Long-Term Prognosis in Comparison to Cerebral Infarction. Eur Neurol. 2016;76:95-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 2. | Novy J, Carruzzo A, Maeder P, Bogousslavsky J. Spinal cord ischemia: clinical and imaging patterns, pathogenesis, and outcomes in 27 patients. Arch Neurol. 2006;63:1113-1120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 242] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 3. | Sampogna G, Tessitore N, Bianconi T, Leo A, Zarbo M, Montanari E, Spinelli M. Spinal cord dysfunction after COVID-19 infection. Spinal Cord Ser Cases. 2020;6:92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 4. | Eissa M, Abdelhady M, Alqatami H, Salem K, Own A, El Beltagi AH. Spinal cord infarction in a 41-year-old male patient with COVID-19. Neuroradiol J. 2021;34:245-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 5. | Bax F, Gigli GL, Iaiza F, Valente M. Spontaneous spinal cord ischemia during COVID-19 infection. J Neurol. 2021;268:4000-4001. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Kahan J, Gibson CJ, Strauss SB, Bronstein M, Winchell RJ, Barie PS, Segal AZ. Cervical spinal cord infarction associated with coronavirus infectious disease (COVID)-19. J Clin Neurosci. 2021;87:89-91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Zhang S, Zhang J, Wang C, Chen X, Zhao X, Jing H, Liu H, Li Z, Wang L, Shi J. COVID19 and ischemic stroke: Mechanisms of hypercoagulability (Review). Int J Mol Med. 2021;47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 55] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 8. | Coperchini F, Chiovato L, Croce L, Magri F, Rotondi M. The cytokine storm in COVID-19: An overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev. 2020;53:25-32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 955] [Cited by in RCA: 939] [Article Influence: 187.8] [Reference Citation Analysis (0)] |
| 9. | World Health Organization. WHO reference number. Available from: https://www.ukwhoswho.com/display/10.1093/ww/9780199540891.001.0001/ww-9780199540884-e-164854. |
| 10. | Nalbandian A, Sehgal K, Gupta A, Madhavan MV, McGroder C, Stevens JS, Cook JR, Nordvig AS, Shalev D, Sehrawat TS, Ahluwalia N, Bikdeli B, Dietz D, Der-Nigoghossian C, Liyanage-Don N, Rosner GF, Bernstein EJ, Mohan S, Beckley AA, Seres DS, Choueiri TK, Uriel N, Ausiello JC, Accili D, Freedberg DE, Baldwin M, Schwartz A, Brodie D, Garcia CK, Elkind MSV, Connors JM, Bilezikian JP, Landry DW, Wan EY. Post-acute COVID-19 syndrome. Nat Med. 2021;27:601-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3262] [Cited by in RCA: 3001] [Article Influence: 750.3] [Reference Citation Analysis (0)] |
| 11. | Bull-Otterson L, Baca S, Saydah S, Boehmer T, Adjei S, Gray S, Harris AM. Post–COVID Conditions Among Adult COVID-19 Survivors Aged 18–64 and ≥65 Years — United States, March 2020–November 2021. MMWR Morb Mortal Wkly Rep. 2022;71:713-717. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 210] [Article Influence: 70.0] [Reference Citation Analysis (0)] |
| 12. | Sneller MC, Liang CJ, Marques AR, Chung JY, Shanbhag SM, Fontana JR, Raza H, Okeke O, Dewar RL, Higgins BP, Tolstenko K, Kwan RW, Gittens KR, Seamon CA, McCormack G, Shaw JS, Okpali GM, Law M, Trihemasava K, Kennedy BD, Shi V, Justement JS, Buckner CM, Blazkova J, Moir S, Chun TW, Lane HC. A Longitudinal Study of COVID-19 Sequelae and Immunity: Baseline Findings. Ann Intern Med. 2022;175:969-979. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 113] [Article Influence: 37.7] [Reference Citation Analysis (0)] |
| 13. | Davis HE, Assaf GS, McCorkell L, Wei H, Low RJ, Re'em Y, Redfield S, Austin JP, Akrami A. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. 2021;38:101019. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1118] [Cited by in RCA: 1575] [Article Influence: 393.8] [Reference Citation Analysis (0)] |
| 14. | Chen Y, Yi Q, Liu G, Shen X, Xuan L, Tian Y. Cerebral white matter injury and damage to myelin sheath following whole-brain ischemia. Brain Res. 2013;1495:11-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 15. | Arana-Guajardo A, Villarreal-Alarcón M. CREST Syndrome: Clinical Expression of the Disease. J Clin Rheumatol. 2017;23:285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Siegel CH, Richey M. Lupus blood test results: What to know: HSS rheumatology. Hospital for Special Surgery. Available from: https://www.hss.edu/conditions_understanding-laboratory-tests-and-results-for-systemic-lupus-erythematosus.asp#aPL. |
| 17. | Andre JB, Bammer R. Advanced diffusion-weighted magnetic resonance imaging techniques of the human spinal cord. Top Magn Reson Imaging. 2010;21:367-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 18. | Epstein LG, Gendelman HE. Human immunodeficiency virus type 1 infection of the nervous system: pathogenetic mechanisms. Ann Neurol. 1993;33:429-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 255] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 19. | Mocchetti I, Bachis A, Avdoshina V. Neurotoxicity of human immunodeficiency virus-1: viral proteins and axonal transport. Neurotox Res. 2012;21:79-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 20. | Siroky MB. Pathogenesis of bacteriuria and infection in the spinal cord injured patient. Am J Med. 2002;113 Suppl 1A:67S-79S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 141] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 21. | Centers for Disease Control and Prevention. (n.d.). Post-covid conditions. Centers for Disease Control and Prevention. [Cited 7 March 2022]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/Long-term-effects/index.html. |
| 22. | Carod-Artal FJ. Neurological complications of coronavirus and COVID-19. Rev Neurol. 2020;70:311-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 199] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 23. | Favas TT, Dev P, Chaurasia RN, Chakravarty K, Mishra R, Joshi D, Mishra VN, Kumar A, Singh VK, Pandey M, Pathak A. Neurological manifestations of COVID-19: a systematic review and meta-analysis of proportions. Neurol Sci. 2020;41:3437-3470. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 146] [Cited by in RCA: 133] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 24. | Tabacof L, Tosto-Mancuso J, Wood J, Cortes M, Kontorovich A, McCarthy D, Rizk D, Rozanski G, Breyman E, Nasr L, Kellner C, Herrera JE, Putrino D. Post-acute COVID-19 Syndrome Negatively Impacts Physical Function, Cognitive Function, Health-Related Quality of Life, and Participation. Am J Phys Med Rehabil. 2022;101:48-52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 208] [Article Influence: 69.3] [Reference Citation Analysis (0)] |
| 25. | Oleson CV, Shermon S, Olsen AC. Spinal Cord Infarction and Ischemia Attributed to Severe Acute Respiratory Syndrome Coronavirus-2, with Progression to Post-Acute Sequelae of COVID-19: A case series. Presented at the Annual Meeting of the American Spinal Injury Association. St. Louis MO, USA. [Cited 8 July 2021]. Available from: https://probiologists.com/Article/Spinal-cord-infarction-associated-with-severe-acute-respiratory-syndrome-coronavirus-2:-A-case-series-of-diagnostic-challenges-and-post-acute-sequelae-of-COVID-19. |
| 26. | Lamb LE, Timar R, Wills M, Dhar S, Lucas SM, Komnenov D, Chancellor MB, Dhar N. Long COVID and COVID-19-associated cystitis (CAC). Int Urol Nephrol. 2022;54:17-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 27. | Lamb LE, Dhar N, Timar R, Wills M, Dhar S, Chancellor MB. COVID-19 inflammation results in urine cytokine elevation and causes COVID-19 associated cystitis (CAC). Med Hypotheses. 2020;145:110375. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |