Published online Dec 26, 2023. doi: 10.12998/wjcc.v11.i36.8527
Peer-review started: September 1, 2023
First decision: November 16, 2023
Revised: November 27, 2023
Accepted: December 13, 2023
Article in press: December 13, 2023
Published online: December 26, 2023
Processing time: 112 Days and 0.8 Hours
Castleman disease (CD) was first reported in 1954. It is a rare non-malignant lymphoproliferative disease with unclear etiology. As the clinical manifestations of CD are different, there are difficulties in its diagnosis and treatment. Therefore, for patients with CD, it is important to establish the diagnosis in order to choose the appropriate treatment.
In this report, three patients with intraperitoneal CD treated at our center from January 2018 to June 2023 were reviewed, and the clinical and paraclinical exa
CD is a complex and rare disease. Because there are no special clinical symptoms and laboratory abnormalities, the diagnosis often depends on routine pathological and immunohistochemical findings.
Core Tip: Castleman disease (CD) is a rare and complex disease. As the clinical signs and laboratory results are not specific, clinical diagnosis is difficult, which often depends on imaging and pathology examinations. This report summarizes the diagnosis and treatment of three cases of CD and reviews the related literature to explore the diagnosis and treatment of CD in order to improve the clinical management of this disease.
- Citation: Gao JW, Shi ZY, Zhu ZB, Xu XR, Chen W. Intraperitoneal hyaline vascular Castleman disease: Three case reports. World J Clin Cases 2023; 11(36): 8527-8534
- URL: https://www.wjgnet.com/2307-8960/full/v11/i36/8527.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i36.8527
Castleman disease (CD) is a group of rare disorders with characteristic histopathological features. Monocentric CD is a benign localized lymphoproliferative disease, which was first reported in 1954 and described as mediastinal localized lymphoproliferative disease[1]. The clinical subtypes of CD include monocentric type and multicentric type, and his
Case 1: A 37-year-old man was admitted to hospital due to "a mass in the pancreas found 1 wk ago".
Case 2: A 38-year-old woman was admitted to hospital due to "an abdominal mass found for 1 mo".
Case 3: A 71-year-old woman was admitted to hospital due to "a gallbladder mass found 2 mo ago".
During the course of the disease, all patients had no paroxysmal palpitations, headache, or other positive symptoms.
Case 1 and Case 2: The patients had no previous history of hypertension or other chronic diseases, and had no obvious abnormal tumor markers after admission.
Case 3: Blood pressure control was satisfactory, and the patient had a history of hypertension for > 3 years.
None of the three patients had personal or family history of the disease.
Cardiopulmonary and abdominal examinations of all three patients showed no abnormalities and no positive signs.
Case 1: Endocrine tests showed the following: The adrenocorticotropin-cortisol rhythm was normal, the renin-an
Case 2: There were no obvious abnormalities in hemoglobin (77 g/L), routine biochemical and urine testing, blood coagulation function, tumor markers, and HIV testing.
Case 3: Laboratory examinations showed that there were no obvious abnormalities in routine biochemical, blood, and urine tests, and tumor markers were normal. Endocrine tests showed the following: The adrenocorticotropin-cortisol rhythm was normal, the renin-angiotensin II-aldosterone test in the decubitus position showed no abnormalities, and HIV testing was negative.
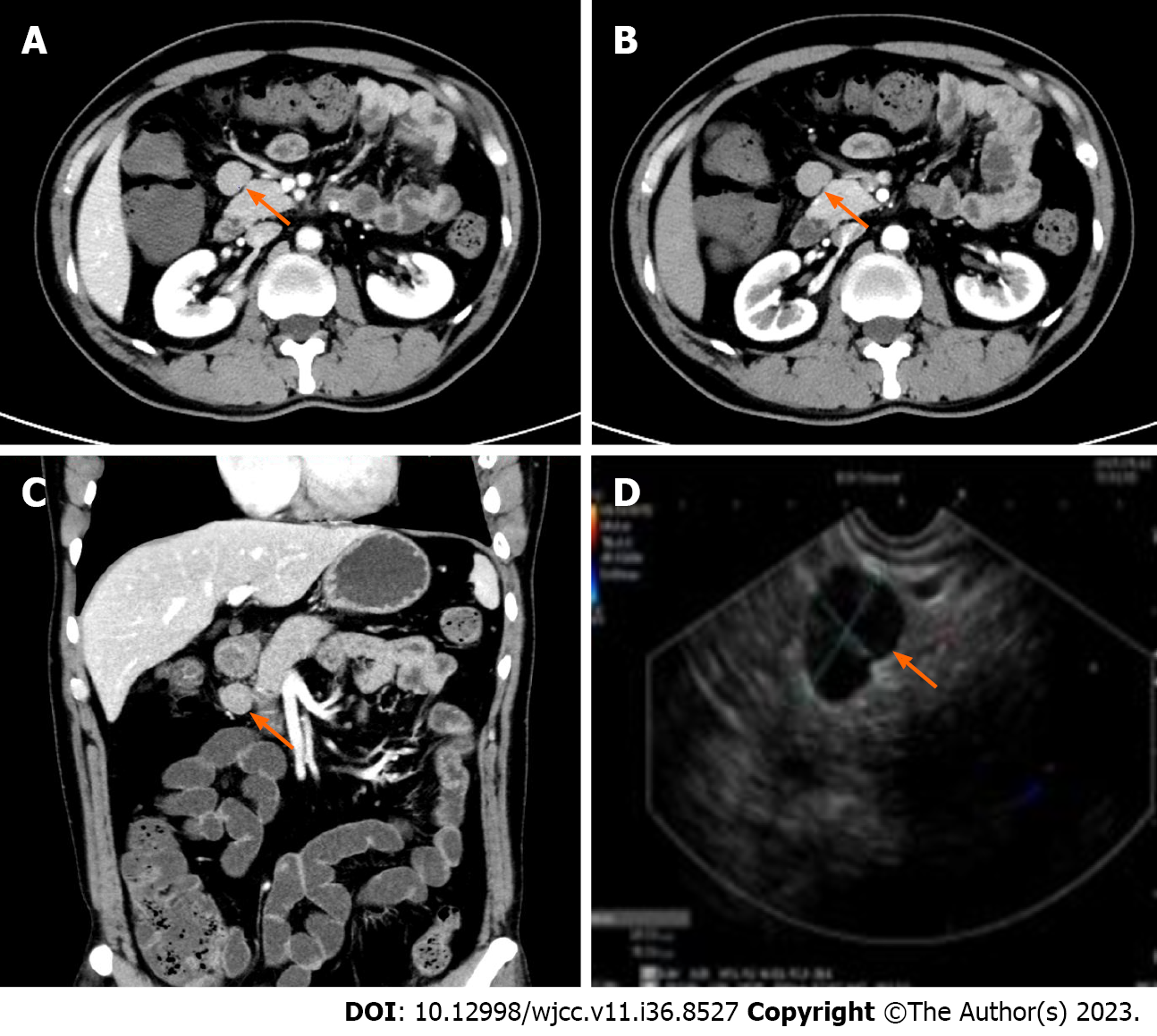
Case 1: A contrast-enhanced computed tomography (CT) scan showed a soft tissue lesion in front of the pancreas with a quasi-circular density, and a diameter of approximately 22 mm, smooth edges, and obvious uniform and continuous enhancement. Similar nodules were seen at the edge of the mass, and there were also several small abdominal lymph nodes with an average diameter of 5 mm. Giant lymph node hyperplasia/ectopic pancreas was considered. In order to further confirm the diagnosis, a painless ultrasonic gastroscopic biopsy was performed. A linear 7.5 MHz ultrasonic gastroscope was used to explore the gastric cavity and duodenum. During the procedure, enlarged lymph nodes above the head of the pancreas were seen, showing a triangular shape, hilar structure could be seen inside, and no obvious blood flow signal was seen in the periphery of the lymph nodes. One cross-section was approximately 20 mm × 15 mm, the lymphatic edge was sharp, showing uniform hypoechoic changes, and 3 Lymph nodes were punctured (Figure 1).
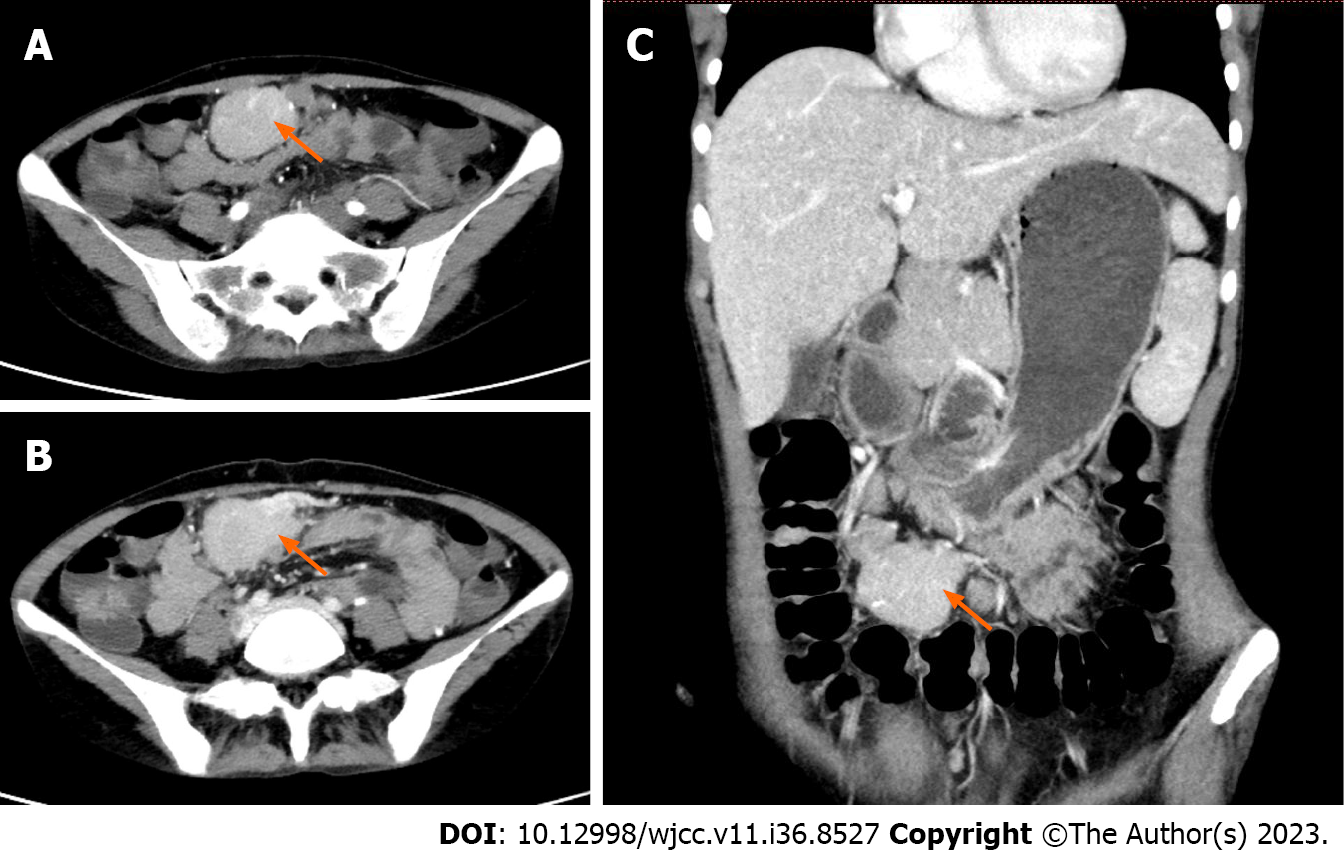
Case 2: Before admission, a head and chest plain CT scan revealed a low density focus in the left lobe of the liver, and the patient was admitted to the hospital for further examination. Enhanced CT suggested multiple masses in the abdominal and pelvic cavities, the largest mass was found in the pelvic cavity which had a clear boundary and irregular shape, and a small nodular calcification was seen around the focus. Moderate enhancement was seen, the enhancement pattern of other abnormal soft tissue nodules was similar, and the possibility of stromal tumor was considered (Figure 2).
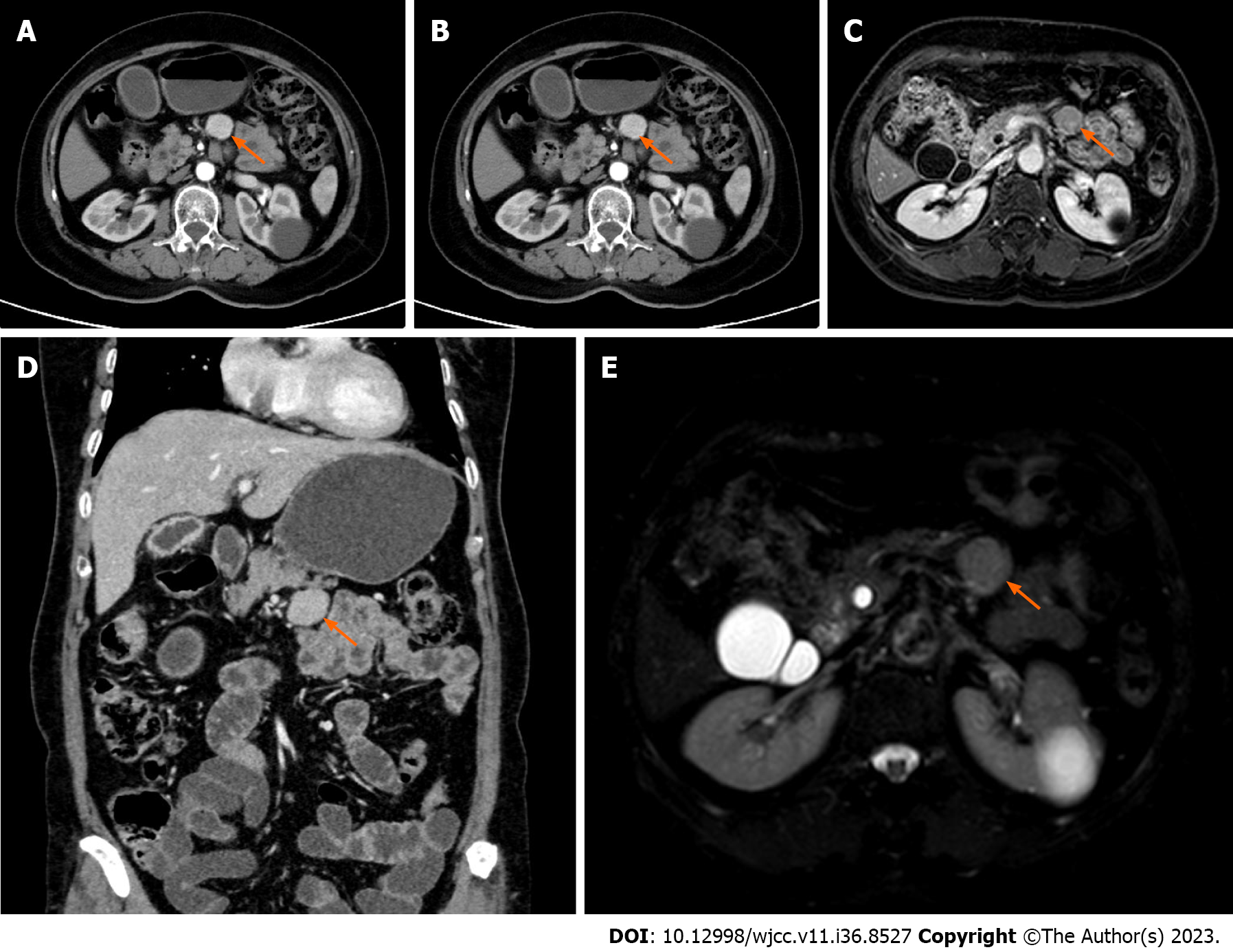
Case 3: The patient underwent B-ultrasound examination more than 2 mo previously, which indicated gallbladder enlargement and thickening of the gallbladder wall. Enhanced magnetic resonance imaging of the upper abdomen was performed, which indicated an abnormal signal focus in the lower part of the pancreas that was circular, approximately 25 mm × 24 mm in size, the edge was smooth and the boundary was clear, and the possibility of giant lymph node hyperplasia was considered. Cholecystitis and thickening of the gallbladder wall did not exclude the possibility of malignancy. The patient was then admitted to hospital to complete the preoperative examination. Enhanced CT indicated a round soft-tissue density focus below the pancreas, with obvious enhancement in the arterial phase and slightly decreased enhancement in the venous phase and delayed phase, with a length of approximately 25 mm. The possibility of giant lymph node hyperplasia was considered and as the gallbladder wall was not uniformly thickened, the possibility of local malignancy was also considered (Figure 3).
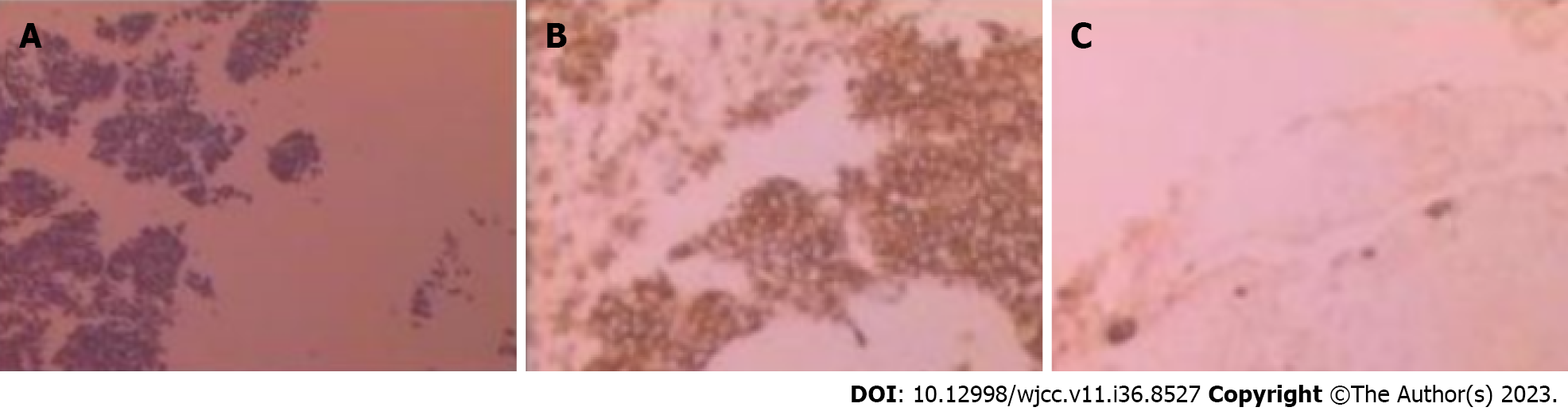
Case 1: Routine postoperative pathology showed that a large number of small round cells were stretched and lymphoproliferative lesions were considered. Immunohistochemical diagnosis showed hyaline vascular CD due to the presence of AE1/AE3 (-), CD10 (-), CD20 (+), CD3 (+), CD45R0 (+), CD79 α (+), LCA (+), Ki-67 (+, 15%), CD5 (+), and Bc1-2 (+) (Figure 4).
Case 2: Following excision of the mass, rapid pathology suggested lymphoproliferative lesions. A postoperative pathological diagnosis of hyaline vascular CD was made due to the presence of CD20 (+), CD3 (+), CD21 (follicular dendritic +), CD23 (follicular dendritic +), CD5 (+), CyclinD1 (-), Bcl-2 (germinal center -), Bcl-6 (germinal center +), Ki67 (+, about 40%), CD10 (-), MuM1(-), Pax-5 (+), CD30 (-), ALK (-), and CD4 (+) (Figure 5).
Case 3: Following excision of the mass, rapid pathology indicated lymphoproliferative lesions. Postoperative pathology showed hyaline vascular CD. Immunohistochemical results were as follows: CK20 (+), Pax5 (+), CD3 (+), CD5 (+), CD21 (germinal center +), CD15 (-), CD30 (-), EMA (-), CD10 (germinal center +), Bcl-6 (germinal center +), MuM1 (-), EBER-ISH (-), and Bcl-2 (-) (Figure 6).
Conservative treatment was provided following communication with the patient.
Exploratory laparotomy was performed after preoperative preparation, and a mass in the greater omentum on the greater curvature of the stomach was found, which was approximately 5 cm × 4 cm in size.
Radical resection of gallbladder cancer and excision of the abdominal lesion were performed. During the operation, a mass of approximately 3 cm in size was found in the lower part of the pancreas, which was closely adhered to the lower margin of the pancreas. The anatomical mass was completely separated using an ultrasonic knife.
The patient's condition was stable and there was no obvious disease progression during the 2-year follow-up.
The patient's condition was stable and there was no obvious disease progression during the 2-year follow-up.
After the operation, anti-inflammatory, stomach protection, and other symptomatic support was provided, and the patient recovered well and was subsequently discharged. CT reexamination 2 mo after surgery showed no signs of recurrence.
CD is a rare lymphoproliferative disease characterized by the proliferation and enlargement of lymphoid tissue[5]. The most common site of CD is the mediastinum, accounting for approximately 70% of all CD patients, while the occurrence of CD in the abdomen is uncommon, and only a few cases have been reported[6]. The exact cause of the disease is unknown, but some studies have suggested that it is related to HIV and human herpes virus-8.
CD can be divided into monocentric and multicentric subtypes, and the different types of CD are characterized by significant lymphatic architecture changes in all lymph node septa. Monocentric type includes hyaline vascular type and plasma cell type. Multicentric CD is mainly characterized by plasma cell variants, with a few cases showing mixed type. In this report, all three patients underwent routine pathological and immunohistochemical examinations, and all showed hyaline vascular type CD. Hyaline vascular CD presents as an affected lymphoid follicle with an enlarged outer layer of concentric rings of small lymphocytes that surround a small germinal center of atrophy or degenerative transformation. Germinal centers usually have penetrating transparent small blood vessels and protruding follicular dendritic cells that may be dilated, destroyed, or have multiple tight connections[7].
In contrast, the germinal center of plasma cell CD is proliferative rather than degenerative, and the interfollicular region of the lymph nodes is vascularized and contains plasma cell sheets with polyclonal characteristics. However, these histopathological findings are not specific to CD and diseases that may cause similar changes in proliferative reactive lymph nodes, such as rheumatoid arthritis and viral lymphadenitis, need to be ruled out[8].
The diagnosis of CD mainly depends on histological examination by excision or puncture of the swollen lymph nodes, and radiological examination, the most common of which is whole-body CT or CT-18F-fluorodeoxyglucose positron emission tomography examination. Currently, monocentric CD is considered a soft tissue mass with clear and regular edges, uniform density, and speckle calcification, or accompanied by bleeding and necrosis on CT[9]. On an enhanced scan, the mass shows enhancement in the arterial phase, with continued enhancement in the venous phase and delayed phase. On CT, multicentric CD usually presents as multiple round and low-density masses of similar size, most of which have uniform enhancement, and partial irregular enhancement may occur if the lesion is large[10]. However, the imaging features of CD are difficult to distinguish from other diseases such as neuroendocrine tumors or lymphomas, such as neurogenic tumors, lymph node metastases, and gastrointestinal stromal tumors. In many cases, the preoperative imag
CD is a complex and rare disease, and a standard treatment is still lacking. For clinicians, it is difficult to make a clear diagnosis before surgery due to the lack of specific radiological markers. Therefore, when the diagnosis is unclear, histopathological examination is important to guide treatment. Complete resection is the gold standard for the treatment of monocentric CD, while multicentric CD requires comprehensive treatment depending on the patient’s condition, but the overall prognosis is poor.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Khalil G S-Editor: Lin C L-Editor: Wang TQ P-Editor: Lin C
| 1. | Shimokihara K, Kawahara T, Kasahara R, Kasuga J, Sugiura S, Tajiri R, Uemura H, Chiba K. Retroperitoneal Castleman's Disease. Case Rep Oncol. 2019;12:885-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 2. | Ridolfini MP, Rotondi F, Gourgiotis S, Alfieri S, Di Miceli D, Larocca LM, Doglietto GB. [Retroperitoneal Castleman's disease. A report of two cases and analysis of the literature]. Chir Ital. 2007;59:53-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 63] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 3. | Wang S, Chen S, Xu J, Cai S. Clinicopathological characteristics of unicentric retroperitoneal Castleman's disease: a study of 14 cases. World J Surg Oncol. 2016;14:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 4. | Iyer S, Bhatti MI, Halliday M. Castleman's disease-A case report. Int J Surg Case Rep. 2010;1:25-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Oida Y, Shimizu K, Mukai M, Imaizumi T, Nakamura M, Makuuchi H. FDG-PET and diffusion-weighted MR imaging appearance in retroperitoneal Castleman's disease: a case report. Clin Imaging. 2008;32:144-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Menenakos C, Braumann C, Hartmann J, Jacobi CA. Retroperitoneal Castleman's tumor and paraneoplastic pemphigus: report of a case and review of the literature. World J Surg Oncol. 2007;5:45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Nguyen DT, Diamond LW, Hansmann ML, Alavaikko MJ, Schröder H, Fellbaum C, Fischer R. Castleman's disease. Differences in follicular dendritic network in the hyaline vascular and plasma cell variants. Histopathology. 1994;24:437-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 52] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | Kojima M, Motoori T, Nakamura S. Benign, atypical and malignant lymphoproliferative disorders in rheumatoid arthritis patients. Biomed Pharmacother. 2006;60:663-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | van Rhee F, Oksenhendler E, Srkalovic G, Voorhees P, Lim M, Dispenzieri A, Ide M, Parente S, Schey S, Streetly M, Wong R, Wu D, Maillard I, Brandstadter J, Munshi N, Bowne W, Elenitoba-Johnson KS, Fössa A, Lechowicz MJ, Chandrakasan S, Pierson SK, Greenway A, Nasta S, Yoshizaki K, Kurzrock R, Uldrick TS, Casper C, Chadburn A, Fajgenbaum DC. International evidence-based consensus diagnostic and treatment guidelines for unicentric Castleman disease. Blood Adv. 2020;4:6039-6050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 126] [Article Influence: 31.5] [Reference Citation Analysis (1)] |
| 10. | Minemura H, Ikeda K, Tanino Y, Hashimoto Y, Nikaido T, Fukuhara A, Yokouchi H, Sato S, Tanino M, Oka T, Hebisawa A, Suzuki H, Ogawa K, Takeishi Y, Munakata M. Multicentric Castleman's disease with impaired lymphocytic apoptosis. Allergol Int. 2015;64:112-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Bracale U, Pacelli F, Milone M, Bracale UM, Sodo M, Merola G, Troiani T, Di Salvo E. Laparoscopic treatment of abdominal unicentric castleman's disease: a case report and literature review. BMC Surg. 2017;17:38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 12. | van Rhee F, Munshi NC. Castleman Disease. Hematol Oncol Clin North Am. 2018;32:xiii-xxiv. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Killian M, Viel S, Chalayer E, Forest F, Grange S, Bonnefoy PB, Oksenhendler E, Cathébras P, Paul S. JAK1/2 Inhibition in Severe TAFRO Syndrome: A Case Report. Ann Intern Med. 2021;174:719-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Lust H, Gong S, Remiker A, Rossoff J. Idiopathic multicentric Castleman disease with TAFRO clinical subtype responsive to IL-6/JAK inhibition: A pediatric case series. Pediatr Blood Cancer. 2021;68:e29261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 15. | Imen BI, Zenaidi H, Abdelwahed Y, Sabeur R, Ayoub Z. Management of isolated retroperitoneal Castelman's disease: A case report. Int J Surg Case Rep. 2020;70:24-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Dong Y, Zhang L, Nong L, Wang L, Liang Z, Zhou D, Fajgenbaum DC, Ren H, Li J. Effectiveness of rituximab-containing treatment regimens in idiopathic multicentric Castleman disease. Ann Hematol. 2018;97:1641-1647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 17. | Jung CP, Emmerich B, Goebel FD, Bogner JR. Successful treatment of a patient with HIV-associated multicentric Castleman disease (MCD) with thalidomide. Am J Hematol. 2004;75:176-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |