Published online Dec 16, 2023. doi: 10.12998/wjcc.v11.i35.8425
Peer-review started: October 11, 2023
First decision: October 24, 2023
Revised: November 4, 2023
Accepted: November 30, 2023
Article in press: November 30, 2023
Published online: December 16, 2023
Processing time: 64 Days and 0.4 Hours
This case report addresses the dearth of effective therapeutic interventions for central nervous system metastases in patients with HER2-negative breast cancer. It presents a unique case of a woman with estrogen receptor-positive, HER2-negative breast cancer who developed brain metastasis. The report highlights her initial favorable response to abemaciclib and letrozole therapy prior to the discontinuation due to drug-induced lung damage (DILD).
In this comprehensive case summary, we present the clinical course of a woman in her 60s, who 11 years following primary breast cancer surgery, was diagnosed with multiple brain metastases. As a third-line systemic therapy, she underwent treatment with abemaciclib and letrozole. This treatment approach yielded a near-partial response in her metastatic brain lesions. However, abemaciclib administration ceased due to the emergence of DILD, as confirmed by a computed tomography scan. The DILD improved after 1 mo of cessation. Despite ongoing therapeutic efforts, the patient’s condition progressively deteriorated, ultimately resulting in death due to progression of the brain metastases.
This case underscores the challenge of managing adverse events in responsive brain metastasis patients, given the scarcity of therapeutic options.
Core Tip: In this case report, we address the critical issue of limited therapeutic options for HER2-negative breast cancer patients with brain metastases. We present a case of a woman with estrogen receptor-positive, HER2-negative breast cancer who initially exhibited an encouraging response to abemaciclib and letrozole therapy for brain metastases. However, this treatment had to be discontinued due to drug-induced lung damage. This study reports the challenges of achieving a balance between efficacy and adverse events in managing brain metastases and highlights the need for alternative treatment strategies in this patient population.
- Citation: Yamashiro H, Morii N. Abemaciclib-induced lung damage leading to discontinuation in brain metastases from breast cancer: A case report. World J Clin Cases 2023; 11(35): 8425-8430
- URL: https://www.wjgnet.com/2307-8960/full/v11/i35/8425.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i35.8425
Central nervous system (CNS) metastasis in primary breast cancer occurs in 10%-15% of advanced/recurrent breast cancer cases[1]. However, drug therapy for brain metastases has been considered less effective due to the presence of the brain-blood barrier (BBB), which restricts the entry of many drugs into the CNS. In certain phenotypes, such as HER2-positive breast cancer, treatment outcomes of CNS metastasis have been improving with the development of new drugs[2]. However, there is currently no drug that has significant efficacy in treating CNS metastasis from HER2 negative breast cancer, and radiation therapy remains the first choice.
Cyclin-dependent kinase (CDK) 4/6 inhibitors, abemaciclib and palbociclib, are available as insurance-covered treat
Here, we report a case of brain metastasis of ER-positive/HER2-negative primary breast cancer that initially responded to concomitant therapy of abemaciclib and letrozole, but required termination owing to drug-induced lung damage (DILD).
A postmenopausal woman in her 60s attended our hospital complaining of unsteadiness or dizziness.
Several months ago, the patient became aware of dizziness and the symptoms gradually worsened. She was not aware of nausea, paralysis, muscle weakness, or memory loss.
The woman underwent mastectomy and axillary lymph node dissection for breast cancer 11 years ago. The pathological diagnosis was mucinous carcinoma, histological grade 2, pT1c, n0(0/7), ER-positive, progesterone receptor-positive, and HER2-negative (score 0). For the 5 years after surgery, she received endocrine therapy and was no longer followed up after being recurrence-free for 10 years postoperatively.
She had no particular personal and family medical history.
On physical examination, no notable abnormalities were observed.
The laboratory examination results were unremarkable.
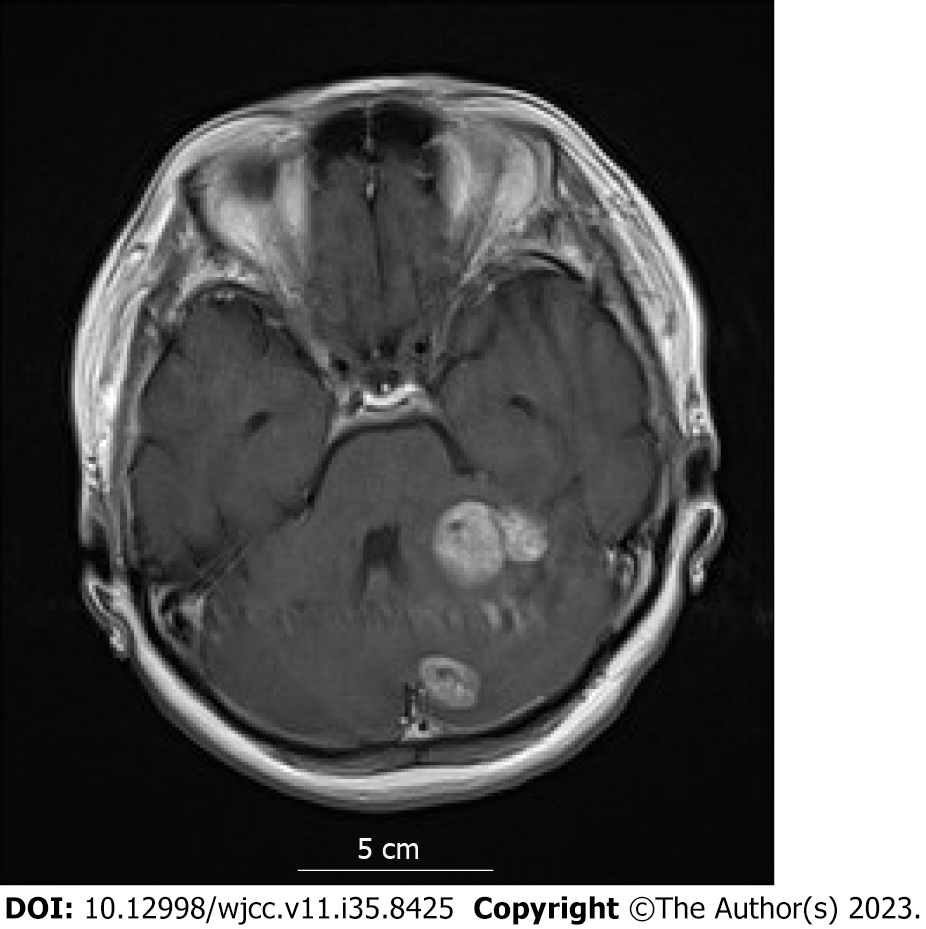
Head magnetic resonance imaging (MRI) indicated multiple brain metastases (Figure 1); no trunk lesions were observed on computed tomography (CT).
Late (more than 10 years after primary radical surgery) recurrence of breast cancer. Multiple brain metastases.
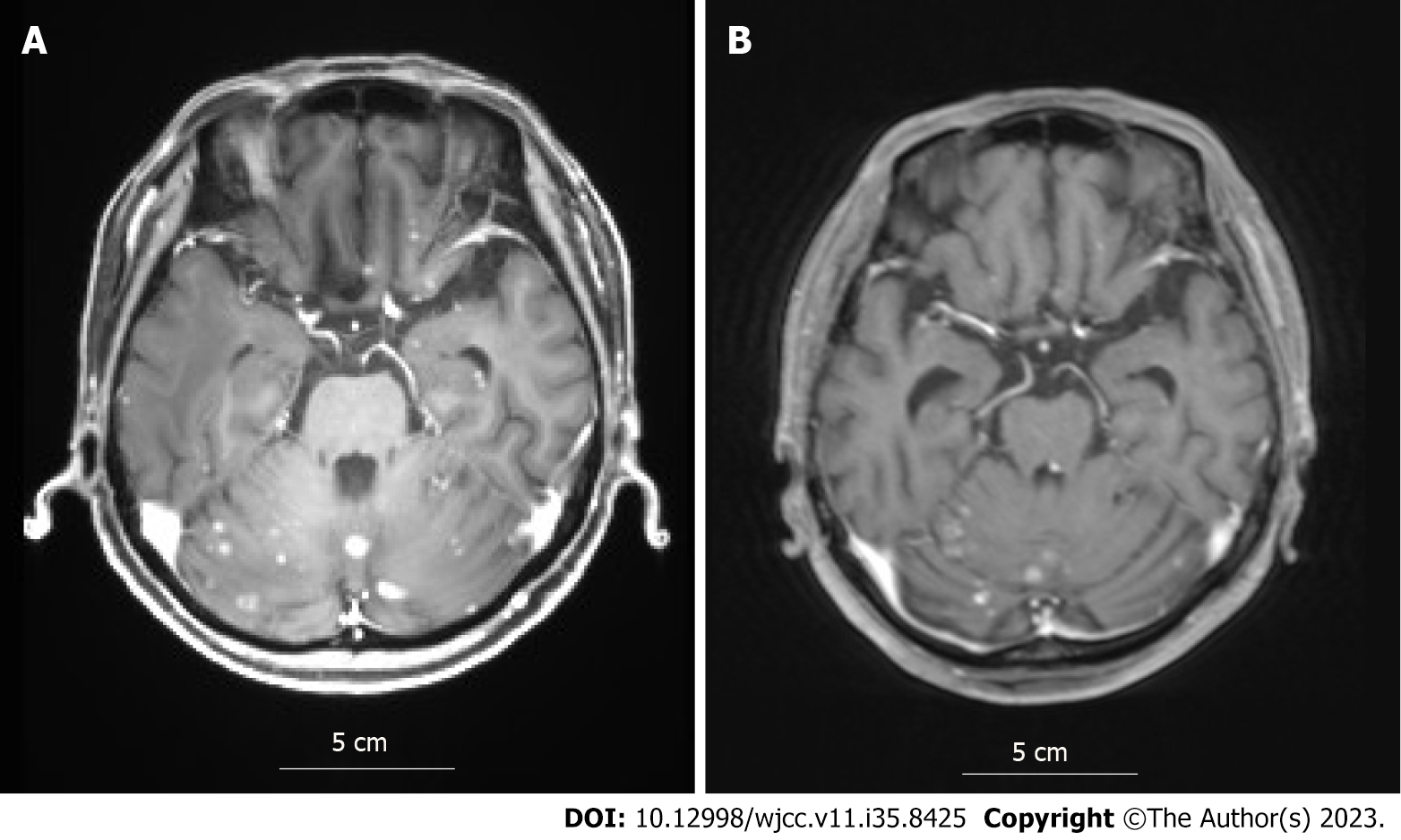
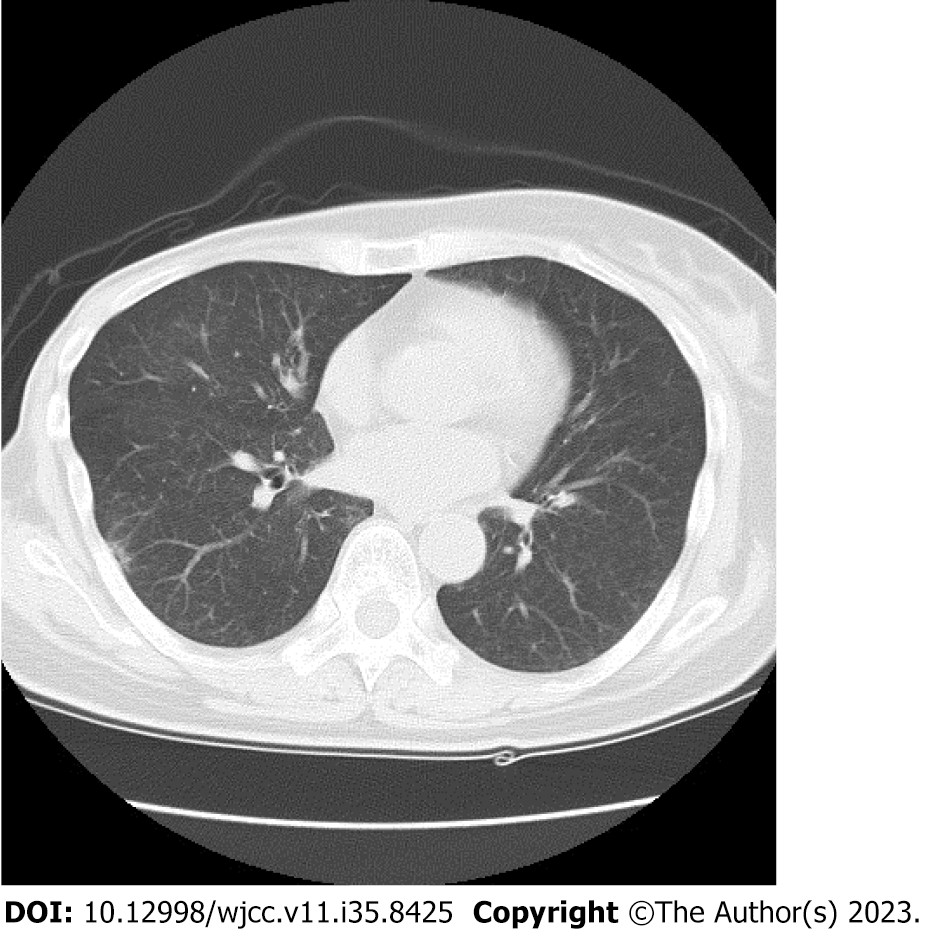
After whole brain irradiation (40 Gy/20 Fr), exemestane treatment was initiated. Although the brain metastases showed partial response (PR), in the 12th year postoperatively, lymph node and adrenal metastases were detected by CT. Consequently, the treatment was changed to palbociclib plus letrozole. Thereafter, owing to deterioration of the pre-existing cerebellar metastasis, localized irradiation was performed. In the 13th year postoperatively, the brain metastases progressed (Figure 2A), so treatment was changed to abemaciclib plus letrozole as the third-line treatment. Three months later, MRI showed attenuation of the brain metastases and the contrast effect (Figure 2B), almost reaching a PR. However, DILD was noted on CT (Figure 3), which necessitated the termination of abemaciclib. No other grade 3 or higher hematological toxicity, diarrhea, or fatigue occurred.
The DILD improved after 1 mo of cessation. The patient continued treatments with TS-1 and fulvestrant but died in the 14th year postoperatively due to progression of the brain metastases.
We experienced a case in which abemaciclib was successful for brain metastasis of ER-positive/HER2-negative breast cancer but had to be discontinued due to DILD. Brain metastasis is reported to occur in 10%-15% of cases of advanced or recurrent breast cancer[1]. The mean survival after the appearance of brain metastasis varies depending on the subtype, ranging from several years for HER2-positive breast cancer to less than 6 mo for triple-negative (HER2, ER, and progesterone receptor all negative) breast cancer[5-7].
Breast cancer is recognized as a solid tumor that often responds well to pharmacotherapy, and advancements in translational research have significantly expanded the array of available drugs. However, conventional drugs are limited in their effectiveness for brain metastases of breast cancer due to the BBB and low cerebrospinal fluid penetration[8].
Some case reports and phase II trials have indicated a response rate of 5.2% to abemaciclib in brain metastases of ER-positive breast cancer and the overall outcomes have been less than satisfactory[9]. Capivasertib (a protein kinase B inhibitor) and alpelisib (a phosphatidylinositol 3-kinase inhibitor) may have some potential for the treatment of brain metastasis of hormone receptor-positive breast cancer.
Trastuzumab-deruxtecan (T-DXd) is an antibody-drug conjugate that covalently binds trastuzumab, a humanized monoclonal antibody, with deruxtecan, a topoisomerase I inhibitor (a derivative of exatecan). T-DXd has shown promising results with a high response rate of 47.1% for brain metastases of HER2-positive breast cancer[10] owing to its unique mechanism of action. With HER2-low (HER2 score of 1 or 2 and fluorescence in situ hybridization-negative) recurrent metastatic breast cancer being added as an indication, T-DXd is expected to show effectiveness in brain metastases of breast cancer previously considered as ER-positive or triple-negative with low HER2 expression[11]. The first-line drug for ER-positive/HER2-negative metastatic/recurrent breast cancer is a CDK4/6 inhibitor, but if accompanied by brain metastases, T-Dxd may be a better first-line choice.
In global trials of abemaciclib (MONARCH 2, 3, and E)[12-14], DILD was reported in approximately 2%-3% of cases. Although DILD is not often fatal with appropriate monitoring and early intervention, in cases of grade 2 or higher severity, treatment must often be terminated without the possibility of it being resumed. Underlying lung diseases such as interstitial pneumonia can be risk factors for DILD, but the exact mechanism of onset is not fully understood, and preventive measures have not yet been established. CDK4/6 inhibitors, abemaciclib, and palbociclib can be prescribed under Japanese insurance; however, palbociclib has a high incidence of hematological toxicity. In particular, neutropenia was reported in 78% (66% grade 3) of cases in a phase III[3] study. Abemaciclib also has hematological toxicity[12-14], however can be clinically characterized by non-hematological toxicity symptoms, such as diarrhea and fatigue.
As this is a case report, our findings have not been validated, however, they may provide an opportunity to consider the factors involved leading to treatment termination due to adverse events. This is critical as there are no drugs that have shown significant efficacy on brain metastases of breast cancer.
We present the case of a patient with brain metastases from primary breast cancer where abemaciclib was terminated owing to DILD. While we anticipate the development of drugs that are effective for brain metastases, exploring strategies to minimize treatment terminations due to toxicities remains an important approach.
This case was presented at the 30th Annual Meeting of the Japanese Breast Cancer Society (June 30, 2022).
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Beeraka NM, India S-Editor: Qu XL L-Editor: A P-Editor: Qu XL
| 1. | Lin NU, Amiri-Kordestani L, Palmieri D, Liewehr DJ, Steeg PS. CNS metastases in breast cancer: old challenge, new frontiers. Clin Cancer Res. 2013;19:6404-6418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 163] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 2. | Ramakrishna N, Temin S, Chandarlapaty S, Crews JR, Davidson NE, Esteva FJ, Giordano SH, Gonzalez-Angulo AM, Kirshner JJ, Krop I, Levinson J, Modi S, Patt DA, Perez EA, Perlmutter J, Winer EP, Lin NU. Recommendations on disease management for patients with advanced human epidermal growth factor receptor 2-positive breast cancer and brain metastases: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2014;32:2100-2108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 148] [Cited by in RCA: 144] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 3. | Finn RS, Martin M, Rugo HS, Jones S, Im SA, Gelmon K, Harbeck N, Lipatov ON, Walshe JM, Moulder S, Gauthier E, Lu DR, Randolph S, Diéras V, Slamon DJ. Palbociclib and Letrozole in Advanced Breast Cancer. N Engl J Med. 2016;375:1925-1936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1472] [Cited by in RCA: 2010] [Article Influence: 223.3] [Reference Citation Analysis (0)] |
| 4. | Patnaik A, Rosen LS, Tolaney SM, Tolcher AW, Goldman JW, Gandhi L, Papadopoulos KP, Beeram M, Rasco DW, Hilton JF, Nasir A, Beckmann RP, Schade AE, Fulford AD, Nguyen TS, Martinez R, Kulanthaivel P, Li LQ, Frenzel M, Cronier DM, Chan EM, Flaherty KT, Wen PY, Shapiro GI. Efficacy and Safety of Abemaciclib, an Inhibitor of CDK4 and CDK6, for Patients with Breast Cancer, Non-Small Cell Lung Cancer, and Other Solid Tumors. Cancer Discov. 2016;6:740-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 419] [Cited by in RCA: 569] [Article Influence: 63.2] [Reference Citation Analysis (0)] |
| 5. | Matsuo S, Watanabe J, Mitsuya K, Hayashi N, Nakasu Y, Hayashi M. Brain metastasis in patients with metastatic breast cancer in the real world: a single-institution, retrospective review of 12-year follow-up. Breast Cancer Res Treat. 2017;162:169-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 6. | Hurvitz SA, O'Shaughnessy J, Mason G, Yardley DA, Jahanzeb M, Brufsky A, Rugo HS, Swain SM, Kaufman PA, Tripathy D, Chu L, Li H, Antao V, Cobleigh M. Central Nervous System Metastasis in Patients with HER2-Positive Metastatic Breast Cancer: Patient Characteristics, Treatment, and Survival from SystHERs. Clin Cancer Res. 2019;25:2433-2441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 73] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 7. | Rostami R, Mittal S, Rostami P, Tavassoli F, Jabbari B. Brain metastasis in breast cancer: a comprehensive literature review. J Neurooncol. 2016;127:407-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 195] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 8. | Sun A, Wang J. Choroid Plexus and Drug Removal Mechanisms. AAPS J. 2021;23:61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 9. | Tolaney SM, Sahebjam S, Le Rhun E, Bachelot T, Kabos P, Awada A, Yardley D, Chan A, Conte P, Diéras V, Lin NU, Bear M, Chapman SC, Yang Z, Chen Y, Anders CK. A Phase II Study of Abemaciclib in Patients with Brain Metastases Secondary to Hormone Receptor-Positive Breast Cancer. Clin Cancer Res. 2020;26:5310-5319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 142] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 10. | Jerusalem G, Park YH, Yamashita T, Hurvitz SA, Modi S, Andre F, Krop IE, Gonzàlez Farré X, You B, Saura C, Kim SB, Osborne CR, Murthy RK, Gianni L, Takano T, Liu Y, Cathcart J, Lee C, Perrin C. Trastuzumab Deruxtecan in HER2-Positive Metastatic Breast Cancer Patients with Brain Metastases: A DESTINY-Breast01 Subgroup Analysis. Cancer Discov. 2022;12:2754-2762. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 71] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 11. | Kabraji S, Ni J, Sammons S, Li T, Van Swearingen AED, Wang Y, Pereslete A, Hsu L, DiPiro PJ, Lascola C, Moore H, Hughes M, Raghavendra AS, Gule-Monroe M, Murthy RK, Winer EP, Anders CK, Zhao JJ, Lin NU. Preclinical and Clinical Efficacy of Trastuzumab Deruxtecan in Breast Cancer Brain Metastases. Clin Cancer Res. 2023;29:174-182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 72] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 12. | Sledge GW Jr, Toi M, Neven P, Sohn J, Inoue K, Pivot X, Burdaeva O, Okera M, Masuda N, Kaufman PA, Koh H, Grischke EM, Frenzel M, Lin Y, Barriga S, Smith IC, Bourayou N, Llombart-Cussac A. MONARCH 2: Abemaciclib in Combination With Fulvestrant in Women With HR+/HER2- Advanced Breast Cancer Who Had Progressed While Receiving Endocrine Therapy. J Clin Oncol. 2017;35:2875-2884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 743] [Cited by in RCA: 824] [Article Influence: 103.0] [Reference Citation Analysis (0)] |
| 13. | Goetz MP, Toi M, Campone M, Sohn J, Paluch-Shimon S, Huober J, Park IH, Trédan O, Chen SC, Manso L, Freedman OC, Garnica Jaliffe G, Forrester T, Frenzel M, Barriga S, Smith IC, Bourayou N, Di Leo A. MONARCH 3: Abemaciclib As Initial Therapy for Advanced Breast Cancer. J Clin Oncol. 2017;35:3638-3646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 736] [Cited by in RCA: 790] [Article Influence: 98.8] [Reference Citation Analysis (0)] |
| 14. | Johnston SRD, Harbeck N, Hegg R, Toi M, Martin M, Shao ZM, Zhang QY, Martinez Rodriguez JL, Campone M, Hamilton E, Sohn J, Guarneri V, Okada M, Boyle F, Neven P, Cortés J, Huober J, Wardley A, Tolaney SM, Cicin I, Smith IC, Frenzel M, Headley D, Wei R, San Antonio B, Hulstijn M, Cox J, O'Shaughnessy J, Rastogi P; monarchE Committee Members and Investigators. Abemaciclib Combined With Endocrine Therapy for the Adjuvant Treatment of HR+, HER2-, Node-Positive, High-Risk, Early Breast Cancer (monarchE). J Clin Oncol. 2020;38:3987-3998. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 218] [Cited by in RCA: 639] [Article Influence: 127.8] [Reference Citation Analysis (0)] |