Published online Dec 16, 2023. doi: 10.12998/wjcc.v11.i35.8416
Peer-review started: October 7, 2023
First decision: November 22, 2023
Revised: November 27, 2023
Accepted: December 4, 2023
Article in press: December 4, 2023
Published online: December 16, 2023
Processing time: 67 Days and 15.9 Hours
Solitary fibrous tumor (SFT) is a rare mesenchymal tumor that is most commonly found in the pleura but can also originate from non-pleural sites. Among the non-pleural localizations, the pancreas is extremely rare. In particular, metastasis to the pancreas from the central nervous system (CNS) is extremely rare, with only two cases reported so far. We report a case of recurrence in the pancreas 14 years after the initial complete surgical removal of a tumor in the CNS.
A 68-year-old man with a past medical history of recurrent meningeal hemangiopericytoma, currently referred to as SFT, presented to the hospital with jaundice. The patient was first diagnosed with an 8cm-sized meningeal hemangiopericytoma fourteen years ago and underwent osteoplastic craniotomy. After 16 mo, imaging showed recurrence and he underwent gamma knife radiosurgery (GKRS). 2 years later, imaging showed recurrence again leading to a second GKRS. 5 years later, recurrence was again suspected leading to a second craniotomy. Since then 3 years had passed, and imaging showed a 3.5cm-sized mass lesion on the pancreatic head with obstruction of the pancreatic and bile ducts. Endosonography with fine-needle aspiration biopsy was done preoperatively and aided in the diagnosis of SFT. The patient underwent pylorus-preserving pancreaticoduodenectomy. Pathologic findings of the resected pancreatic specimen, consistent with the previously resected brain specimen, confirmed the diagnosis of SFT.
The rarity and lack of knowledge about SFTs make suspecting and diagnosing this disease challenging. We believe that a report of metastatic pancreatic SFT from the CNS will contribute to a better understanding of this rare disease.
Core Tip: Solitary fibrous tumor (SFT) is a rare mesenchymal tumor most commonly found in the pleura. Among non-pleural localizations, the pancreas is extremely rare. However, if the patient has a known history of primary intracranial SFT and imaging shows a hypervascular, well-encapsulated lesion in distant organs such as the pancreas, metastasis should always be considered. Imaging can be inconclusive, in which cases, endosonography with fine-needle aspiration biopsy can be a safe and effective diagnostic tool preoperatively. There are only two case reports on metastatic SFTs from the central nervous system, and we believe this case will further contribute to a better understanding.
- Citation: Yi K, Lee J, Kim DU. Metastatic pancreatic solitary fibrous tumor: A case report. World J Clin Cases 2023; 11(35): 8416-8424
- URL: https://www.wjgnet.com/2307-8960/full/v11/i35/8416.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i35.8416
Solitary fibrous tumor (SFT) is a rare mesenchymal tumor that can arise in various organs and tissues[1]. SFT is mostly found in the pleura but can also occur in non-pleural sites[2]. The pancreas is an extremely rare non-pleural localization for SFT, with the majority of the tumors being secondary lesions[3,4]. According to the case report Yamashita et al[5] published in 2019, 21 cases of primary and secondary SFTs of the pancreas were reported in the English literature. Among them, only 4 cases were secondary, with 2 cases originating from the central nervous system. Although most SFTs are known to be benign, about 10%-15% of SFTs are reported to show local recurrence or distant metastasis[5]. Including cases published after that time, about 35 cases of pancreatic SFTs have been reported to date[6]. However, to our knowledge, there have been no additional reports of metastatic pancreatic SFTs since the report in 2019. Our case is interesting in that it shows a similar disease course to the two previously published cases. Here we present an additional similar case of metastatic SFT of the pancreas originating from the central nervous system treated at our hospital, discussing its diagnostic methods, histopathological features, and therapeutic approaches in the hope of a better understanding of this disease. Due to the rarity and lack of knowledge about SFTs, it is challenging to suspect and diagnose this disease. We emphasize the importance of the need for a more precise and deep understanding of these rare tumors, providing valuable insight into clinical practice.
A 68-year-old man presented with features of jaundice without abdominal pain.
The patient had no unusual symptoms other than jaundice and was referred to our hospital for further evaluation of a pancreatic head tumor found on abdominal computed tomography (CT).
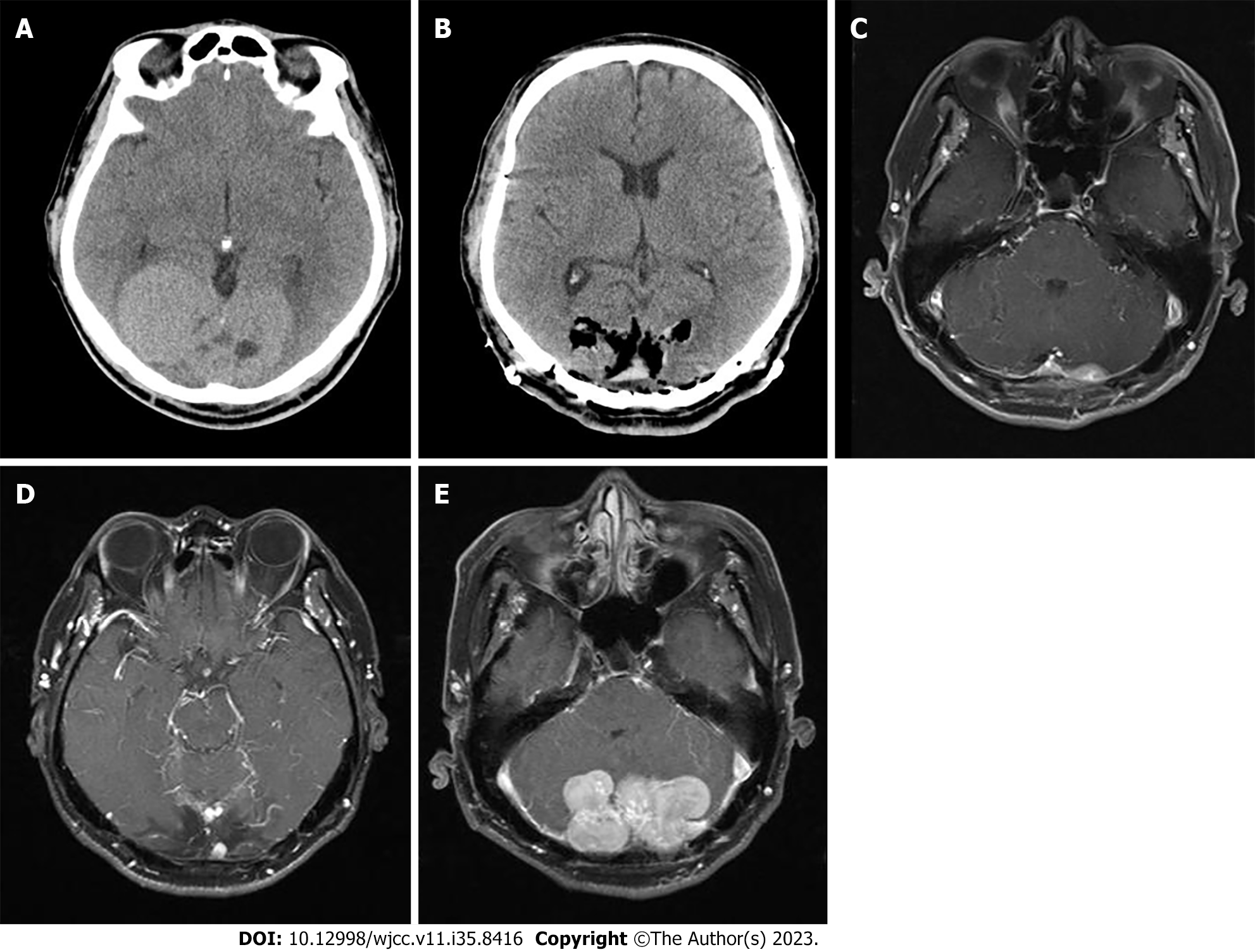
Fourteen years ago, the patient presented to our hospital with a complaint of decreased visual acuity. Brain CT revealed an 8 cm × 4.7 cm multilobulated heterogeneous mass at both parieto-occipital lobes (Figure 1A). The mass was initially suspected as meningioma and the patient underwent osteoplastic craniotomy for removal (Figure 1B). The pathologic diagnosis was solitary fibrous tumor with hypercellularity, focal moderate to marked cellular atyia, 0-1 mitosis per 10 HPF, and margins indeterminate. Immunohistochemical stain showed positive for vimentin, Bcl-2, and CD34, and negative for epithelial membrane antigen (EMA), glial fibrillary acidic protein (GFAP), S-100, CD99, smooth muscle actin (SMA), and CD56. On 3 and 6-mo follow-up CTs, abnormal enhancement was observed at the surgical site, which was thought to be a remnant tumor or postoperative change. Follow-up was continued and on the 16-mo follow-up brain magnetic resonance imaging (MRI), a 1.7 cm sized abnormal nodular enhancing lesion at the left cerebellar area highly suggested remnant mass (Figure 1C). The patient underwent GKRS and continued follow-up. 2 years later, brain MRI showed an interval decrease in the extent of the enhancing lesion at the left cerebellar area. However, a new 0.6 cm-sized enhancing mass was observed, suggesting recurrence (Figure 1D). The patient underwent a second GKRS. 5 years later, brain MRI showed multiple new masses in both cerebellar areas (Figure 1E). The patient underwent a second osteoplastic craniotomy and immunohistochemical staining showed positive for vimentin and STAT6, focal positive for CD34, and negative for GFAP, S-100, and EMA, leading to the diagnosis of hemangiopericytoma. The patient continued surveillance.
The patient denied any family history of malignancies.
Regular physical examination revealed no unusual findings except jaundice. The patient had no complaint of abdominal pain on examination.
The laboratory results on the day of the first visit to our hospital showed elevated levels of aspartate aminotransferase (223 U/L; reference range: 0-40 U/L), alanine aminotransferase (394 U/L; reference range: 0-40 U/L), alkaline phosphatase (762 U/L; reference range: 40-129 U/L), total bilirubin (4.18 mg/dL; reference range: 0.1-1.2 mg/dL), and gamma-glutamyl transpeptidase (1990 U/L; reference range: 11-73 U/L). These results comprehensively suggested a mixed pattern of hepatocellular and cholestatic liver disease. The serum tumor marker level of carcinoembryonic antigen was within the normal range and carbohydrate antigen 19-9 was slightly elevated to 42.20 U/mL (reference: 0-39 U/mL). Serum amylase and lipase were within normal limits.
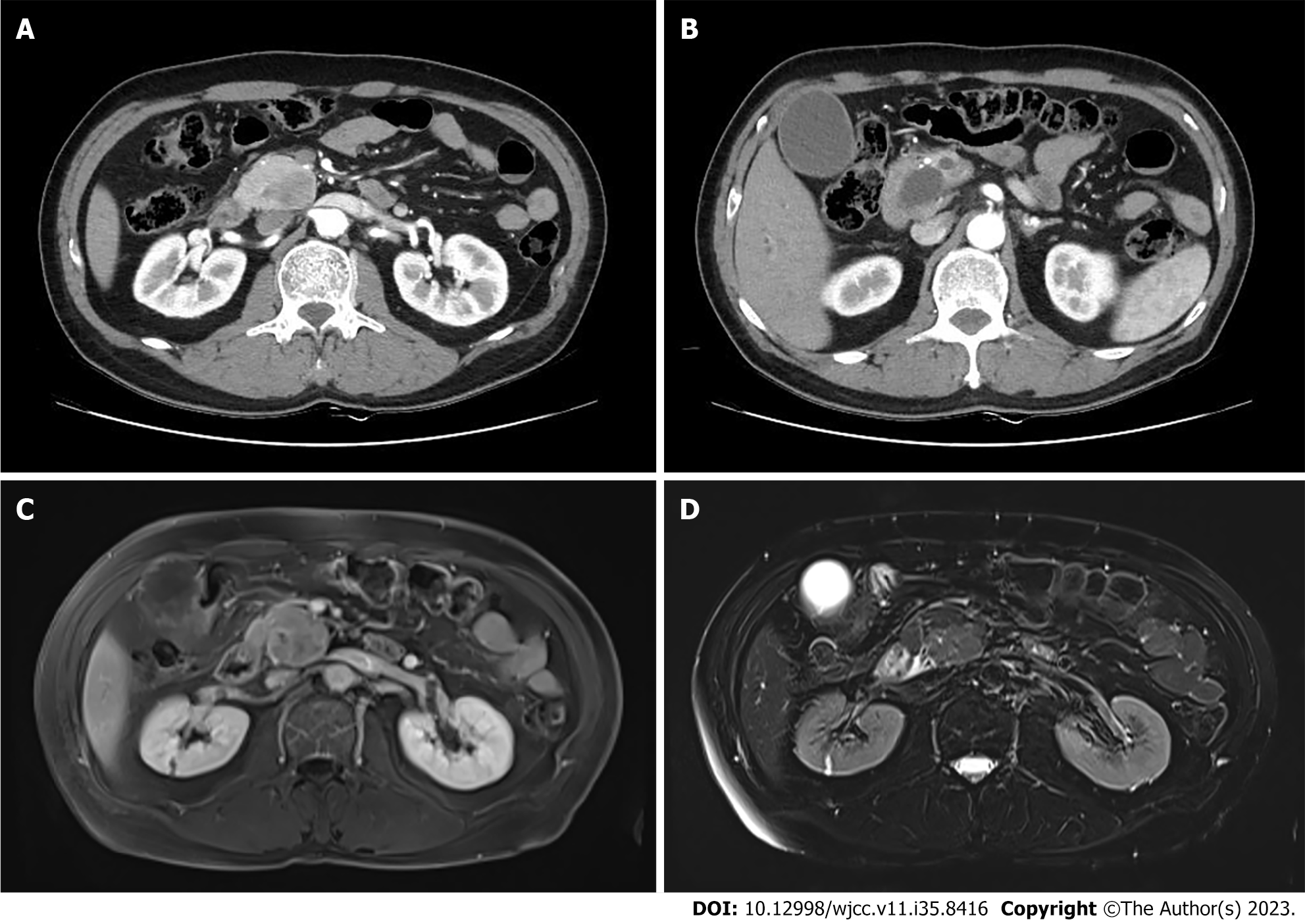
Abdominal CT showed a 3.5 cm-sized mass lesion on the pancreas head (Figure 2A). As the mass lesion compressed the pancreatic and bile ducts, the ducts were dilatated, resulting in the appearance of a double duct sign (Figure 2B). Compared to the surrounding parenchyma of the pancreas, the mass lesion showed hypervascularity during the arterial phase and isovascularity on the delayed phase of the CT. Compared to the usual CT findings of pancreatic cancer, its size was larger and the boundaries were relatively clear, so the possibility of other malignancies such as neuroendocrine tumor or acinar cell carcinoma were considered for differential diagnosis. For further investigation, an MRI was done. MRI showed low signal intensity on T1-weighted imaging and iso-signal intensity on T2-weighted imaging compared to the surrounding pancreas parenchyma (Figure 2C and D).
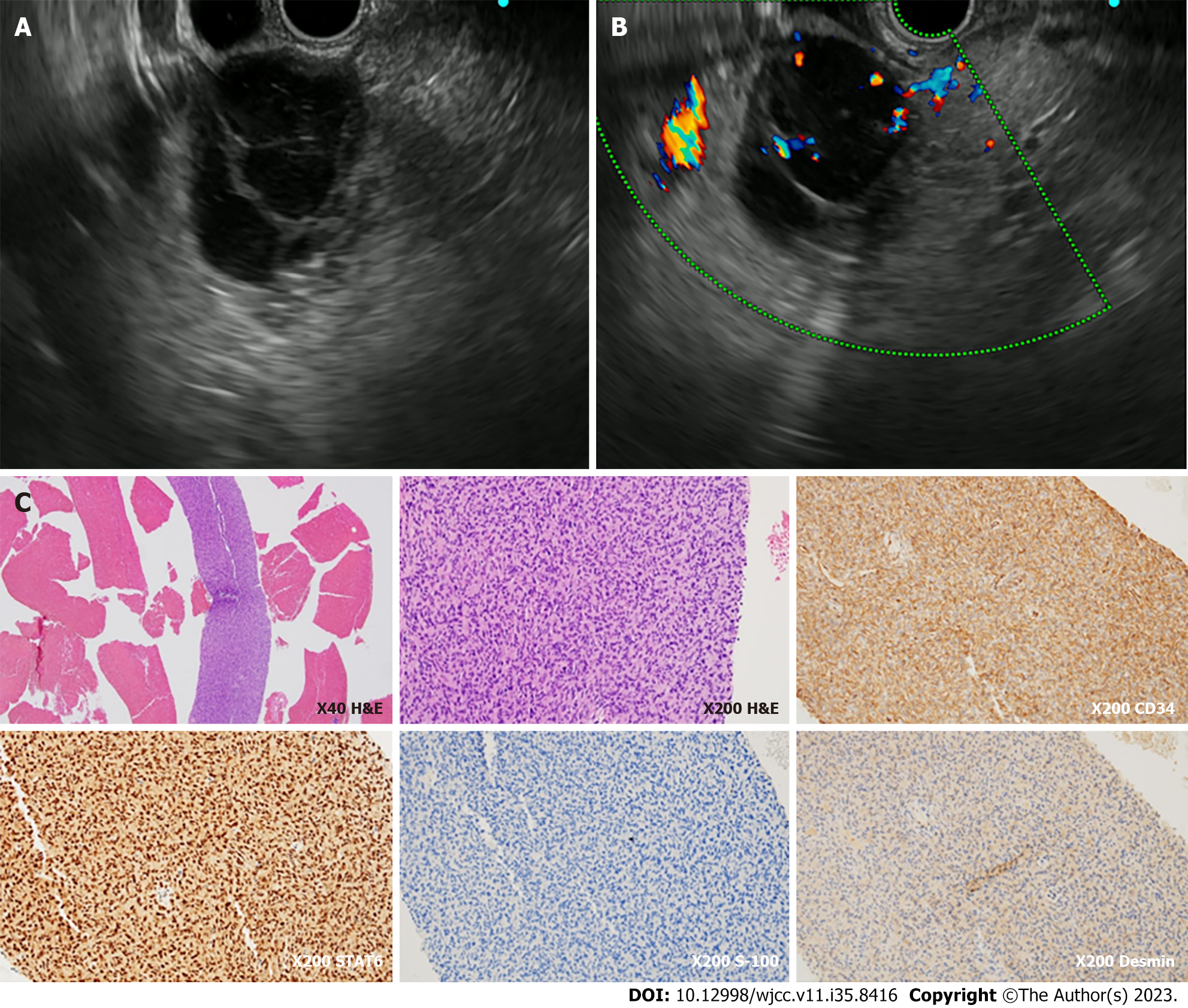
Pathological examination of the endosonography with fine-needle aspiration biopsy (EUS-FNA) specimen showed proliferation of spindle to ovoid cells. Immunohistochemically, the specimen was positive for SMA, STAT6, and CD34 but negative for DOG-1, C-kit, S-100, and desmin (Figure 3C). Based on histologic and immunohistochemistry results, the lesion was consistent with the findings of a hemangiopericytoma, currently referred to as SFT.
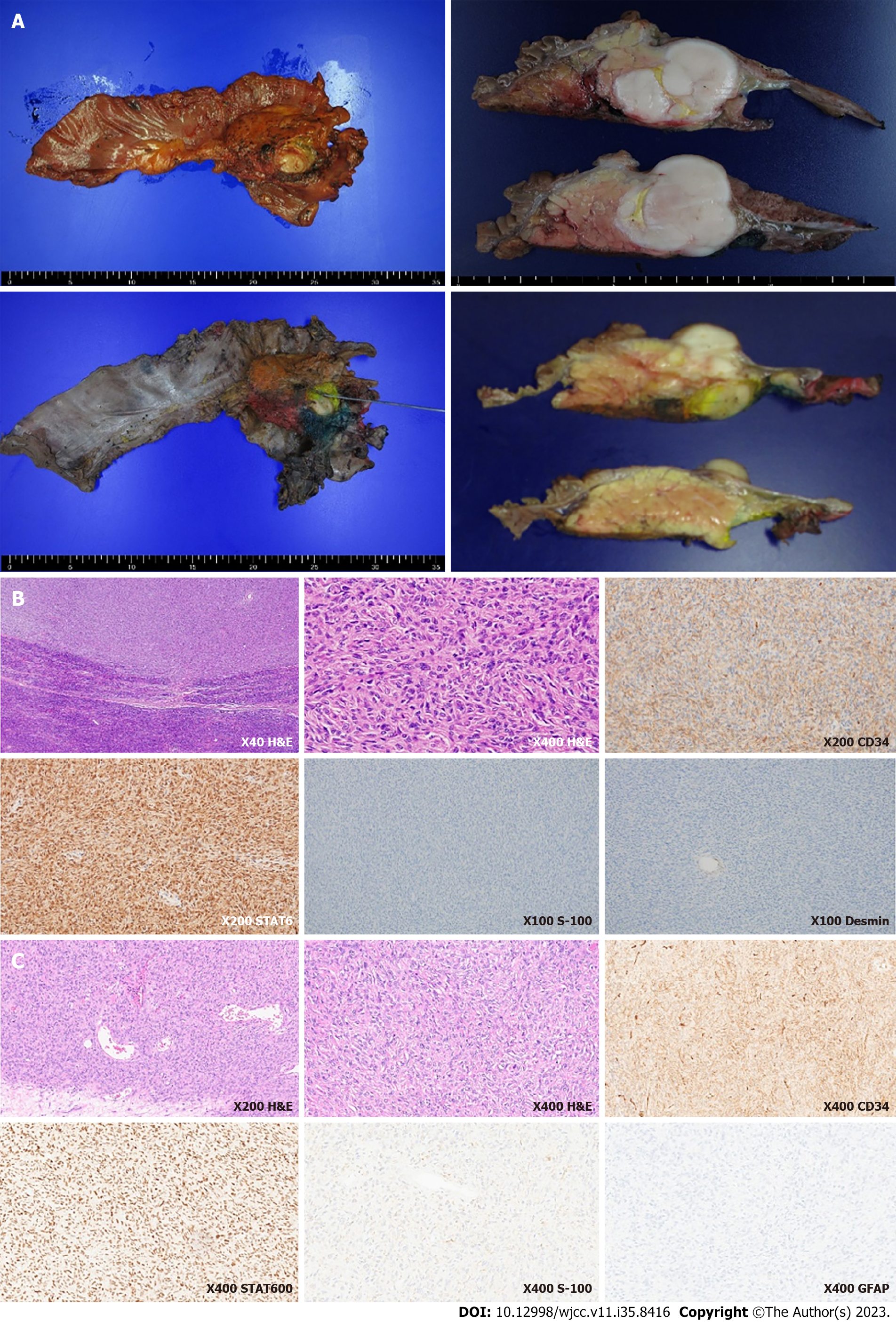
During hospitalization, the level of total bilirubin continued to rise because of the obstruction of the bile ducts due to the mass lesion. In combination with antibiotic treatment, endoscopic retrograde biliary drainage and pancreatic drainage were done to relieve the obstruction. The patient was referred for surgery and underwent pylorus-preserving pancreaticoduodenectomy. The cut surface of the resected specimen showed a 3.9 cm × 3.6 cm well-circumscribed, gray to white-colored mass (Figure 4A). The resected specimen demonstrated similar features as the EUS-FNA specimen, showing dense spindle to ovoid cells with positivity to CD34, EMA, and STAT6 and negativity to desmin, SMA, DOG-1, c-kit, and S-100 (Figure 4B). Pathology demonstrated moderate nuclear atypia, high cellularity, a mitotic rate of 5 per 10 high-power fields (HPFs), and absent necrosis. The distal side of the pancreatic margin was involved by the tumor, so the patient underwent additional radiotherapy.
The immunohistochemical staining of the meningeal hemangiopericytoma tissue resected 5 years ago showed positive for vimentin and STAT6, focal positive for CD34, and negative for GFAP, S-100, EMA, leading to the diagnosis of hemangiopericytoma. As the histological and immunohistochemical staining of the resected pancreatic tissue was similar to that of the meningeal hemangiopericytoma tissue resected 5 years ago, it could be assumed that the pancreatic lesion was a metastatic lesion from the meninges (Figure 4C).
The patient has been under surveillance for 20 mo after surgery, and there is no evidence of local pancreas recurrence or metastasis.
Most pancreatic tumors are of epithelial origin, with more than 90% being pancreatic ductal adenocarcinomas[3]. However, tumors of mesenchymal origin can also involve the pancreas, accounting for 1%-2% of all tumors[3]. Mesenchymal tumors can be derived from the connective, lymphatic, neuronal, and vascular tissues, and show different clinicopathologic manifestations and outcomes[3]. Primary mesenchymal tumors of the pancreas that are known to show benign or borderline behavior include schwannomas, inflammatory myofibroblastic tumors, hamartomas, and solitary fibrous tumors in order of frequency[4]. Primary mesenchymal tumors of the pancreas that are reported malignant are leiomyosarcomas, Ewing sarcomas, and malignant fibrous histiocytomas[4]. However, there is a lack of information about the frequency or characteristics of secondary pancreatic mesenchymal tumors depending on whether they are benign or malignant.
Among the various mesenchymal tumors, SFT is a rare spindle cell neoplasm that was first reported in the pleura by Klemperer et al[7] in 1931. SFT originates most commonly from the pleura (65%), and it can also arise from extrapleural sites, but pancreatic localization is very rare among them[8]. It is known to affect adults between the ages of 20 to 70 years and the incidence is hard to estimate[9]. SFTs were previously called hemangiopericytoma and they were frequently mistaken as meningiomas because of the similar radiological findings, but they tend to have a high recurrence rate and risk for metastases[10]. The 2016 World Health Organization Classification of the central nervous system included hemangiopericytomas into the family of solitary fibrous tumors because the NAB2-STAT6 fusion was identified as defining molecular modifications in both tumors, suggesting they were overlapping entities[11].
The diagnosis of SFT relies on a combination of clinical, radiological, and histopathological findings. Symptoms may vary depending on the location and size of the mass[12]. Radiological features are non-specific, with contrast-enhanced CT showing arterial and venous phase enhancement, and MRI displaying T1 hypointensity and T2 hyperintensity[13]. Given these non-specific radiologic findings, pancreatic SFTs can be mistaken for other hypervascular pancreatic masses such as a neuroendocrine tumor[14]. Therefore, biopsy and histopathology are crucial for definitive diagnosis, with EUS-FNA as a safe preoperative tool[1,6,15]. There has been a report of a series of four patients who underwent EUS-FNA of the pancreas including differential diagnoses of metastatic leiomyosarcoma, metastatic liposarcoma, metastatic solitary fibrous tumor, and metastatic alveolar rhabdomyosarcoma[16]. Histologically, SFTs exhibit spindle-shaped cells, CD34, Bcl-2, and CD99 positivity, and STAT6 nuclear expression[5].
The differential diagnosis of benign and malignancy is important, however, there is no diagnostic criteria established to date[17]. One study investigated 82 patients and suggested that malignant histology, compared to benign, was associated with larger tumor size, higher mitotic counts, and metastatic disease at diagnosis, while gender, age, and tumor site showed no significant difference[18]. Another study reported that approximately 12%-22% of SFTs were malignant[9], and were associated with features like nuclear atypia, marked hypercellularity, tumor size larger than 5 cm, a mitotic rate greater than 4 per 10 HPFs, and tumor necrosis[19,20]. It is difficult to determine whether SFT is malignant or benign, and even when it is considered benign, it can recur aggressively[21]. In our case, the brain hemangiopericytoma that first occurred was the size of 8 cm and pathology showed hypercellularity, nuclear atypia, and indeterminate margins. The recurred brain hemangiopericytoma showed moderate nuclear atypia and moderate cellularity. Both cases showed factors that were reported to be associated with malignancy. Metastases occurred most frequently in the lung (32.1%) followed by the brain (2.3%)[22]. The recommended treatment for SFTs of the pancreas is surgical resection, aiming for complete removal. For unresectable or metastatic cases, systemic chemotherapy and radiation therapy can be considerable options[23].
As aforementioned, there have been two case reports of intracranial SFTs metastasizing to the pancreas (Table 1). One case was of metastasis to the pancreas in a patient who had undergone GKRS due to intracerebral recurrence 9 and 4 years ago after surgery of the intracranial SFT first diagnosed 16 years ago[24]. The other case was of metastasis to the pancreas after surgery for intracerebral recurrence 16 and 26 years after initial surgery for intracranial SFT diagnosed and removed 27 years ago[5]. Including this current case, all three patients had a past medical history of recurrent meningeal hemangiopericytoma and the resected pancreatic lesions showed malignant findings of a high mitotic rate. Currently, the prognosis of SFTs is unpredictable and surveillance methods are yet established[9]. A review article of 29 studies including 368 patients, it was reported that primary intracranial and spinal SFT/hematopoietic progenitor cells metastasized in 13.3% of cases during a median period of 3.25 years (range 0.5-12.3 years) after the diagnosis of the primary lesion. However, it is interesting that in the three cases in Table 1, including ours, the period for metastasis was much longer, at 16, 27, and 14 years, respectively. Therefore, more caution should be taken in the progress of surveillance for a longer duration in cases of recurrent meningeal SFTs, and the pancreas should be considered as a possible site for metastasis.
| Author | Age, Sex | Symptoms | Size (cm) | Preoperative biopsy | Treatment of primary (intracranial) lesion | Treatment of secondary (pancreatic) lesion | Malignant findings | Time to metastastis (years) | |
| 1 | Osuga et al[7] | F, 62 | Asymptomatic | 3.3 | EUS-FNA | Resection, GKRS | Resection | High mitotic rate | 16 |
| 2 | Yamashita et al[5] | F, 58 | Asymptomatic | 5.5 | None | Resection | Resection | High mitotic rate | 27 |
| 3 | Current case | M, 68 | Jaundice | 8 | EUS-FNA | Resection, GKRS | Resection, radiation | High mitotic rate, nuclear atypia, hypercellularity | 14 |
In the case of pancreatic tumors, adenocarcinoma is most common, and neuroendocrine tumors or mesenchymal tumors are very rare[1]. Because imaging findings are non-specific and in most cases, a diagnosis can be made only through histopathological findings, it is difficult to even think of SFT as a differential diagnosis when a pancreatic tumor is found. However, if the patient has a known history of primary SFT and has a hypervascular, well-encapsulated lesion in distant organs such as the pancreas, metastasis of SFT should always be considered. EUS-FNA can be a safe and effective tool used preoperatively for the diagnosis of pancreatic mesenchymal tumors. There are only two case reports on metastatic SFTs from the central nervous system, and we hope that this case report will further contribute to a better understanding of metastatic SFTs. Further research is warranted to better understand the pathogenesis of SFT and develop more effective therapeutic approaches to improve patient outcomes.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Covantsev S, Russia S-Editor: Liu JH L-Editor: A P-Editor: Yu HG
| 1. | Srinivasan VD, Wayne JD, Rao MS, Zynger DL. Solitary fibrous tumor of the pancreas: case report with cytologic and surgical pathology correlation and review of the literature. JOP. 2008;9:526-530. [PubMed] |
| 2. | Tasdemir A, Soyuer I, Yurci A, Karahanli I, Akyildiz H. A huge solitary fibrous tumor localized in the pancreas: a young women. JOP. 2012;13:304-307. [PubMed] |
| 3. | Askan G, Basturk O. Mesenchymal Tumors Involving the Pancreas: A Clinicopathologic Analysis and Review of the Literature. Turk Patoloji Derg. 2022;38:46-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 4. | Kim JY, Song JS, Park H, Byun JH, Song KB, Kim KP, Kim SC, Hong SM. Primary mesenchymal tumors of the pancreas: single-center experience over 16 years. Pancreas. 2014;43:959-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 5. | Yamashita H, Fujino Y, Ohara T, Kakinoki K, Sugimoto T, Kajimoto K, Tominaga M. A rare case of metastatic solitary fibrous tumor of the pancreas manifesting as a cystic neoplasm: a case report. Surg Case Rep. 2019;5:142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Wang W, Zhou S, Wu X, Wang L, Ruan Y, Lu J, Li H, Li X, Qiu L, Zhou X. Imaging, pathology, and diagnosis of solitary fibrous tumor of the pancreas: a literature review. 2023. [DOI] [Full Text] |
| 7. | Klemperer P, Coleman BR. Primary neoplasms of the pleura. A report of five cases. Am J Ind Med. 1992;22:1-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 199] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 8. | Kwon HJ, Byun JH, Kang J, Park SH, Lee MG. Solitary fibrous tumor of the pancreas: imaging findings. Korean J Radiol. 2008;9 Suppl:S48-S51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 9. | D'Amico FE, Ruffolo C, Romano M, DI Domenico M, Sbaraglia M, Dei Tos AP, Garofalo T, Giordano A, Bassi I, Massani M. Rare Neoplasm Mimicking Neuoroendocrine Pancreatic Tumor: A Case Report of Solitary Fibrous Tumor with Review of the Literature. Anticancer Res. 2017;37:3093-3097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Wen PY, Huse JT. 2016 World Health Organization Classification of Central Nervous System Tumors. Continuum (Minneap Minn). 2017;23:1531-1547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 71] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 11. | Fritchie K, Jensch K, Moskalev EA, Caron A, Jenkins S, Link M, Brown PD, Rodriguez FJ, Guajardo A, Brat D, Velázquez Vega JE, Perry A, Wu A, Raleigh DR, Santagata S, Louis DN, Brastianos PK, Kaplan A, Alexander BM, Rossi S, Ferrarese F, Haller F, Giannini C. The impact of histopathology and NAB2-STAT6 fusion subtype in classification and grading of meningeal solitary fibrous tumor/hemangiopericytoma. Acta Neuropathol. 2019;137:307-319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 12. | Han SH, Baek YH, Han S-y, Lee SW, Jeong JS, Cho JH, Kwon H-J. Solitary Fibrous Tumor of the Pancreas: A Case Report and Review of the Literature. Korean Journal of Medicine. 2015;88. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | McKenzie J, Bates W, Kruse EJ. Solitary Fibrous Tumor of the Pancreas. Am Surg. 2019;85:e468-e469. [PubMed] |
| 14. | Trout AT, Elsayes KM. Multidetector CT of pancreatic hemangiopericytoma. Cancer Imaging. 2009;9:15-18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Spadaccini M, Conti Bellocchi MC, Mangiavillano B, Fantin A, Rahal D, Manfrin E, Gavazzi F, Bozzarelli S, Crinò SF, Terrin M, Di Leo M, Bonifacio C, Facciorusso A, Realdon S, Cristofori C, Auriemma F, Fugazza A, Frulloni L, Hassan C, Repici A, Carrara S. Secondary Tumors of the Pancreas: A Multicenter Analysis of Clinicopathological and Endosonographic Features. J Clin Med. 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Varghese L, Ngae MY, Wilson AP, Crowder CD, Gulbahce HE, Pambuccian SE. Diagnosis of metastatic pancreatic mesenchymal tumors by endoscopic ultrasound-guided fine-needle aspiration. Diagn Cytopathol. 2009;37:792-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Shabunin AV, Dolidze DD, Lebedinsky IN, Bagatelia ZA, Sukhotko AS, Kovaleva MV, Covantsev S. Surgical treatment of a patient with hemangiopericytoma and subsequent abdominoplasty: a clinical case. Academic Journal of Health Sciences: Medicina Balear. 2023;38:166-169. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 18. | DeVito N, Henderson E, Han G, Reed D, Bui MM, Lavey R, Robinson L, Zager JS, Gonzalez RJ, Sondak VK, Letson GD, Conley A. Clinical Characteristics and Outcomes for Solitary Fibrous Tumor (SFT): A Single Center Experience. PLoS One. 2015;10:e0140362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 19. | Gold JS, Antonescu CR, Hajdu C, Ferrone CR, Hussain M, Lewis JJ, Brennan MF, Coit DG. Clinicopathologic correlates of solitary fibrous tumors. Cancer. 2002;94:1057-1068. [PubMed] |
| 20. | Vallat-Decouvelaere AV, Dry SM, Fletcher CD. Atypical and malignant solitary fibrous tumors in extrathoracic locations: evidence of their comparability to intra-thoracic tumors. Am J Surg Pathol. 1998;22:1501-1511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 490] [Cited by in RCA: 442] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 21. | Davanzo B, Emerson RE, Lisy M, Koniaris LG, Kays JK. Solitary fibrous tumor. Transl Gastroenterol Hepatol. 2018;3:94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 134] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 22. | Giordan E, Marton E, Wennberg AM, Guerriero A, Canova G. A review of solitary fibrous tumor/hemangiopericytoma tumor and a comparison of risk factors for recurrence, metastases, and death among patients with spinal and intracranial tumors. Neurosurg Rev. 2021;44:1299-1312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 23. | Qian X, Zhou D, Gao B, Wang W. Metastatic solitary fibrous tumor of the pancreas in a patient with Doege-Potter syndrome. Hepatobiliary Surg Nutr. 2020;9:112-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 24. | Osuga T, Hayashi T, Ishiwatari H, Ono M, Yoshida M, Kimura Y, Hasegawa T, Sato Y, Sato T, Miyanishi K, Takimoto R, Kobune M, Kato J. Pancreatic metastasis from a solitary fibrous tumor of the central nervous system. JOP. 2014;15:58-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |