Published online Dec 16, 2023. doi: 10.12998/wjcc.v11.i35.8372
Peer-review started: August 27, 2023
First decision: September 13, 2023
Revised: September 18, 2023
Accepted: December 1, 2023
Article in press: December 1, 2023
Published online: December 16, 2023
Processing time: 109 Days and 1.6 Hours
Thoracic empyema and malignant pleural mesothelioma (MPM) are distinct medical conditions with similar symptoms, including cough, chest pain, and breathing difficulty. We present a rare MPM case mimicking thoracic empyema. Physicians must consider MPM risks for patients exposed to building material who exhibit lobulated pleural effusions, indicating thoracic empyema.
A 68-year-old retired male construction worker suffered from shortness of breath and chest tightness over 10 d, particularly during physical activity. A poor appetite and 4 kg weight loss over the past 3 wk were also reported. Chest images and laboratory data concluded a tentative impression of empyema thoracis (right). Video-assisted thoracic surgery with decortication and delobulation (right) was conducted. The pathological report yielded an MPM diagnosis. Refractory pleural bilateral effusions and respiratory failure developed postoperatively, and the patient died three weeks after the operation.
Thoracic empyema and MPM are distinct medical conditions that can present similar symptoms, and video-assisted thoracic surgery facilitates an accurate diagnosis. Empyema-mimicking presentations and postoperative refractory pleural effusion may indicate a poor MPM outcome.
Core Tip: Malignant pleural mesothelioma (MPM) shows similar clinical symptoms and imaging features to thoracic empyema. In the present case, the final diagnosis was confirmed by the thoracoscopic surgery. This article could offer clinicians a hint to consider a diagnosis of MPM when treating thoracic empyema and consider thoracoscopic surgery for accurate diagnosis.
- Citation: Yao YH, Kuo YS. Malignant pleural mesothelioma mimics thoracic empyema: A case report. World J Clin Cases 2023; 11(35): 8372-8378
- URL: https://www.wjgnet.com/2307-8960/full/v11/i35/8372.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i35.8372
Malignant pleural mesothelioma (MPM) is a rare pleural disease associated with environmental or occupational asbestos exposure and maintains one of the poorest prognoses among respiratory diseases[1,2]. Past studies have primarily linked MPM to asbestos exposure, theorizing that inhaling asbestos prompts repeated pleural inflammation, proto-oncogene activation, and free radical production[2].
Image studies [i.e., chest computed tomography (CT) with intravenous contrast] and invasive procedures (i.e., thoracoscopic pleural biopsy and thoracentesis of pleural effusion with cytologic analysis) are often required to diagnose MPM[3,4]. However, not all patients with pleural effusion receive invasive procedures, such as pleural biopsy. Clinically, MPM has a nonspecific presentation and can be misdiagnosed as other conditions, such as pulmonary empyema or metastatic pleural lung cancer, without a thorough patient history[5].
Hence, we present the case of a patient initially suspected to have lobulated pleural effusion on his right side due to empyema or tumor. However, the biopsy performed during video-assisted thoracic surgery revealed poorly differentiated pleural mesothelioma (Table 1).
| Time | Events |
| January 13, 2023 | Visited emergent department; tube thoracostomy, right and admission |
| January 16, 2023 | Video-assisted thoracoscopic decortication and pleural biopsy, right |
| January 31, 2023 | Acute respiratory failure; endotracheal intubation |
| February 1, 2023 | Thoracic tumor board conference discussion; chemoport implantation |
| February 2, 2023 | Cisplatin based chemotherapy |
| February 17, 2023 | Percutaneous tracheostomy |
| February 22, 2023 | Ventilator-associated pneumonia, bilateral with severe acute respiratory distress syndrome, and septic shock |
| February 23, 2023 | Expired |
A 68-year-old retired male construction worker suffered from shortness of breath and chest tightness, particularly during physical activity, for 10 d. A recent appetite loss, resulting in a 4 kg weight loss over the past 3 wk, was also reported.
The patient entered the emergency department after 10 d of shortness of breath and chest tightness, particularly during physical activity. He also reported a recent appetite loss, resulting in a 4 kg weight loss over the past 3 wk.
The patient had a past coronary artery disease medical history managed by percutaneous coronary artery intervention with one stent implantation. He also experienced an abdominal aortic aneurysm, which was treated by endovascular aneurysm repair.
The patient, a non-smoker, denied any family cancer history.
The breathing sound was decreased in the right side chest.
The patient's blood examination revealed an elevated leukocyte count of 24020/µL, with 92.5% neutrophils, a low hemoglobin level at 8.9 g/dL, and a high C-reactive protein level at 27.08 mg/dL. As a result, a tube thoracostomy was performed on the right side, and the pleural effusion was collected for analysis. The subsequent analysis indicated a total cell count of 154686, red blood cell count of 154000/μL, total protein level at 4.2 g/dL, lactate dehydrogenase fluid level at 1801 U/L, polymorphonuclear leukocyte percentage of 76%, and morphonuclear leukocyte percentage of 24%. No pathogens were cultured from the pleural effusion, but the Rivalta test was positive, confirming the exudate and raising suspicion of empyema. Influenza A and B virus antigen detection was arranged, but all tests were negative.
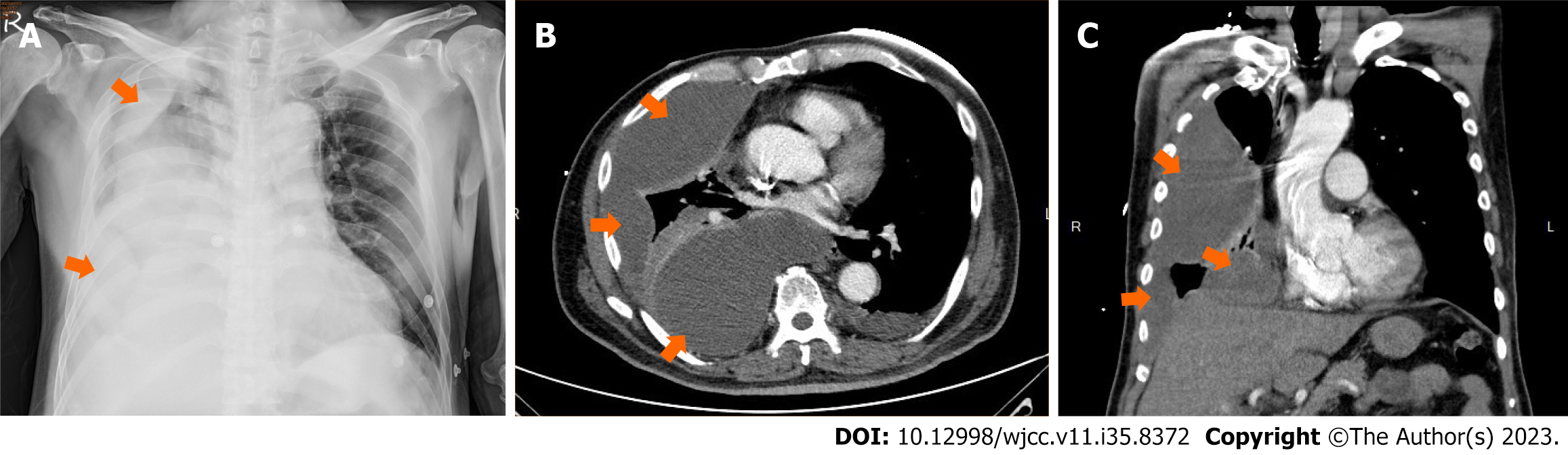
The chest plain film (Figure 1A) and CT (Figure 1B and C) confirmed multiple lobulated pleural effusions with the reverse D sign, raising suspicion of thoracic empyema on the right side.
The thoracic tumor board conference at the Tri-Service General Hospital suggested that the impression of postoperative tumor-related leukocytosis was considered rather than postoperative empyema. Cisplatin based chemotherapy was suggested for the patient.
Malignant pleura mesothelioma with bilateral malignant pleural effusion, epithelioid subtype (AJCC Cancer Staging Manual, 8th Edition), clinical stage T4N0M0, stage IIIB.
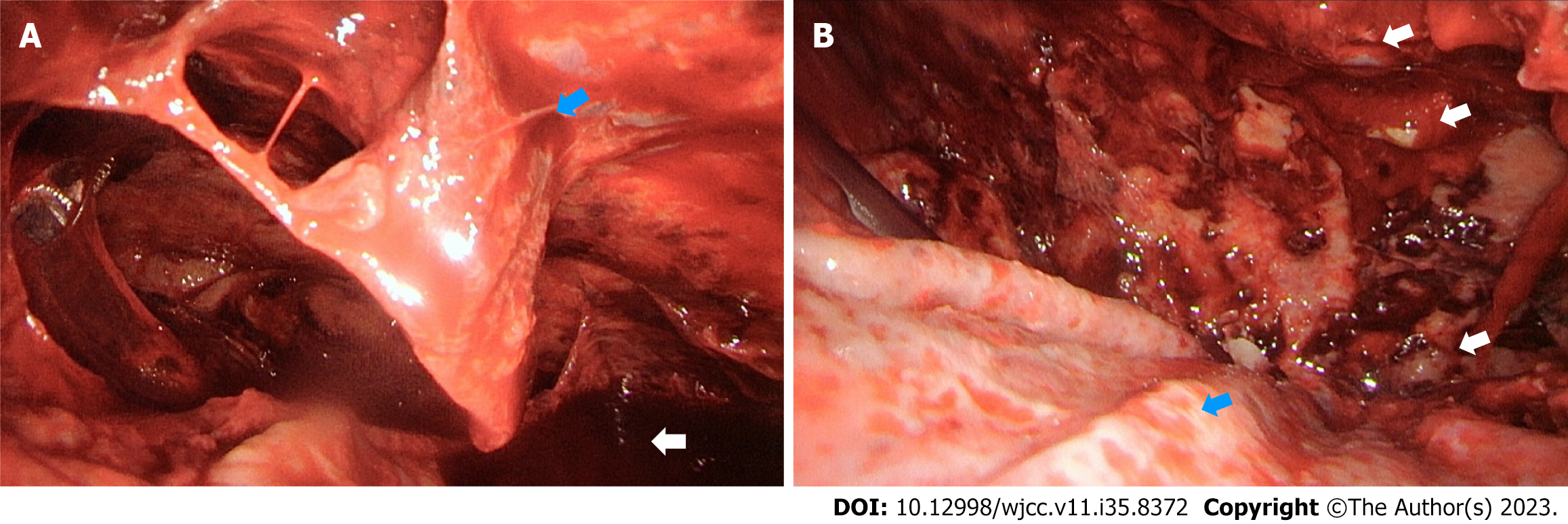
Due to symptom persistence and the lobulated pleural effusion in the right-side pleural cavity, which was suspected to be caused by the tentative impression of thoracic empyema, the patient underwent video-assisted thoracic surgery with decortication and delobulation. After entering the right pleural cavity, multiple lobulated bloody pleural effusions were observed (Figure 2A). In addition, several tiny pleural nodules were observed during thoracoscopic surgery (Figure 2B), and intra-operative frozen sections from the pleural biopsy displayed positive malignancy.
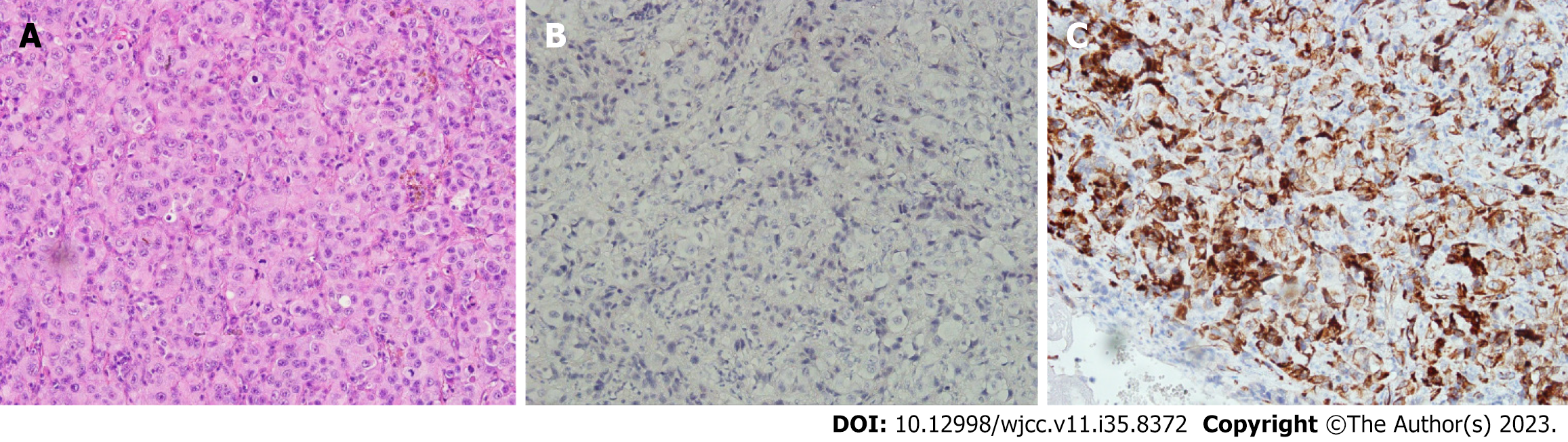
The chest plain film on the fifth postoperative day showed a massive pleural effusion on the left side, and a pig-tail drainage tube was inserted into the left pleural cavity. The left-side pleural effusion also exhibited bloody fluid, and the cytology analysis suggested malignancy. The pleural nodule pathological report (Figure 3) revealed poorly differentiated carcinoma compatible with MPM. The diagnosis of MPM with bilateral malignant pleural effusion, epithelioid subtype (AJCC Cancer Staging Manual, 8th Edition), clinical stage T4N0M0, stage IIIB was determined after the series of examinations, including blood tests, abdominal CT, brain magnetic resonance imaging (MRI), and a bone scan.
In addition, follow-up chest plain films indicated no remission of the right-side pleural effusion. The refractory bilateral massive pleural effusion was observed following the postoperative course. Intravenous diuretics, frequent bilateral thoracentesis, and pleural catheter insertion were conducted during this period. Then, an acute hypoxemic respiratory failure developed on the tenth postoperative day. Emergent endotracheal intubation was performed, and the patient was transferred to the intensive care unit. The laboratory data revealed persistent leukocytosis with leukocyte counts of 20000/µL to 26000/µL and mildly elevated serum C reactive protein levels of 2 mg/dL to 4 mg/dL post operation. However, no definitive toxic signs, such as fever, were evident, and tachycardia was observed clinically.
Following the thoracic tumor board conference discussion at our hospital, tumor-related leukocytosis was considered rather than postoperative empyema.
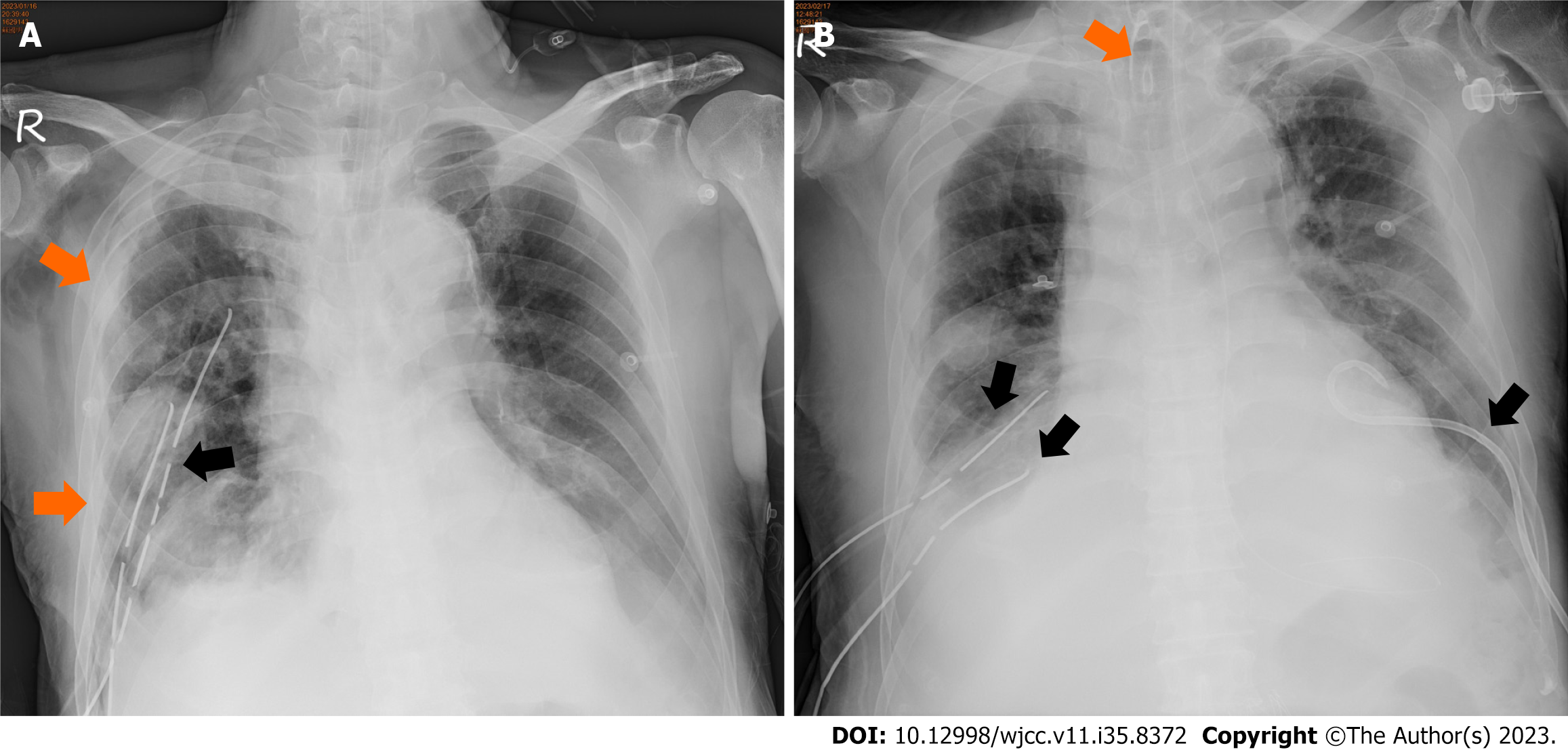
A chemo port was implemented on the 14th postoperative day (Figure 4). On the 15th postoperative day, a first course of cisplatin-based chemotherapy was performed. After the first chemotherapy course, ventilator-associated pneumonia, bilateral with severe acute respiratory distress syndrome, and septic shock developed. The patient died on the 21st postoperative day.
Thoracic empyema and MPM are distinct medical conditions with similar symptoms, such as cough, chest pain, and breathing difficulty. Thoracic empyema occurs when pus collects in the pleural cavity, the space between the lungs and the chest wall. Bacterial infection or other underlying conditions, such as pneumonia, lung abscess, or tuberculosis, can beget this condition. In contrast, MPM is a type of cancer that affects the lining of the lungs, chest wall, or abdomen, often caused by asbestos exposure and can take decades to develop.
Although thoracic empyema and MPM symptoms can be similar, their underlying causes and treatment options vary. Thoracic empyema is typically treated with antibiotics and pus drainage, whereas mesothelioma may require surgery, chemotherapy, or radiation therapy. MPM is usually diagnosed through needle biopsy rather than surgery. Diagnostic imaging methods for pleural mesothelioma include standard X-rays, CT scans, MRI, and positron emission tomography scans[6-8]. However, the chest CT scan for our patient revealed no bilateral pleura thickening but a typical lobulated pleural effusion appearance on his right side. Still, deciding the optimal site for CT-guided core biopsy is challenging.
Finally, the pathological diagnosis was determined with video-assisted thoracoscopic surgery (VATS). VATS may be the most reliable method for a final tissue diagnosis[9-11] and could also serve as a curative treatment method. We conclude that VATS has unique diagnostic value for empyema-mimicking pleural mesothelioma than the CT-guided core biopsy. Based on NCCN guidelines and Zhou et al's study (2022) concerning MPM management, pleurectomy/decortication (PDC) and extrapleural pneumonectomy (EPP) have comparable long-term oncologic outcomes, but PDC has much lower perioperative mortality[12].
Our patient underwent a PDC-like surgery. However, further surgical intervention, such as EPP, was not considered due to his poor postoperative conditions. Zhou et al[12] reported that the surgical cytoreduction goal for pleural mesothelioma should be to achieve macroscopic complete resection with the safest operation that the patient can tolerate. In addition, our patient suffered from bilateral refractory pleural effusion caused by MPM following the VATS procedure. Maione et al[13] discussed that chemical pleurodesis and pleural catheter insertion may be an ideal option for patients like ours. However, the patient later became unfit for surgery due to deteriorating health conditions before this attempt could be made.
In this case, we faced a dilemma in distinguishing empyema-related leukocytosis from tumor-related leukocytosis during the postoperative course. Tumor-related leukocytosis is a paraneoplastic syndrome that was reported as a poor negative prognostic factor in the clinical course of patients with lung cancer[13,14]. Thus, chemotherapy was performed under the curative intent after the thoracic tumor board conference discussion.
Matsuoka et al[15] described a patient initially diagnosed with acute empyema and severe leukocytosis but ultimately diagnosed with MPM and opted for palliative care. Our patient had a similar poor outcome and died on the 21st postoperative day. We theorize that empyema-mimicking presentations and postoperative refractory pleural effusion indicate a poor outcome.
A recent study by Lapidot et al[16] published in the Annals of Thoracic Surgery reported postoperative empyema rates after PDC for MPM up to 10% and identified several risk factors, including prolonged air leak, diabetes, and previous radiation therapy. Although the study focused on postoperative empyema following PDC, its findings highlight the necessity for developing novel strategies to prevent empyema in MPM patients who have undergone other treatments, such as chemotherapy.
In the Journal of Clinical Oncology, Javidfar et al[17] revealed a different treatment option: Repeated ambulatory intrapleural chemotherapy for patients with MPM who are not eligible for surgery or unresponsive to prior treatments. The procedure involves placing a catheter into the pleural cavity and infusing chemotherapy drugs directly into the affected area on an outpatient basis. Several studies have demonstrated that this approach can effectively control pleural effusions, extend survival, and improve patients' quality of life, as measured by various scales and questionnaires[18,19]. However, this treatment is not without risks and potential complications. Some patients may experience pain or discomfort during the procedure, while others may develop infections or other adverse events, as in our case. Furthermore, there is limited data on the long-term safety and efficacy of repeated intrapleural chemotherapy, and further research is needed to evaluate its comprehensive benefits and risks.
Considering these studies, combining induction chemotherapy, EPP, and adjuvant radiotherapy has emerged as a treatment option for select patients with resectable diseases. In a retrospective analysis, Guy and St. Thomas Hospital researchers chronicled their experience with this tri-modality approach in 36 patients with MPM[20]. All patients received induction chemotherapy with cisplatin and pemetrexed, followed by EPP and adjuvant hemi-thoracic radiotherapy. The median overall survival was 20.2 mo, with a 43% 2-year survival rate. However, the high morbidity associated with EPP and adjuvant radiotherapy should be considered when contemplating this treatment strategy.
In summary, clinicians should have a high level of suspicion for MPM risks in patients with thoracic empyema or pleural effusions. MPM can mimic empyema, delaying diagnosis and resulting in missed treatment opportunities and poor outcomes. Thoracoscopic surgery may be crucial for pathological diagnosis.
Although thoracic empyema and MPM are distinct medical conditions exhibiting similar symptoms, VATS can offer an accurate diagnosis. In addition, empyema-mimicking presentations and postoperative refractory pleural effusion may signal poor outcomes for MPM.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Surgery
Country/Territory of origin: Taiwan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hoffmann M, Germany S-Editor: Yan JP L-Editor: Wang TQ P-Editor: Yan JP
| 1. | Fournel L, Charrier T, Huriet M, Iaffaldano A, Lupo A, Damotte D, Arrondeau J, Alifano M. Prognostic impact of inflammation in malignant pleural mesothelioma: A large-scale analysis of consecutive patients. Lung Cancer. 2022;166:221-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 2. | Ahmed I, Ahmed Tipu S, Ishtiaq S. Malignant mesothelioma. Pak J Med Sci. 2013;29:1433-1438. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Woolhouse I, Maskell NA. Introducing the new BTS guideline: the investigation and management of pleural malignant mesothelioma. Thorax. 2018;73:210-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Ettinger DS, Wood DE, Akerley W, Bazhenova LA, Borghaei H, Camidge DR, Cheney RT, Chirieac LR, D'Amico TA, Dilling T, Dobelbower M, Govindan R, Hennon M, Horn L, Jahan TM, Komaki R, Lackner RP, Lanuti M, Lilenbaum R, Lin J, Loo BW Jr, Martins R, Otterson GA, Patel JD, Pisters KM, Reckamp K, Riely GJ, Schild SE, Shapiro TA, Sharma N, Swanson SJ, Stevenson J, Tauer K, Yang SC, Gregory K, Hughes M. NCCN Guidelines Insights: Malignant Pleural Mesothelioma, Version 3.2016. J Natl Compr Canc Netw. 2016;14:825-836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 73] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 5. | Odisio EG, Marom EM, Shroff GS, Wu CC, Benveniste APA, Truong MT, Benveniste MF. Malignant Pleural Mesothelioma: Diagnosis, Staging, Pitfalls and Follow-up. Semin Ultrasound CT MR. 2017;38:559-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Klawiter A, Damaszke T. Pleural mesothelioma - case report. Pol J Radiol. 2010;75:61-63. [PubMed] |
| 7. | Kawashima A, Libshitz HI. Malignant pleural mesothelioma: CT manifestations in 50 cases. AJR Am J Roentgenol. 1990;155:965-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 81] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Ng CS, Munden RF, Libshitz HI. Malignant pleural mesothelioma: the spectrum of manifestations on CT in 70 cases. Clin Radiol. 1999;54:415-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Cantó A, Guijarro R, Arnau A, Galbis J, Martorell M, García Aguado R. Videothoracoscopy in the diagnosis and treatment of malignant pleural mesothelioma with associated pleural effusions. Thorac Cardiovasc Surg. 1997;45:16-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 25] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Travis WD, Brambilla E, Burke AP, Marx A, Nicholson AG. Introduction to The 2015 World Health Organization Classification of Tumors of the Lung, Pleura, Thymus, and Heart. J Thorac Oncol. 2015;10:1240-1242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 411] [Cited by in RCA: 1095] [Article Influence: 109.5] [Reference Citation Analysis (0)] |
| 11. | van Zandwijk N, Clarke C, Henderson D, Musk AW, Fong K, Nowak A, Loneragan R, McCaughan B, Boyer M, Feigen M, Currow D, Schofield P, Nick Pavlakis BI, McLean J, Marshall H, Leong S, Keena V, Penman A. Guidelines for the diagnosis and treatment of malignant pleural mesothelioma. J Thorac Dis. 2013;5:E254-E307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 85] [Reference Citation Analysis (0)] |
| 12. | Zhou N, Rice DC, Tsao AS, Lee PP, Haymaker CL, Corsini EM, Antonoff MB, Hofstetter WL, Rajaram R, Roth JA, Swisher SG, Vaporciyan AA, Walsh GL, Mehran RJ, Sepesi B. Extrapleural Pneumonectomy Versus Pleurectomy/Decortication for Malignant Pleural Mesothelioma. Ann Thorac Surg. 2022;113:200-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 13. | Maione P, Rossi A, Di Maio M, Gridelli C. Tumor-related leucocytosis and chemotherapy-induced neutropenia: linked or independent prognostic factors for advanced non-small cell lung cancer? Lung Cancer. 2009;66:8-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Brundage MD, Davies D, Mackillop WJ. Prognostic factors in non-small cell lung cancer: a decade of progress. Chest. 2002;122:1037-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 418] [Cited by in RCA: 434] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 15. | Matsuoka K. Malignant pleural mesothelioma presenting as acute empyema with severe leukocytosis. Ann Thorac Cardiovasc Surg. 2014;20 Suppl:513-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Lapidot M, Mazzola E, Freyaldenhoven S, De León LE, Jaklitsch MT, Bueno R. Postoperative Empyema After Pleurectomy Decortication for Malignant Pleural Mesothelioma. Ann Thorac Surg. 2022;114:1214-1219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Javidfar J, Sonett JR, Ginsburg ME, Miller J, Hare E, Bacchetta M, Fawwaz R, Taub RN. Repeated ambulatory intrapleural chemotherapy for malignant pleural mesothelioma. J Clinic Oncol. 2011;29 (15_suppl):7088-7088. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 18. | Weder W, Stahel RA, Bernhard J, Bodis S, Vogt P, Ballabeni P, Lardinois D, Betticher D, Schmid R, Stupp R, Ris HB, Jermann M, Mingrone W, Roth AD, Spiliopoulos A; Swiss Group for Clinical Cancer Research. Multicenter trial of neo-adjuvant chemotherapy followed by extrapleural pneumonectomy in malignant pleural mesothelioma. Ann Oncol. 2007;18:1196-1202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 222] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 19. | Muers MF, Stephens RJ, Fisher P, Darlison L, Higgs CM, Lowry E, Nicholson AG, O'Brien M, Peake M, Rudd R, Snee M, Steele J, Girling DJ, Nankivell M, Pugh C, Parmar MK; MS01 Trial Management Group. Active symptom control with or without chemotherapy in the treatment of patients with malignant pleural mesothelioma (MS01): a multicentre randomised trial. Lancet. 2008;371:1685-1694. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 196] [Cited by in RCA: 173] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 20. | Bille A, Belcher E, Raubenheimer H, Landau D, Cane P, Spicer J, Lang-Lazdunski L. Induction chemotherapy, extrapleural pneumonectomy, and adjuvant radiotherapy for malignant pleural mesothelioma: experience of Guy's and St Thomas' hospitals. Gen Thorac Cardiovasc Surg. 2012;60:289-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |