Published online Dec 16, 2023. doi: 10.12998/wjcc.v11.i35.8330
Peer-review started: September 13, 2023
First decision: November 1, 2023
Revised: November 12, 2023
Accepted: November 28, 2023
Article in press: November 28, 2023
Published online: December 16, 2023
Processing time: 91 Days and 11.5 Hours
Heart failure (HF), an end-stage manifestation of various cardiac diseases, poses an enormous economic and health burden on society. Vericiguat may be an effective drug in the treatment of HF.
To explore by meta-analysis the efficacy and safety of Vericiguat in treating chronic heart failure.
Databases, including PubMed, EMBASE, Web of Science, and Cochrane Library, were searched to collect all published randomized controlled trials (RCTs) on Vericiguat treatment of chronic heart failure from the earliest electronic records to those published in March 2023. Two investigators independently screened the literature according to inclusion and exclusion criteria, evaluated the quality of the studies, and extracted valid data before conducting a meta-analysis using RevMan5.4.
Four RCTs with 5919 patients were included, and the meta-analysis showed that treatment with 10 mg Vericiguat reduced the incidence of the primary endpoint (a composite of cardiovascular mortality and first heart-failure-related hospitalization) in patients with chronic heart failure compared to placebo [relative risk (RR) = 0.91, 95% confidence interval (CI): 0.85–0.98, P = 0.01], and reduced the incidence of heart-failure-related hospitalization (RR = 0.92, 95%CI: 0.84–1.00, P = 0.05). However, for the incidence of cardiovascular and all-cause death, there were no significant differences between the Vericiguat and placebo groups. In addition, the two groups did not show significant differences in blood pressure, heart rate, and Kansas Cardiomyopathy Questionnaire physical limitation score. In terms of safety, 10 mg Vericiguat did not increase the risk of adverse effects in patients with chronic heart failure. Vericiguat may increase the risk of symp
Vericiguat (10 mg) was more effective than placebo in treating patients with chronic heart failure and had a better safety profile.
Core Tip: Heart failure is a major public health problem and a leading cause of mortality worldwide. Vericiguat may be a novel drug effective in slowing the progression of heart failure. We performed a meta-analysis and found that commonly used doses of Vericiguat resulted in a significant benefit in heart failure patients with reduced ejection fraction. Further studies are needed to confirm the long-term efficacy and safety of Vericiguat in heart failure.
- Citation: Yang H, Luo C, Lan WQ, Tang YH. Vericiguat treatment of heart failure: A systematic review and meta-analysis. World J Clin Cases 2023; 11(35): 8330-8342
- URL: https://www.wjgnet.com/2307-8960/full/v11/i35/8330.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i35.8330
Chronic heart failure represents the advanced stage of many cardiac diseases and stands as one of the most prevalent chronic disorders on a global scale[1,2]. Heart failure patients with reduced ejection fraction face a 5-year mortality rate of around 50%[3]. The worldwide population affected by heart failure amounts to an estimated 64.3 million individuals, and this number continues to rise due to escalating risk factors and a younger demographic profile[4]. The substantial rates of hospitalization and mortality among heart failure patients impose a considerable burden on both families and society. Despite advances in heart failure treatment and the standardization of management strategies, chronic heart failure remains a major public health concern due to its high prevalence and poor prognosis.
Vericiguat, an agonist targeting soluble guanylyl cyclase (sGC), has been used to treat heart failure characterized by reduced ejection fraction[5]. The nitric oxide-guanosine cyclase-cyclic GMP pathway (NO-sGC-cGMP) has essential regulatory functions in the cardiovascular system. Under normal physiological conditions, endothelial cells release NO, which binds to sGC, thereby catalyzing the production of the second messenger cGMP[6,7]. This process regulates normal cardiac and vascular functions. However, in the context of heart failure, endothelial dysfunction and inflammatory responses contribute to reduced NO production and bioavailability, subsequently causing microcirculatory disturbances that may exacerbate cardiac fibrosis and the progression of heart failure[8,9]. Vericiguat exhibits cardiovascular benefits by directly activating sGC and promoting independent cGMP production regardless of NO levels. In 2020, the VICTORIA clinical trial results were published by the American College of Cardiology, demonstrating that Vericiguat reduced the incidence of the composite endpoint event (cardiovascular death or initial hospitalization for heart failure) by 10% in patients with heart failure characterized by reduced ejection fraction[5]. In January 2021, Vericiguat received official marketing approval from the US FDA. To offer evidence-based medical support for clinical practice, we conducted a meta-analysis to explore the effectiveness and safety of Vericiguat in heart failure treatment.
Inclusion criteria: (1) Randomized controlled trials (RCTs) evaluating the efficacy of Vericiguat for treatment of chronic heart failure; (2) enrollment of patients with a definitive diagnosis of chronic heart failure, including both heart failure with preserved ejection fraction (HFpEF) and heart failure with reduced ejection fraction (HFrEF); (3) The trial group received Vericiguat at a maintenance dose of 10 mg, while the control group was administered a placebo or other control treatments; and (4) study considered various outcome indicators, encompassing effectiveness measures such as cardiovascular death, heart-failure-related hospitalization, and changes in cardiac function. Safety indicators, such as symptomatic hypotension and syncope, were also assessed. Exclusion criteria were: (1) Repetitive publications or duplicate data within the literature; (2) non-RCT study types, including reviews, case reports, and basic animal experiments; (3) literature lacking essential information regarding the dose of pharmacological interventions, implementation methods, types of controls, or duration of follow-up; and (4) literature with incomplete or ambiguous data that could be rectified through various means to obtain valid data.
A comprehensive computerized search was conducted across several databases, including Pubmed, EMBASE, Web of Science, and Cochrane Library, to identify literature pertaining to the utilization of Vericiguat for the treatment of chronic heart failure. The search encompassed articles published from the inception of the databases to March 2023. The search terms utilized combined subject terms with free words, incorporating the following: Vericiguat, Verquvo, BAY 1021189, Heart Failure, Cardiac Failure, Heart Decompensation, Myocardial Failure, and Heart Failure. Furthermore, terms such as Myocardial Failure, HFrEF, HFpEF, and CHF were used to refine the search. Lastly, terms related to RCTs, such as Randomised control trial, Randomised, and RCT, were included to ensure relevant studies were identified.
The process of literature screening was conducted independently by two researchers. In instances where discrepancies arose, the final decision was reached through collaborative discussions between the two screeners or by seeking consultation with a third-party expert. Relevant data pertaining to RCTs were documented, encompassing essential information such as the study name, publication date, treatment methodology, number of participants, age distribution, gender ratio, treatment duration, follow-up period, and outcome measures. Following the guidelines presented in the Cochrane Risk of Bias Assessment Tool, the risk of bias associated with the included RCT studies was evaluated across six dimensions.
The statistical analysis used Revman 5.4 software to perform a meta-analysis. The weighted mean difference and corresponding 95% confidence interval (95%CIs) were calculated for continuous variables. Dichotomous variables were analyzed using relative risk (RR), accompanied by a 95%CI for interval estimation. In the event that there was minimal heterogeneity observed between the studies (indicated by a Q test P value > 0.10 and I2 < 50%), the fixed-effects model was used to combine the effect sizes. Conversely, when substantial heterogeneity was observed among studies (Q test P value ≤ 0.10 and I2 ≥ 50%), sensitivity or subgroup analyses were conducted to identify the source of heterogeneity. The random-effects model was utilized to combine the data. Statistical significance was considered if the Z-test P value was < 0.05.
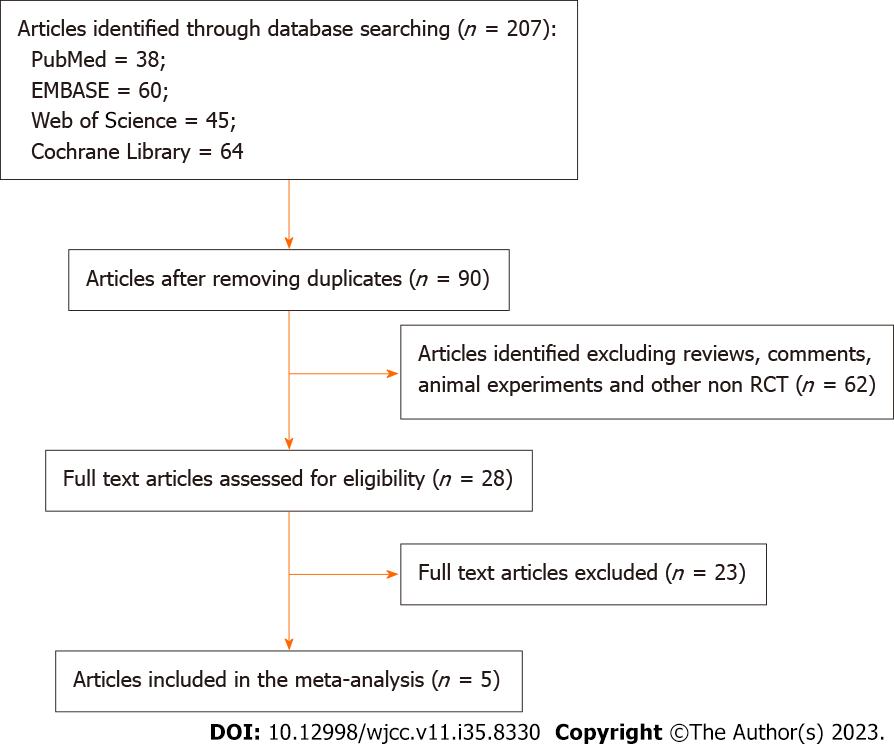
A comprehensive literature search was conducted, resulting in the identification of 207 relevant articles. Following the removal of 179 duplicates and non-RCTs, a total of 28 articles were selected for full-text examination. Among these, four clinical studies comprising five articles were included for analysis[5,10-13] (Figure 1).
The four RCTs encompassed a collective sample size of 5919 patients. These studies exhibited varying sample sizes, ranging from 161 to 5050 participants. Furthermore, the mean age of the patients enrolled in the studies ranged from 67.3 to 73 years, with the female population constituting a proportion ranging from 18% to 46.7%. Considering the prescribed maintenance dose of Vericiguat, which stands at 10 mg, the analysis focused solely on data pertaining to the 10 mg Vericiguat group and the corresponding control group. For comprehensive details, please refer to Table 1.
| Included study | Year | Sample size | Mean age | Female (%) | Treatment regimen | Control group | Mean follow up duration (wk) | Patients |
| VITALITY-HFpEF | 2020 | 525 | 72.5 | 46.7 | 2.5-10 mg, QD | Placebo | 24 | EF ≥ 45%, HFpEF |
| SOCRATES-PRESERVED | 2017 | 161 | 73 | 46.0 | 2.5-10 mg, QD | Placebo | 12 | EF ≥ 45%, HFpEF |
| SOCRATES-REDUCED | 2015 | 183 | 68 | 18.0 | 2.5-10 mg, QD | Placebo | 12 | EF < 45%, HFrEF |
| VICTORIA | 2020 | 5050 | 67.3 | 23.9 | 2.5-10 mg, QD | Placebo | 43 | EF < 45%, HFrEF |
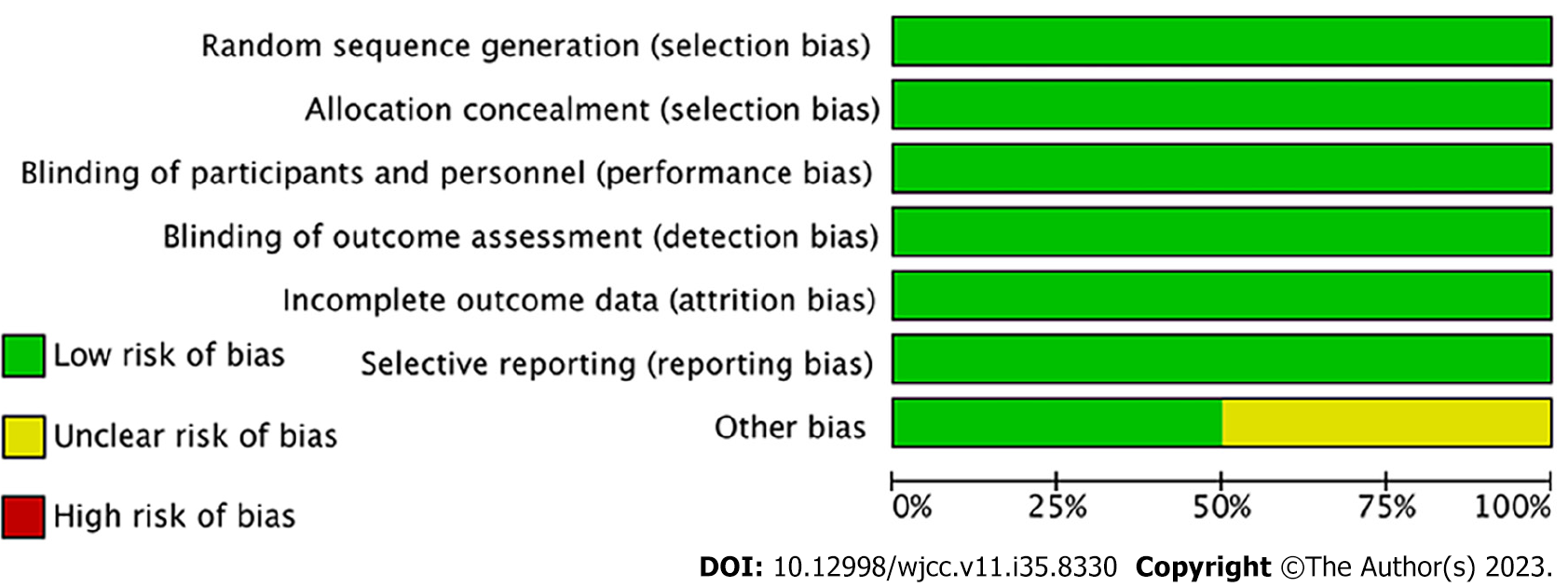
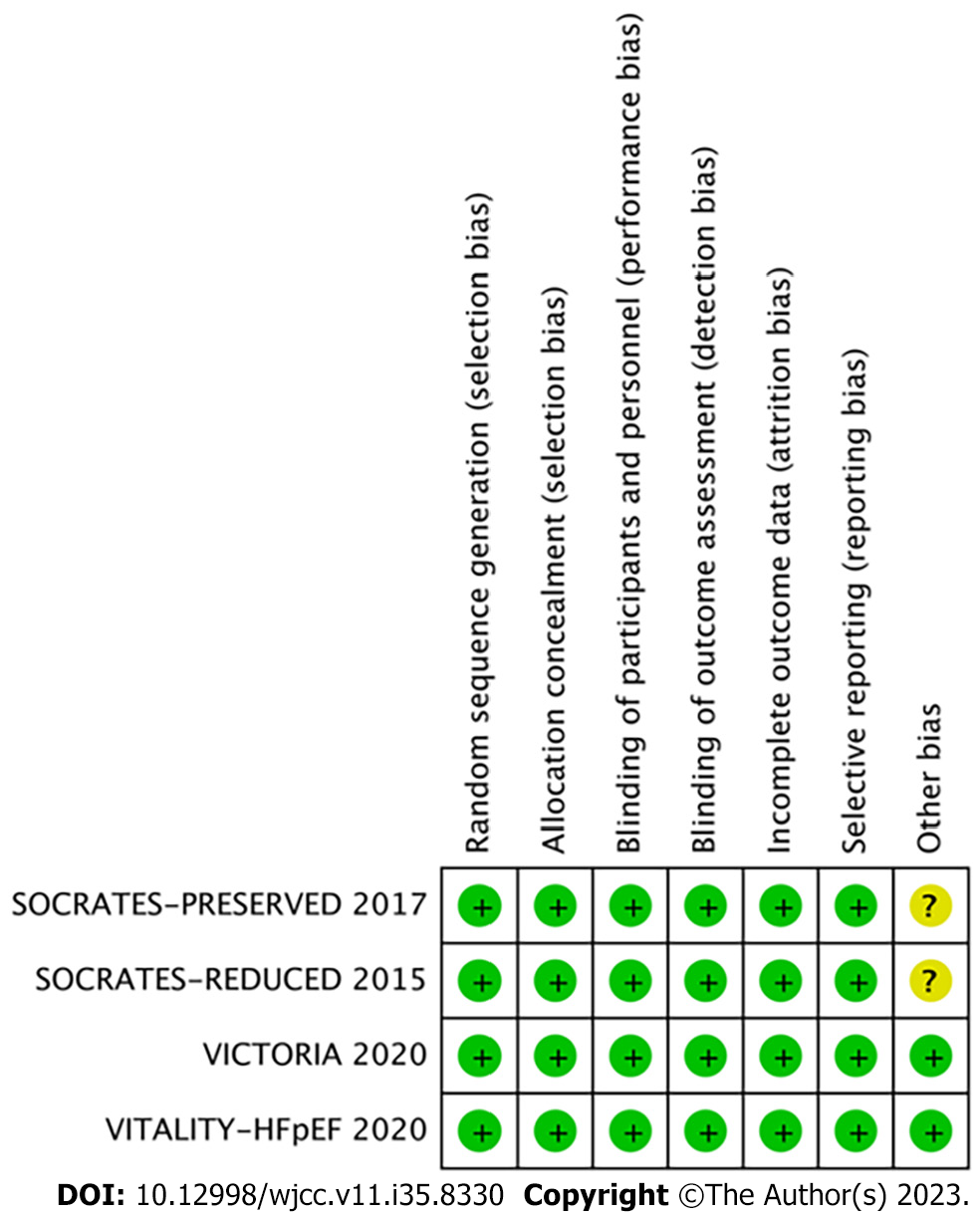
All four studies incorporated in this analysis were characterized as multicenter, randomized, double-blind, and placebo-controlled trials. Methodological appraisal of the included studies indicated a high level of quality, with bias scores ranging from 6 to 7. The evaluation of potential bias across the studies was visually represented using RevMan 5.4, as illustrated in Figures 2 and 3.
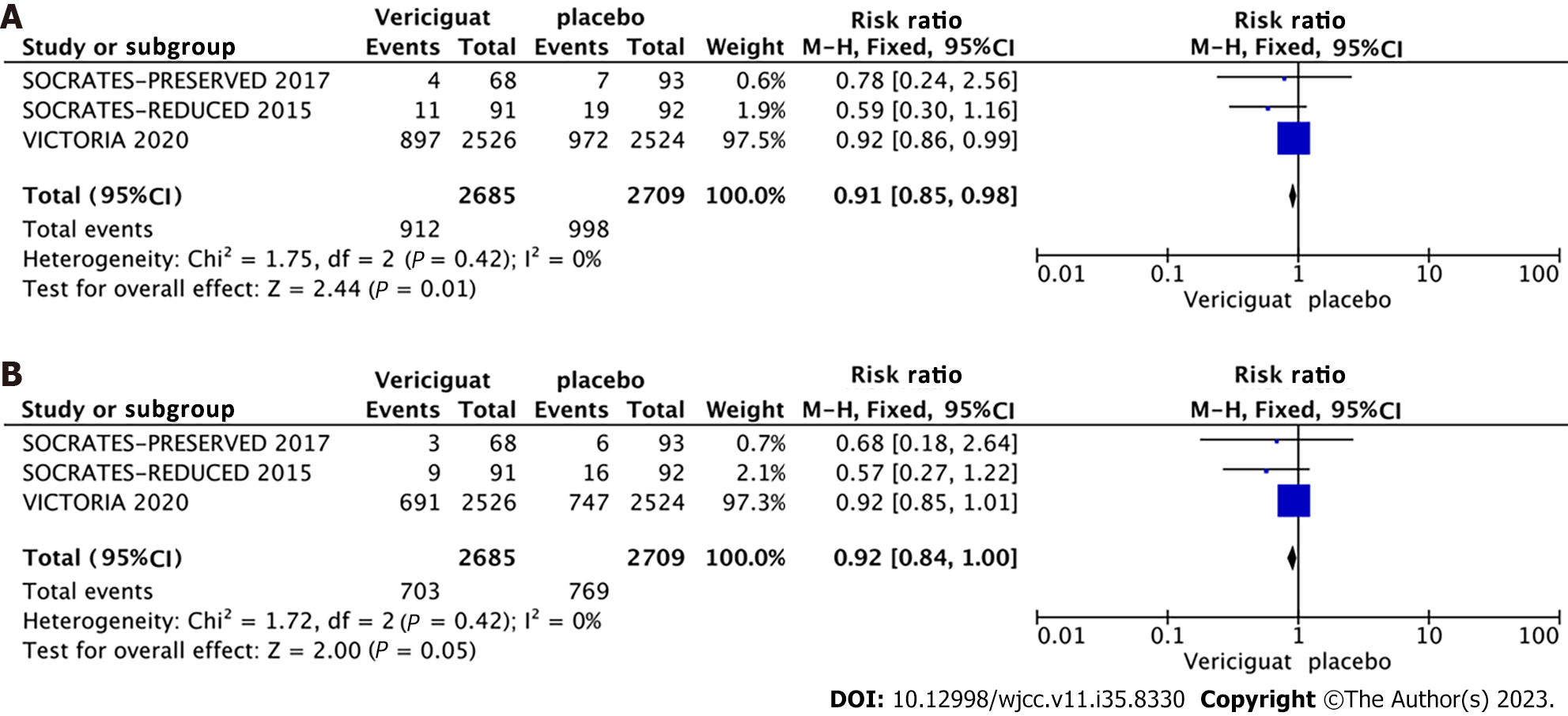
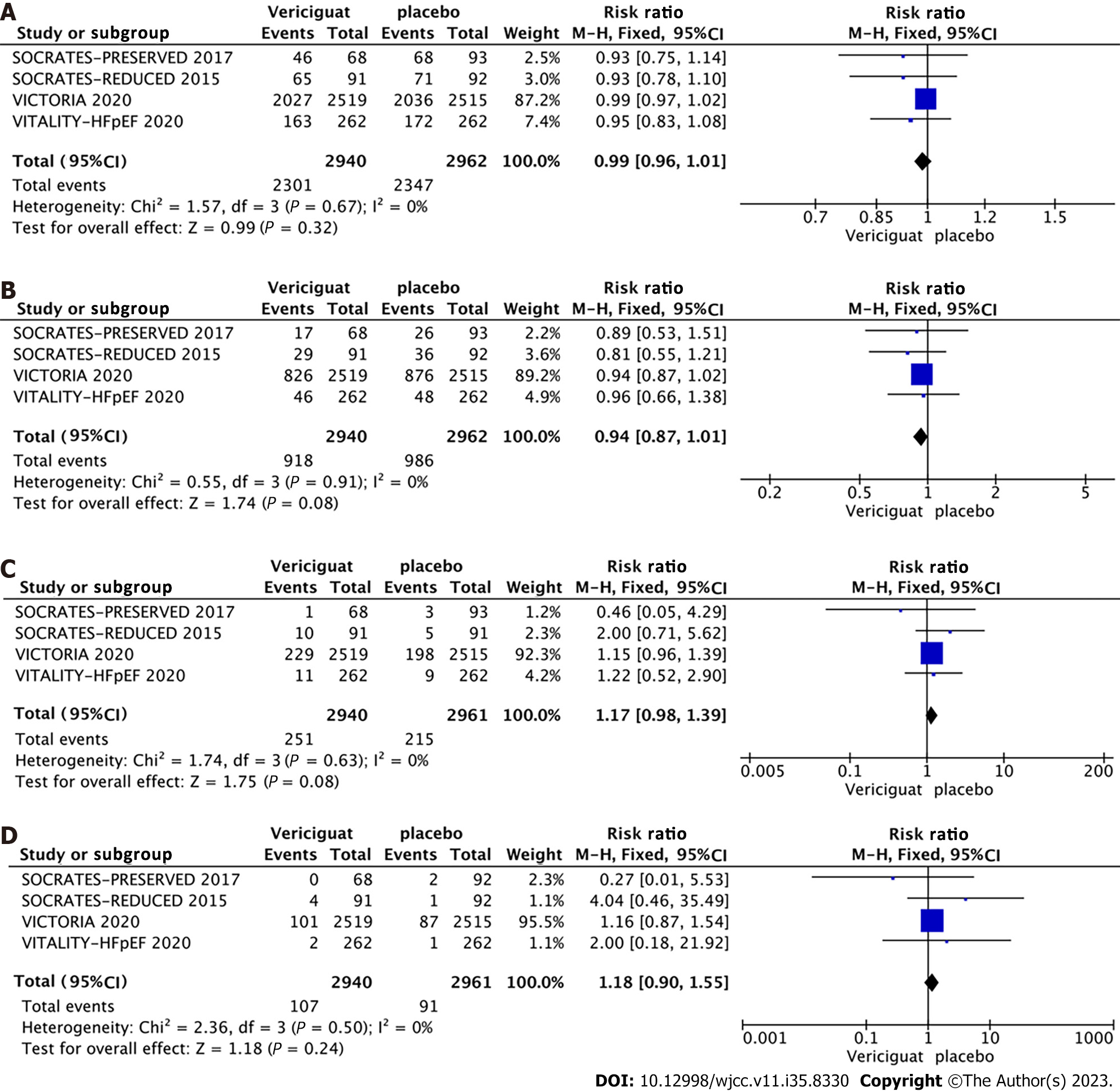
Primary outcomes: A combined effect size analysis and generation of forest plots for each outcome indicator were performed using the RevMan 5.4 software. Among the included studies, three reported the composite outcome event comprising cardiovascular death or initial hospitalization due to heart failure, three studies reported hospitalization specifically for heart failure (Figure 4), and four studies reported the outcomes of cardiovascular death and all-cause mortality (Figure 5). A fixed-effects model indicated by a heterogeneity test displaying I2 < 50% and a Q-test with P > 0.1, the meta-analysis revealed a 9% reduction in the rate of the composite endpoint event for patients in the 10-mg Vericiguat group compared to the placebo group (RR = 0.91, 95%CI: 0.85–0.98, P = 0.01) (Figure 4A). Additionally, there was an 8% reduction in hospitalizations for heart failure (RR = 0.92, 95%CI: 0.84–1.00, P = 0.05) (Figure 4B); both of which were significant. However, for cardiovascular death (Figure 5A) and all-cause mortality events (Figure 5B) between the two groups, the differences were not significant.
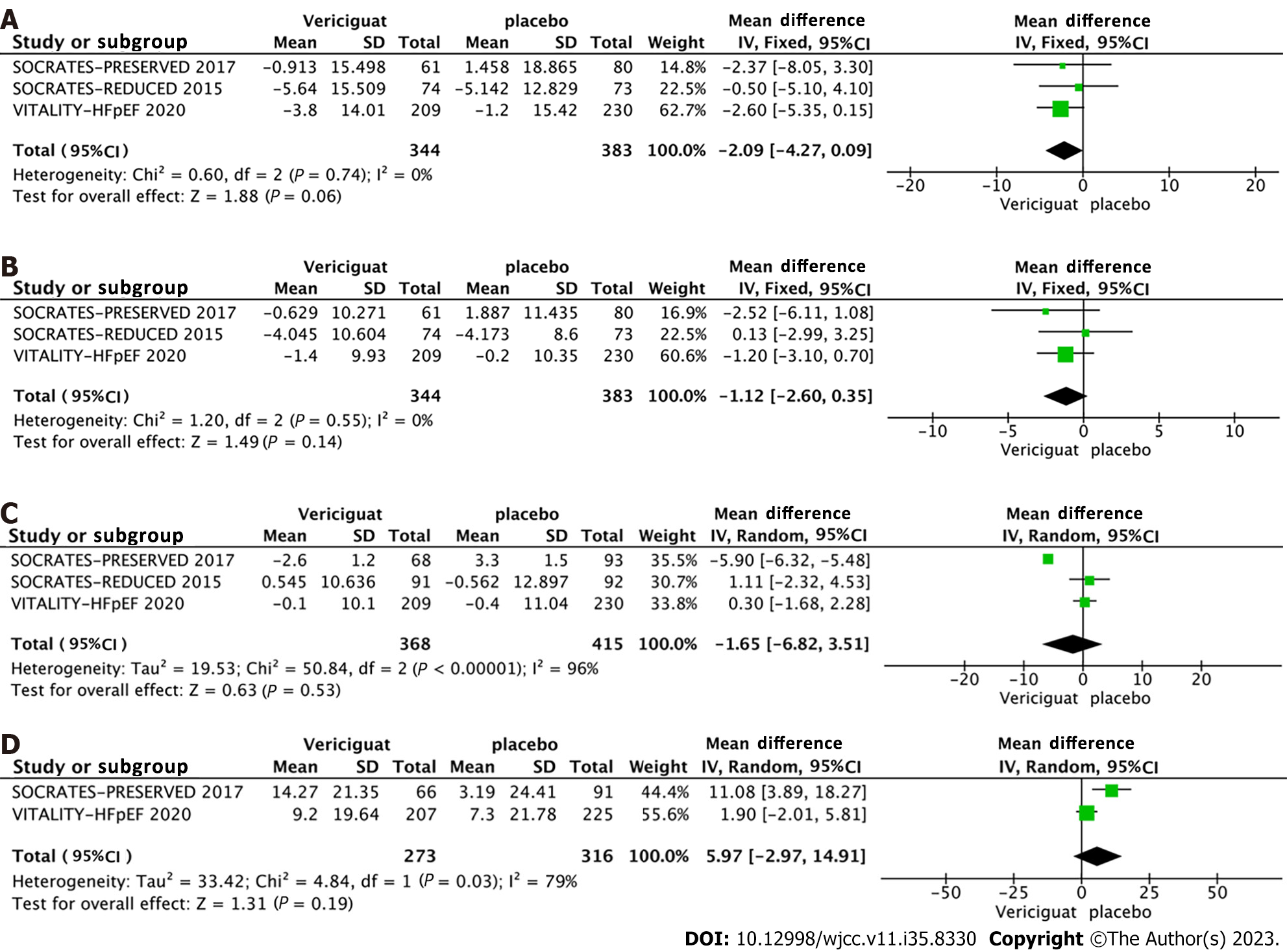
Other relevant outcomes: Regarding other pertinent indicators, three studies reported changes in systolic blood pressure, diastolic blood pressure, and heart rate at the end of the follow-up period for both groups. Two studies reported the changes in the Kansas Cardiomyopathy Questionnaire (KCCQ) physical limitation score (PLS) (Figure 6). For systolic blood pressure and diastolic blood pressure, no significant heterogeneity was observed among the studies (I2 = 0, Q-test P > 0.1), thus a fixed-effects model was used for analysis. Conversely, significant heterogeneity was identified across the studies for heart rate and KCCQ PLS indicators (I2 > 50%, Q-test P < 0.1), necessitating the use of a random-effects model for analysis. The findings demonstrated that the administration of 10 mg Vericiguat did not significantly influence systolic blood pressure (Figure 6A), diastolic blood pressure (Figure 6B), heart rate (Figure 6C), or KCCQ PLS (Figure 6D).
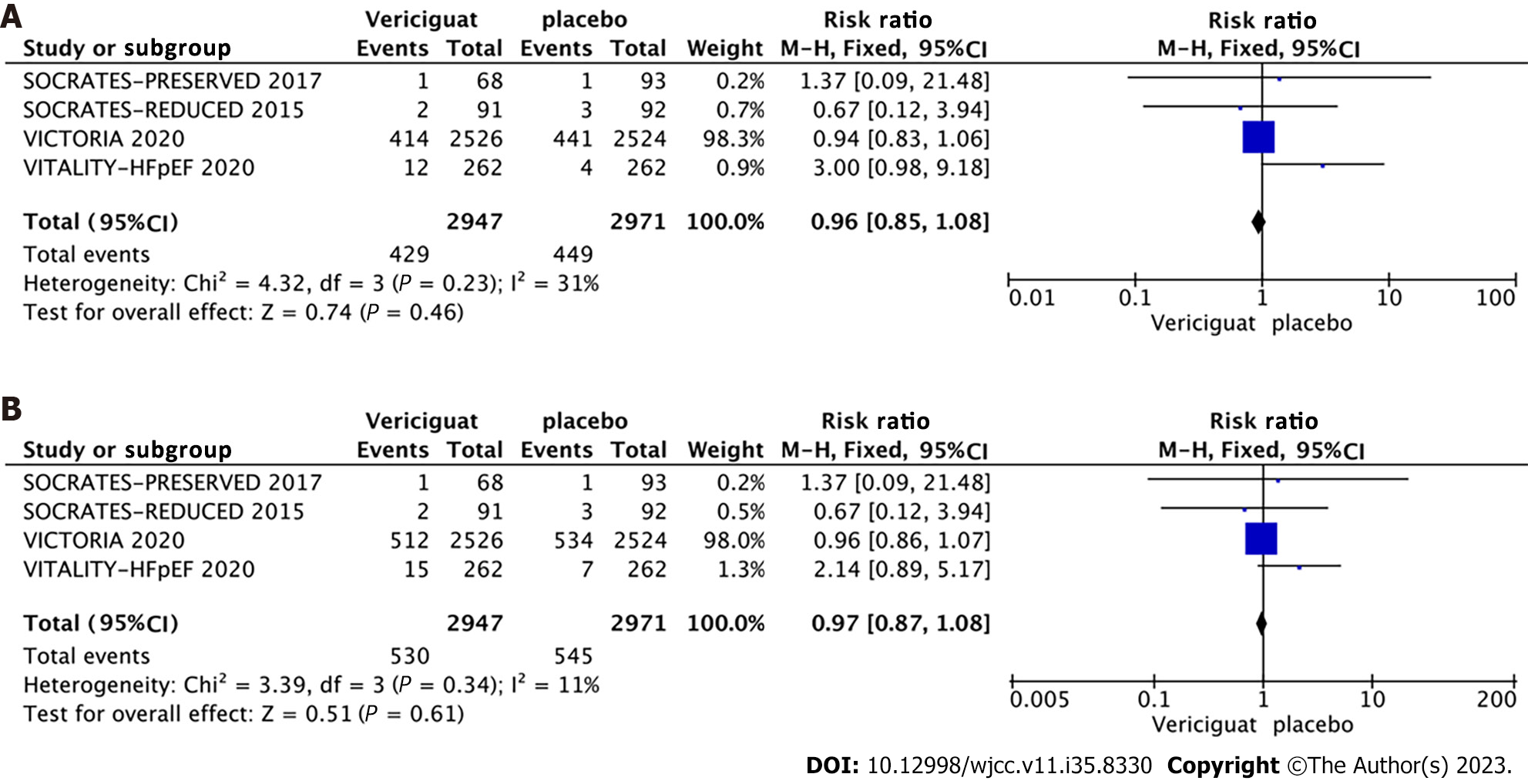
Safety outcomes: All four studies included in the analysis provided data on adverse events, serious adverse events, symptomatic hypotension, and syncope for both treatment groups. The studies did not exhibit significant heterogeneity
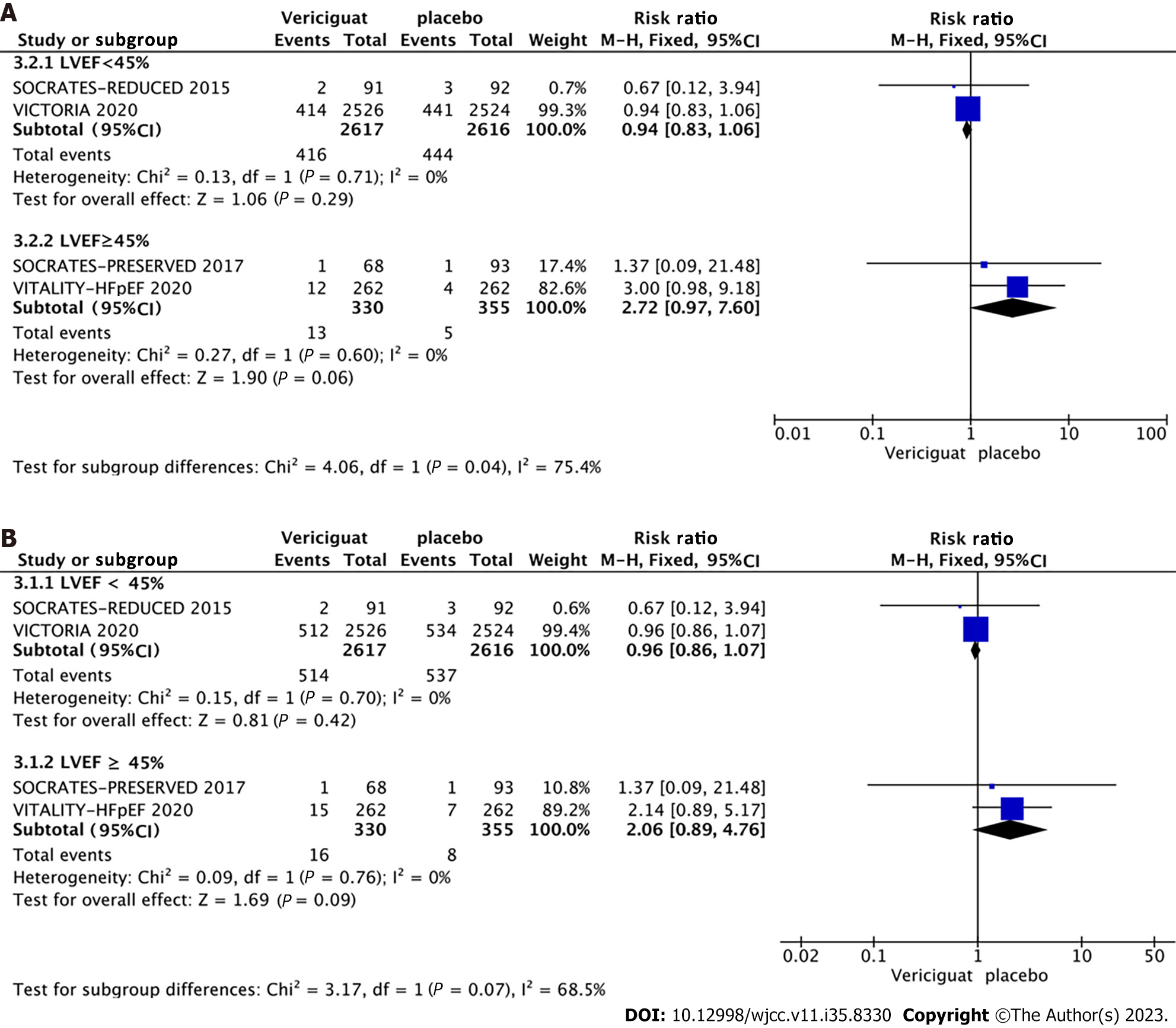
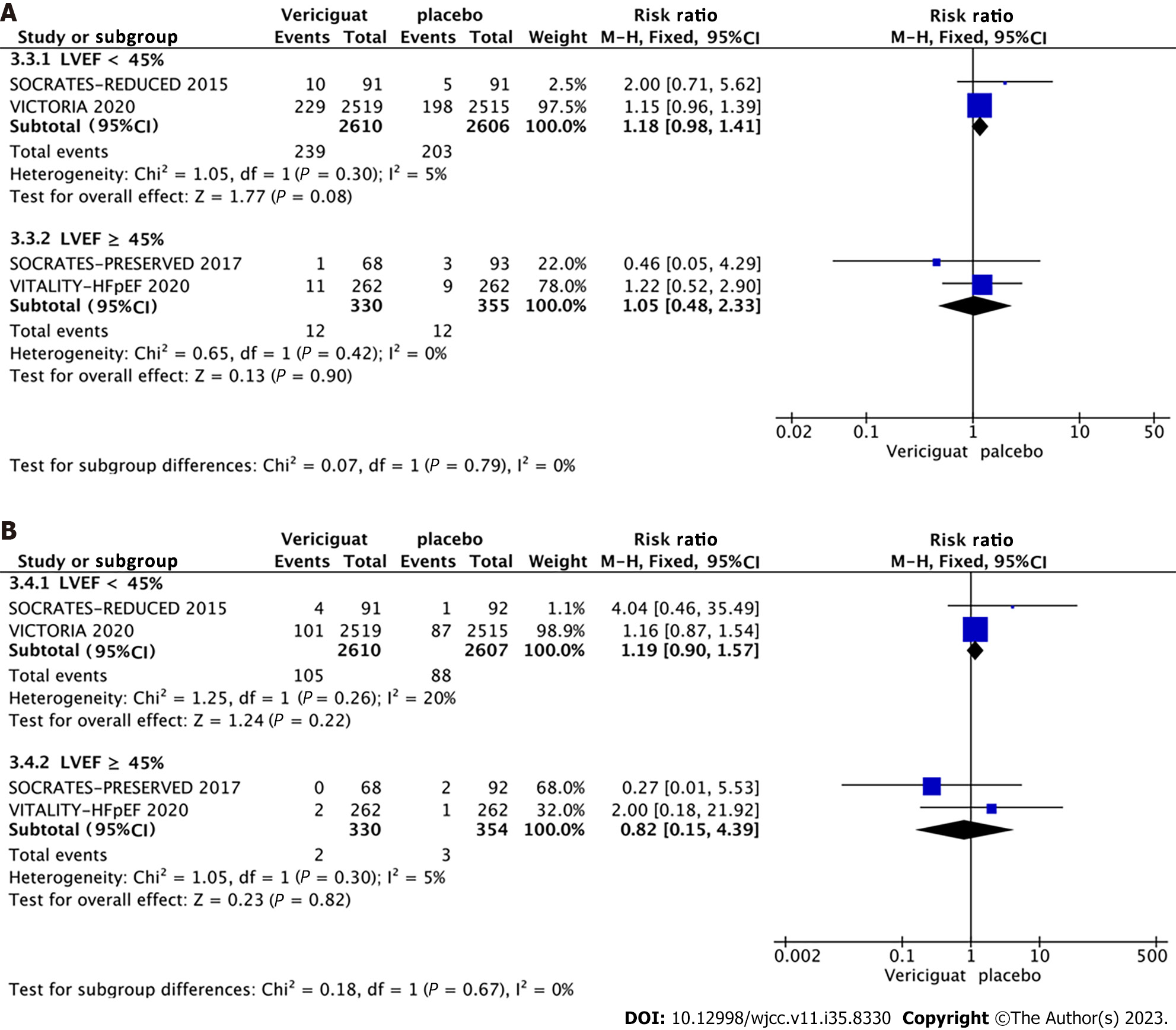
Among the four included RCTs, two involved patients with HFrEF (LVEF < 40%), while the remaining two included patients with HFpEF (LVEF ≥ 40%) (Figure 8A and 8B). Accordingly, the studies were categorized into two groups based on LVEF (< 40% and ≥ 40%), and subgroup analyses were conducted to assess the occurrence of cardiovascular death, all-cause mortality, symptomatic hypotension, and syncope. The combined effect sizes demonstrated that in the LVEF < 40% subgroup, treatment with 10 mg Vericiguat reduced the risk of cardiovascular death (RR = 0.94, 95%CI: 0.83–1.06, P = 0.29) (Figure 8A) and all-cause mortality (RR = 0.96, 95%CI: 0.86–1.07, P = 0.42) (Figure 8B) by 6% and 4%, respectively. In the LVEF ≥ 40% subgroup, patients in the Vericiguat group demonstrated a 2.72-fold and 2.06-fold increased risk of cardiovascular death (RR = 2.72, 95%CI: 0.97–7.60, P = 0.06) and all-cause mortality (RR = 2.06, 95%CI: 0.89–4.76, P = 0.09), respectively, although these results did not reach significance. Furthermore, administration of 10 mg Vericiguat did not elevate the risk of symptomatic hypotension (Figure 9A) or syncope (Figure 9B) in either subgroup.
Given the inclusion of only four RCTs in this study, it as challenging to conclusively ascertain the presence of publication bias. However, to provide a visual representation of the potential publication bias, a funnel plot was generated using RevMan5.4 (Figure 10).
Chronic heart failure encompasses a range of severe clinical syndromes that can develop from various causes of cardiac injury or persistent exacerbation of cardiac dysfunction. Despite receiving standardized treatment regimens for heart failure, patients often face a grim prognosis, with a significant portion succumbing to worsening heart failure or sudden cardiac death. Studies have revealed that within 1 year of diagnosis, up to 40% of patients with symptomatic heart failure die, and within 5 years, the mortality rate reaches 60%–70%[14,15]. Given the substantial economic and health burden imposed on society by chronic heart failure, numerous medical practitioners and researchers have been dedicated to the exploration of drugs or methods capable of enhancing heart failure prognosis. Over the past decade, substantial progress has been made in therapeutic approaches and management options for heart failure, transitioning from the traditional “golden triangle” (comprising β-blockers, angiotensin-converting enzyme inhibitors/angiotensin II receptor antagonists, and aldosterone receptor antagonists) to the current new quadruple therapy (involving a renin-angiotensin system inhibitor, β-blocker, aldosterone receptor antagonist, and SGLT2i)[16-18]. Within this context, Vericiguat, a novel sGC agonist, has exhibited promising efficacy in heart failure patients with reduced EF.
This study encompassed four RCTs evaluating the impact of a 10 mg dose of Vericiguat on chronic heart failure treatment. The combined patient count for these trials was 5919, and all studies adhered to rigorous standards as multicenter, randomized, double-blind, placebo-controlled trials. Notably, aside from heart rate and KCCQ PLS, other measured variables did not exhibit significant heterogeneity. The findings indicated that a 10 mg dose of Vericiguat resulted in a 9% reduction in the incidence of composite endpoint events (cardiovascular death or first heart-failure-related hospitalization) and an 8% decrease in the risk of hospitalization due to heart failure among patients with heart failure. However, statistical significance was not observed regarding the risk of cardiovascular death or all-cause mortality. To investigate further, subgroup analyses were conducted based on LVEF criteria. Two of the four RCTs[5,12] included patients with chronic heart failure and LVEF < 40%, while the remaining two[10,11,13] enrolled patients with LVEF ≥ 45%. These subgroup analyses indicated that, although not reaching statistical significance, the administration of 10 mg Vericiguat may result in a 2.72- and 2.06-fold increase in the risk of cardiovascular death and all-cause mortality, respectively, among patients with LVEF ≥ 40%. In the LVEF < 40% group, a 6% reduction in the risk of cardiovascular death and a 4% reduction in the risk of all-cause mortality were observed, suggesting a potentially more pronounced benefit of Vericiguat in patients with HFrEF.
The pharmacokinetic properties of Vericiguat, an oral medication used in the treatment of heart failure, have been extensively investigated. It exhibits a half-life of up to 30 h in patients with heart failure, necessitating only once-daily administration. With a high bioavailability of approximately 96%, Vericiguat achieves steady-state blood concentration within approximately 6 h following a 10-mg oral dose. Coadministration with food has been shown to increase both the area under the blood concentration curve and the maximal blood concentration of Vericiguat by 44% and 41%, respectively. Metabolically, over half of Vericiguat is eliminated as inactive metabolites through renal excretion, while nearly half is excreted in its original form through feces. This unique pharmacokinetic and pharmacogenetic profile may account for the modest impact of Vericiguat on blood pressure in heart failure patients. A meta-analysis evaluating the effects of 10 mg Vericiguat on symptomatic hypotension and syncope risk in heart failure patients revealed no significant increase in these adverse events. Moreover, no significant differences in adverse effects or severe adverse reactions were observed between the Vericiguat and control groups.
Notably, two phase II clinical studies demonstrated good tolerability of Vericiguat in patients with HFrEF or HFpEF[11,13]. The VITALITY-HFpEF trial[10] introduced a 15-mg Vericiguat group for individuals with HFpEF, but no significant improvements in the KCCQ PLS or 6-min walking distance were observed at the end of the 24-wk follow-up. Nevertheless, the higher dose of Vericiguat did not increase the incidence of adverse events, suggesting a favorable safety profile. In the phase III clinical trial, VICTORIA study, it was noted that 10 mg Vericiguat elevated the risk of anemia at the 16-week follow-up (7.6% vs 5.7%, P < 0.01)[5]. However, no further decrease in hemoglobin levels was observed after 96 w of follow-up, necessitating further investigation into the actual impact of Vericiguat on anemia through observational studies[19]. Additionally, researchers examined the combined use of Vericiguat with warfarin and aspirin in a healthy population and found no significant alterations in the pharmacokinetic profile of Vericiguat. Vericiguat did not augment the bleeding risk associated with warfarin or aspirin administration[20,21].
The efficacy and safety of Vericiguat in the treatment of chronic heart failure were investigated in the VICTORIA study; a rigorous clinical phase III trial[5]. The study demonstrated that 10 mg of Vericiguat resulted in a 10% reduction in the incidence of cardiovascular death or the composite endpoint of heart-failure-related hospitalization in patients with heart failure and LVEF < 45%. Moreover, no additional adverse effects were observed. Subgroup analyses of the VICTORIA trial were conducted to explore the role of Vericiguat in patients with concurrent coronary artery disease, atrial fibrillation, renal insufficiency, or varying levels of N-Terminal Pro-Brain Natriuretic Peptide (NT-proBNP)[22-25]. The results revealed that patients with comorbid coronary artery disease, atrial fibrillation, or renal insufficiency had a poorer prognosis but did not experience a diminished benefit from Vericiguat in managing heart failure. Vericiguat exhibited a more pronounced ability to reduce the composite endpoint event in patients with NT-proBNP levels £8000 pg/mL. Although the VICTORIA study indicated a similar risk of symptomatic hypotension between the Vericiguat and placebo groups, post hoc analyses were conducted to assess the risk in patients predisposed to hypotension, such as those aged > 75 years, patients with a baseline systolic blood pressure 100-110 mmHg, and patients receiving a combination of angiotensin receptor neprilysin inhibitor. Vericiguat did not significantly increase the risk of hypotension in these patients. Moreover, these patients did not exhibit a substantial increase in the risk of symptomatic hypotension, and the benefits of Vericiguat on the primary composite outcome remained consistent regardless of the baseline systolic blood pressure[26].
There were several limitations to this study. Although the quality of the included studies was high, the number was small. Additionally, there was considerable variation in the sample sizes of each study, making it challenging to assess publication bias accurately. Finally, the guidelines recommend incorporating Vericiguat in patients with symptomatic HFrEF, yet only two studies included heart failure patients with LVEF < 45%, resulting in a limited amount of data. Consequently, the real-world effect of Vericiguat needs to be observed more comprehensively.
Administration of 10 mg Vericiguat has favorable efficacy and tolerability among patients with chronic heart failure, particularly in those with HFrEF, with LVEF < 45%. However, additional research is warranted to thoroughly examine the long-term effectiveness and safety profile of Vericiguat.
Heart failure (HF) causes a substantial socioeconomic burden, and there is an urgent need for effective treatment.
Vericiguat may be an effective therapeutic agent for HF. This study aims to investigate the benefits of Vericiguat in patients with chronic heart failure (CHF).
To assess the efficacy and safety of Vericiguat in the treatment of CHF.
We searched for randomized controlled trials of Vericiguat for the treatment of CHF and performed meta-analyses using Revman 5.4 and the Cochrane Handbook.
A total of 5919 patients were enrolled in this study. The results showed that Vericiguat treatment reduced the composite endpoints of cardiovascular mortality and first HF-related hospitalization [RR = 0.91, 95%CI: (0.85, 0.98), P = 0.01] and the risk of HF-related hospitalizations [RR = 0.92, 95%CI: (0.84, 1.00), P = 0.05] in patients with CHF. Vericiguat did not increase the risk of adverse reactions and serious adverse events.
Vericiguat is effective and safe in treating heart failure patients with reduced ejection fraction.
Current therapeutic measures for CHF are improving, but patients still have a dismal prognosis. As a novel therapeutic agent for heart failure, Vericiguat may help to slow down the progression of heart failure and promote patient recovery.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Pharmacology and pharmacy
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ong H, Malaysia; Nashwan AJ, Qatar S-Editor: Liu JH L-Editor: A P-Editor: Yu HG
| 1. | Metra M, Tomasoni D, Adamo M, Bayes-Genis A, Filippatos G, Abdelhamid M, Adamopoulos S, Anker SD, Antohi L, Böhm M, Braunschweig F, Gal TB, Butler J, Cleland JGF, Cohen-Solal A, Damman K, Gustafsson F, Hill L, Jankowska EA, Lainscak M, Lund LH, McDonagh T, Mebazaa A, Moura B, Mullens W, Piepoli M, Ponikowski P, Rakisheva A, Ristic A, Savarese G, Seferovic P, Sharma R, Tocchetti CG, Yilmaz MB, Vitale C, Volterrani M, von Haehling S, Chioncel O, Coats AJS, Rosano G. Worsening of chronic heart failure: definition, epidemiology, management and prevention. A clinical consensus statement by the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2023;25:776-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 95] [Reference Citation Analysis (0)] |
| 2. | Roger VL. Epidemiology of Heart Failure: A Contemporary Perspective. Circ Res. 2021;128:1421-1434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 509] [Article Influence: 127.3] [Reference Citation Analysis (0)] |
| 3. | Batra G, Aktaa S, Benson L, Dahlström U, Hage C, Savarese G, Vasko P, Gale CP, Lund LH. Association between heart failure quality of care and mortality: a population-based cohort study using nationwide registries. Eur J Heart Fail. 2022;24:2066-2077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 4. | GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789-1858. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9354] [Cited by in RCA: 8390] [Article Influence: 1198.6] [Reference Citation Analysis (4)] |
| 5. | Armstrong PW, Pieske B, Anstrom KJ, Ezekowitz J, Hernandez AF, Butler J, Lam CSP, Ponikowski P, Voors AA, Jia G, McNulty SE, Patel MJ, Roessig L, Koglin J, O'Connor CM; VICTORIA Study Group. Vericiguat in Patients with Heart Failure and Reduced Ejection Fraction. N Engl J Med. 2020;382:1883-1893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 541] [Cited by in RCA: 835] [Article Influence: 167.0] [Reference Citation Analysis (0)] |
| 6. | Emdin M, Aimo A, Castiglione V, Vergaro G, Georgiopoulos G, Saccaro LF, Lombardi CM, Passino C, Cerbai E, Metra M, Senni M. Targeting Cyclic Guanosine Monophosphate to Treat Heart Failure: JACC Review Topic of the Week. J Am Coll Cardiol. 2020;76:1795-1807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 78] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 7. | Sandner P. From molecules to patients: exploring the therapeutic role of soluble guanylate cyclase stimulators. Biol Chem. 2018;399:679-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 64] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 8. | Ghionzoli N, Gentile F, Del Franco AM, Castiglione V, Aimo A, Giannoni A, Burchielli S, Cameli M, Emdin M, Vergaro G. Current and emerging drug targets in heart failure treatment. Heart Fail Rev. 2022;27:1119-1136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 9. | Marti CN, Gheorghiade M, Kalogeropoulos AP, Georgiopoulou VV, Quyyumi AA, Butler J. Endothelial dysfunction, arterial stiffness, and heart failure. J Am Coll Cardiol. 2012;60:1455-1469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 342] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 10. | Armstrong PW, Lam CSP, Anstrom KJ, Ezekowitz J, Hernandez AF, O'Connor CM, Pieske B, Ponikowski P, Shah SJ, Solomon SD, Voors AA, She L, Vlajnic V, Carvalho F, Bamber L, Blaustein RO, Roessig L, Butler J; VITALITY-HFpEF Study Group. Effect of Vericiguat vs Placebo on Quality of Life in Patients With Heart Failure and Preserved Ejection Fraction: The VITALITY-HFpEF Randomized Clinical Trial. JAMA. 2020;324:1512-1521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 208] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 11. | Filippatos G, Maggioni AP, Lam CSP, Pieske-Kraigher E, Butler J, Spertus J, Ponikowski P, Shah SJ, Solomon SD, Scalise AV, Mueller K, Roessig L, Bamber L, Gheorghiade M, Pieske B. Patient-reported outcomes in the SOluble guanylate Cyclase stimulatoR in heArT failurE patientS with PRESERVED ejection fraction (SOCRATES-PRESERVED) study. Eur J Heart Fail. 2017;19:782-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 82] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 12. | Gheorghiade M, Greene SJ, Butler J, Filippatos G, Lam CS, Maggioni AP, Ponikowski P, Shah SJ, Solomon SD, Kraigher-Krainer E, Samano ET, Müller K, Roessig L, Pieske B; SOCRATES-REDUCED Investigators and Coordinators. Effect of Vericiguat, a Soluble Guanylate Cyclase Stimulator, on Natriuretic Peptide Levels in Patients With Worsening Chronic Heart Failure and Reduced Ejection Fraction: The SOCRATES-REDUCED Randomized Trial. JAMA. 2015;314:2251-2262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 286] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 13. | Pieske B, Maggioni AP, Lam CSP, Pieske-Kraigher E, Filippatos G, Butler J, Ponikowski P, Shah SJ, Solomon SD, Scalise AV, Mueller K, Roessig L, Gheorghiade M. Vericiguat in patients with worsening chronic heart failure and preserved ejection fraction: results of the SOluble guanylate Cyclase stimulatoR in heArT failurE patientS with PRESERVED EF (SOCRATES-PRESERVED) study. Eur Heart J. 2017;38:1119-1127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 216] [Cited by in RCA: 275] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 14. | Ambrosy AP, Fonarow GC, Butler J, Chioncel O, Greene SJ, Vaduganathan M, Nodari S, Lam CSP, Sato N, Shah AN, Gheorghiade M. The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol. 2014;63:1123-1133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1248] [Cited by in RCA: 1571] [Article Influence: 142.8] [Reference Citation Analysis (0)] |
| 15. | Jones NR, Roalfe AK, Adoki I, Hobbs FDR, Taylor CJ. Survival of patients with chronic heart failure in the community: a systematic review and meta-analysis. Eur J Heart Fail. 2019;21:1306-1325. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 356] [Article Influence: 59.3] [Reference Citation Analysis (0)] |
| 16. | McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR; PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4078] [Cited by in RCA: 4741] [Article Influence: 431.0] [Reference Citation Analysis (0)] |
| 17. | McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, Böhm M, Chiang CE, Chopra VK, de Boer RA, Desai AS, Diez M, Drozdz J, Dukát A, Ge J, Howlett JG, Katova T, Kitakaze M, Ljungman CEA, Merkely B, Nicolau JC, O'Meara E, Petrie MC, Vinh PN, Schou M, Tereshchenko S, Verma S, Held C, DeMets DL, Docherty KF, Jhund PS, Bengtsson O, Sjöstrand M, Langkilde AM; DAPA-HF Trial Committees and Investigators. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N Engl J Med. 2019;381:1995-2008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2953] [Cited by in RCA: 4451] [Article Influence: 741.8] [Reference Citation Analysis (0)] |
| 18. | Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, Januzzi J, Verma S, Tsutsui H, Brueckmann M, Jamal W, Kimura K, Schnee J, Zeller C, Cotton D, Bocchi E, Böhm M, Choi DJ, Chopra V, Chuquiure E, Giannetti N, Janssens S, Zhang J, Gonzalez Juanatey JR, Kaul S, Brunner-La Rocca HP, Merkely B, Nicholls SJ, Perrone S, Pina I, Ponikowski P, Sattar N, Senni M, Seronde MF, Spinar J, Squire I, Taddei S, Wanner C, Zannad F; EMPEROR-Reduced Trial Investigators. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N Engl J Med. 2020;383:1413-1424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1875] [Cited by in RCA: 3183] [Article Influence: 636.6] [Reference Citation Analysis (0)] |
| 19. | Ezekowitz JA, Zheng Y, Cohen-Solal A, Melenovský V, Escobedo J, Butler J, Hernandez AF, Lam CSP, O'Connor CM, Pieske B, Ponikowski P, Voors AA, deFilippi C, Westerhout CM, McMullan C, Roessig L, Armstrong PW. Hemoglobin and Clinical Outcomes in the Vericiguat Global Study in Patients With Heart Failure and Reduced Ejection Fraction (VICTORIA). Circulation. 2021;144:1489-1499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 20. | Boettcher M, Gerisch M, Lobmeyer M, Besche N, Thomas D, Gerrits M, Lemmen J, Mueck W, Radtke M, Becker C. Metabolism and Pharmacokinetic Drug-Drug Interaction Profile of Vericiguat, A Soluble Guanylate Cyclase Stimulator: Results From Preclinical and Phase I Healthy Volunteer Studies. Clin Pharmacokinet. 2020;59:1407-1418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 21. | Boettcher M, Loewen S, Gerrits M, Becker C. Pharmacodynamic and Pharmacokinetic Interaction Profile of Vericiguat: Results from Three Randomized Phase I Studies in Healthy Volunteers. Clin Pharmacokinet. 2021;60:337-351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 22. | Ezekowitz JA, O'Connor CM, Troughton RW, Alemayehu WG, Westerhout CM, Voors AA, Butler J, Lam CSP, Ponikowski P, Emdin M, Patel MJ, Pieske B, Roessig L, Hernandez AF, Armstrong PW. N-Terminal Pro-B-Type Natriuretic Peptide and Clinical Outcomes: Vericiguat Heart Failure With Reduced Ejection Fraction Study. JACC Heart Fail. 2020;8:931-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 89] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 23. | Ponikowski P, Alemayehu W, Oto A, Bahit MC, Noori E, Patel MJ, Butler J, Ezekowitz JA, Hernandez AF, Lam CSP, O'Connor CM, Pieske B, Roessig L, Voors AA, Westerhout C, Armstrong PW; VICTORIA Study Group. Vericiguat in patients with atrial fibrillation and heart failure with reduced ejection fraction: insights from the VICTORIA trial. Eur J Heart Fail. 2021;23:1300-1312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 24. | Saldarriaga C, Atar D, Stebbins A, Lewis BS, Abidin IZ, Blaustein RO, Butler J, Ezekowitz JA, Hernandez AF, Lam CSP, O'Connor CM, Pieske B, Ponikowski P, Roessig L, Voors AA, Anstrom KJ, Armstrong PW; VICTORIA Study Group. Vericiguat in patients with coronary artery disease and heart failure with reduced ejection fraction. Eur J Heart Fail. 2022;24:782-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 25. | Voors AA, Mulder H, Reyes E, Cowie MR, Lassus J, Hernandez AF, Ezekowitz JA, Butler J, O'Connor CM, Koglin J, Lam CSP, Pieske B, Roessig L, Ponikowski P, Anstrom KJ, Armstrong PW; VICTORIA Study Group. Renal function and the effects of vericiguat in patients with worsening heart failure with reduced ejection fraction: insights from the VICTORIA (Vericiguat Global Study in Subjects with HFrEF) trial. Eur J Heart Fail. 2021;23:1313-1321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 64] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 26. | Lam CSP, Mulder H, Lopatin Y, Vazquez-Tanus JB, Siu D, Ezekowitz J, Pieske B, O'Connor CM, Roessig L, Patel MJ, Anstrom KJ, Hernandez AF, Armstrong PW; VICTORIA Study Group. Blood Pressure and Safety Events With Vericiguat in the VICTORIA Trial. J Am Heart Assoc. 2021;10:e021094. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |