Published online Dec 6, 2023. doi: 10.12998/wjcc.v11.i34.8158
Peer-review started: July 16, 2023
First decision: October 24, 2023
Revised: November 1, 2023
Accepted: November 24, 2023
Article in press: November 24, 2023
Published online: December 6, 2023
Processing time: 142 Days and 23.3 Hours
Methylmalonic acidemia (MMA) is characterized by non-specific symptoms such as vomiting, and feeding difficulties, along with delayed mental and physical development. However, no case of MMA combined with pulmonary fungal infection has been reported yet.
We report the case of a neonate who presented pulmonary fungal infection along with the non-specific features of MMA. Exome sequencing revealed a c.331C>T variant in exon 3 of MMACHC from the father, and a c.658-c.660delAAG variant in exon 4 from the mother, which confirmed the diagnosis of cblC type MMA combined with hyperhomocysteinemia.
Invasive fungal infection might occur in some infants with MMA. Therefore, early diagnosis is recommended for unexplained pulmonary infection.
Core Tip: We have retrospectively reported the case of a neonate with pulmonary Aspergillus infection as the main clinical manifestation, which was later confirmed as methylmalonic acidemia (MMA) by full exon sequencing and blood amino acid/urine organic acid analysis. Our findings provide a novel clinical diagnostic approach for neonatal MMA.
- Citation: Gao CF, Wang D, Zeng LK, Tao XW. Pulmonary fungal infection in a neonate with methylmalonic acidemia: A case report. World J Clin Cases 2023; 11(34): 8158-8163
- URL: https://www.wjgnet.com/2307-8960/full/v11/i34/8158.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i34.8158
Methylmalonic acidemia (MMA) is an autosomal/X-linked recessive disorder of organic acid metabolism that occurs due to mutations in methylmalonyl-CoAmutase or its coenzyme vitamin B12 (cobalamin)[1]. It is characterized by the accumulation of metabolites such as methylmalonic acid, 3-hydroxypropionic acid, and methylcitrate. Aberrant cobalamin metabolism can be classified into defects in adenosylcobalamin synthesis, including mitochondrial cobalamin deficiency (cblA) and mitochondrial cobalamin adenosyltransferase deficiency (cblB), and defects in adenosylcobalamin and methylcobalamin synthesis due to abnormal cytoplasmic and lysosomal cobalamin metabolism (cblC, cblD, and cblF). The coding genes for these types are MMAA, MMAB, MMACHC, MMADHC, and LMBRD1, respectively. The cblC type predominantly occurs in neonates and typically presents with non-specific symptoms. We report a case of a neonate admitted to the Department of Neonatology, Wuhan Children’s Hospital, with pulmonary Aspergillus infection as the main clinical manifestation, which was later confirmed as MMA through full exon sequencing and blood amino acid/urine organic acid analysis. Our findings provide a novel clinical diagnostic approach for neonatal MMA.
The baby developed shortness of breath for one day.
The patient was a 25-day-old girl, G2P1, G36W, cesarean delivered, with Apgar score 9 after 1 min and 10 after 5 min of birth. The birth weight was 2.06 kg (P10, -1.2SD), head circumference was 32 cm (P50, 0SD), and body length was 46 cm (P50, 0SD). The baby developed shortness of breath without any notable history and was subsequently admitted to our hospital.
No previous history.
No personal and family history.
On admission, the body temperature was 37.6 °C, heart rate was 162 beats/min, blood pressure was 70/37 mmHg, weight was 2.88 kg (P20), head circumference was 33 cm (P10), and body length was 46 cm (P3). The only abnormal finding on examination was coarse respiratory sounds in both lungs. Evaluation of other systems did not reveal any irregularities.
Routine blood count, biochemical parameters, Torch-immunoglobulin M test, blood cultures, 1,3-D glucan test (G test), and galactomannan polysaccharide antigen detection (GM test) showed no obvious abnormalities. Respiratory exudates tested negative for influenza virus, respiratory syncytial virus, and other respiratory viruses. Electrocardiography, electrocorticography, and echocardiography results were normal. The hypersensitive protein level upon admission was relatively high at 21.7 mg/L (< 3 mg/L) but returned to normal after 3 d.
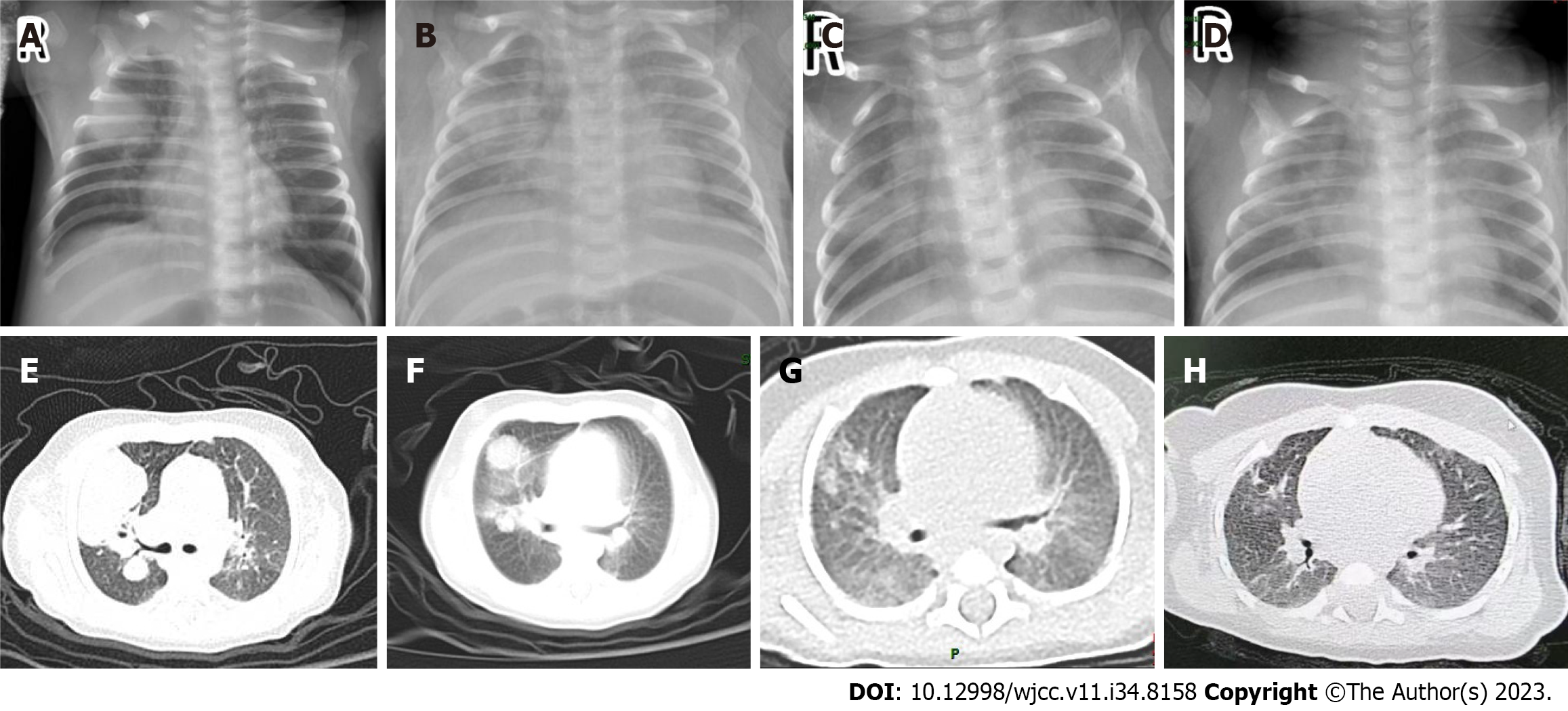
Lung ultrasound and X-ray examination revealed multiple round-like high-density shadows in both lungs, while computed tomography (CT)-scan showed infectious lesions in both lungs with air-containing cavities (Figure 1).
cblC type MMA combined with homocysteinemia.
The infant experienced intermittent fever for 8 d after hospitalization and vomited after formula feeding. The main clinical symptom was shortness of breath with a respiratory rate of 60-70 breaths/min in a calm state. Empirically, ceftazidime combined with vancomycin was administered, but no improvement was observed after one week. Fiberoptic bronchoscopy showed caseous material and pseudomembranous structures in the subbranch lumen of the anterior bronchial. Metagenomics next-generation sequencing of alveolar lavage fluid confirmed Aspergillus infection, leading to a switch in treatment to Voriconazole.
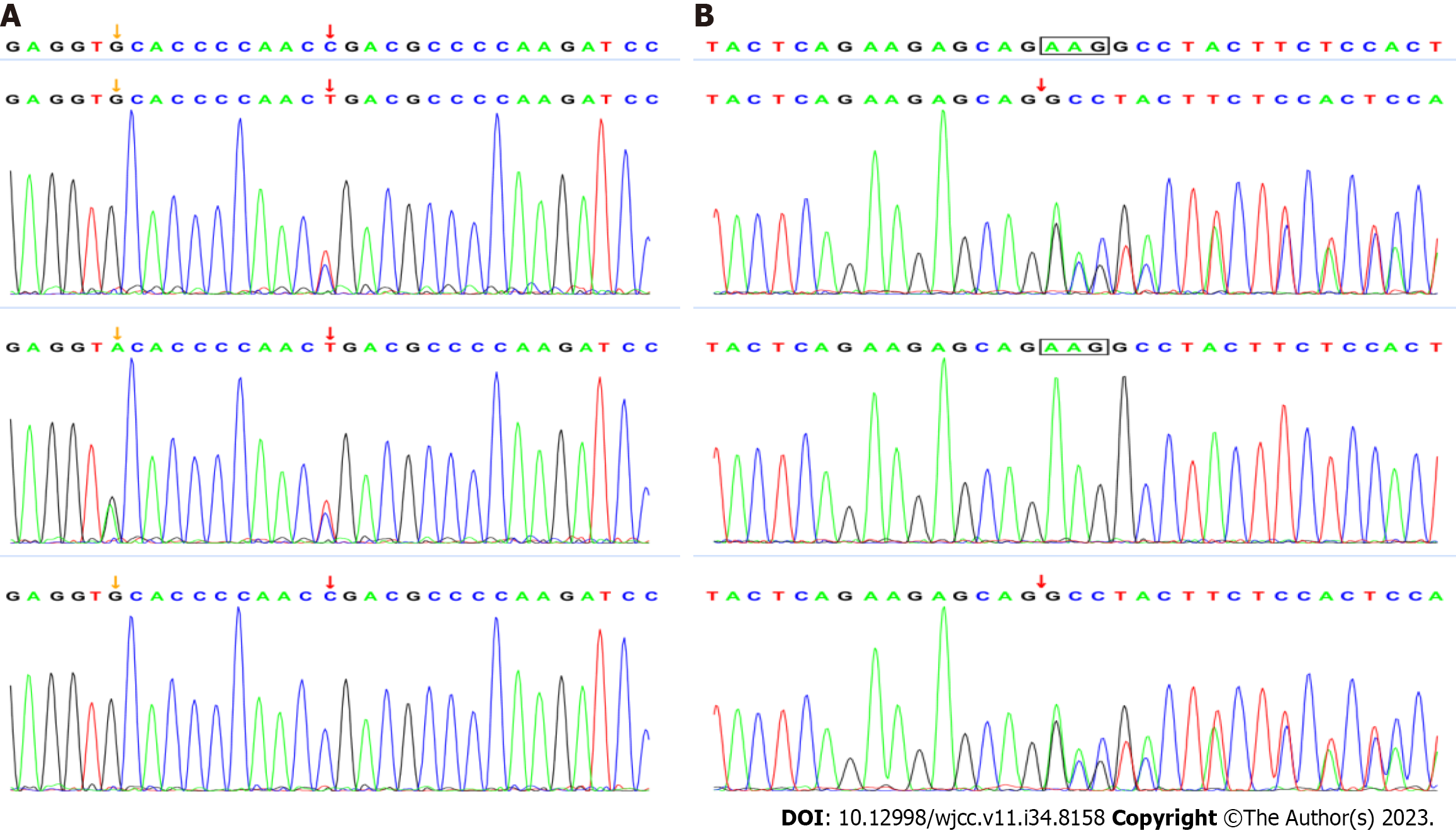
Regular outpatient follow-ups were conducted after discharge. Lung CT-scan was performed after 15 d and in the 2nd mo of follow-up, revealing improvements (Figure 1). Considering the unexplained fungal infection, full exon sequencing was performed to investigate the possibility of an immunodeficiency disease. The results confirmed a compound heterozygous variant in the MMACHC gene, with c.331C>T in exon 3 inherited from the father and c.658-c.660delAAG in exon 4 inherited from the mother, indicating cblC type MMA combined with homocysteinemia (Figure 2). Elevated blood malonylcarnitine, malonylcarnitine/acetylcarnitine ratio, urinary methylmalonic acid, urinary methylcitrate, and urinary homocysteine levels further confirmed the diagnosis. The patient received levocarnitine, betaine, calcium folinic acid, and hydroxocobalamin. At the 6-mo follow-up, the metabolic indicators showed improvement, and the infant exhibited normal physical and neurological development thereafter.
MMA is the most commonly diagnosed organic aciduria in China, and presents a diverse and complex clinical phenotype and genotype. The accumulation of methylmalonic acid and its metabolites can cause damage to the central nervous system, as well as the cardiovascular, renal, pulmonary vascular and hematological tissues, resulting in high mortality and disability rates[2]. The cblC type is the most common form of MMA combined with homocysteinemia (combined MMA). It is caused by mutations in the MMACHC gene[3], which is located on chromosome 1p34 and consists of 4 coding exons and 1 non-coding exon. The cblC protein is composed of 282 amino acids and is primarily found in the cytoplasm. Its main functions include catalyzing the reductive decyanation of cyanocobalamin and the synthesis of adenosine drillamin and methylcobalamin[4]. To date, nearly 80 different MMACHC gene mutations have been identified[5]. In China, the most common early-onset mutations are c.609G>A (48.1%-67.8%) and c.658_660delAAG (7.1%-13.9%). These mutations lead to sequence changes in the C-terminal region of cb1C and may impact the proton pump energy conversion required for cobalamin transport. Surviving children typically have compound heterozygous mutations, and carriers of these mutations often exhibit microcephaly, epilepsy, and severe developmental delay. More than 90% of MMA patients experience delayed intellectual and motor development, convulsions, abnormal psychiatric behavior, and, in some cases, feeding difficulties, vomiting, and malnutrition[6]. Only one published report exists of a neonatal MMA case combined with oral Candida infection after hospitalization[7]. Our neonate patient, on the other hand, presented with pulmonary fungal infection and exhibited atypical symptoms such as fever, lagging physical development, and vomiting during hospitalization. No neurological abnormalities were observed. Neutrophil deficiency is a known risk factor for invasive Aspergillus infection; however, in our case, no significant changes were observed in the neutrophil count or ratio, even during periods of intermittent fever. While we cannot rule out the possibility of neutrophil dysfunction, it is likely that the observed findings are due to decreased endothelial function resulting from the MMACHC mutation. Totoskovic et al[8] proposed a potential relationship between neutrophil morphometric indices and impaired cobalamin status. Vitamin B12 deficiency is known to cause hypersegmentation of the neutrophil nucleus and an increase in cell volume[9,10]. Based on these observations, the authors hypothesized that these changes may impact neutrophil Cell Population Data (CPD). CPD refers to the mean values and standard deviations of volume, conductivity, and light scatter for different leukocyte subpopulations. The study found a strong correlation between hypersegmented neutrophils and vitamin B12 deficiency. However, it remains unclear to what extent neutrophil scatter distribution width could be utilized to assess cobalamin status in patients with inflammatory or infectious conditions, requiring further investigation. This report presents the first documented case of neonatal methylmalonate acidemia combined with pulmonary Aspergillus infection, highlighting the need for future research into potential impairments in neutrophil function caused by methylmalonic acid and vitamin B12 deficiency. In a study involving six children with MMA combined with diffuse pulmonary disease as the primary presentation, four of them were identified as heterozygous or homozygous for the c.80A>G mutation, which has been previously reported in Chinese patients with pulmonary hypertension[11]. The accumulation of methylmalonic acid and homocysteine is often associated with nonspecific symptoms like anemia and poor feeding, while reduced methionine serves as a phenotype of MMACHC mutation[12-14]. Homocysteine, a sulfhydryl amino acid, can accumulate and lead to vascular endothelial damage and hematologic abnormalities, including thrombocytopenia, hemolytic-uremic syndrome, and bone marrow suppression[15]. Consequently, aberrant hematocrit levels can be observed. Anemia is commonly manifested due to factors such as bone marrow suppression, the accumulation of metabolic intermediates that harm the vascular endothelium and erythrocytes, impaired vitamin B12 utilization, and iron deficiency resulting from inadequate feeding[16,17].
It is worth mentioning that although the infant was being effectively treated, the non-specific symptoms such as vomiting and delayed development were considered as possible infection-related and ignored, which delayed the diagnosis. Therefore, a multipronged clinical approach is recommended for neonates with unexplained fungal infection.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Pandey A, India S-Editor: Wang JJ L-Editor: A P-Editor: Zhao S
| 1. | Liu Y, Liu YP, Zhang Y, Song JQ, Zheng H, Dong H, Ma YY, Wu TF, Wang Q, Li XY, Ding Y, Li DX, Jin Y, Li MQ, Wang ZX, Yuan Y, Li HX, Qin J, Yang YL. [Heterogeneous phenotypes, genotypes, treatment and prevention of 1 003 patients with methylmalonic acidemia in the mainland of China]. Zhonghua Er Ke Za Zhi. 2018;56:414-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 2. | Sheng ZQ, Yuan YR, Zhao B. [Diagnostic features of cb1C-type mthylmalonic acidemia with homocysteinemia and literature review]. Chin J Diagnostics (Electronic Edition). 2017;5:281-284. [DOI] [Full Text] |
| 3. | Wang C, Li D, Cai F, Zhang X, Xu X, Liu X, Zhang C, Wang D, Lin S, Zhang Y, Shu J. Mutation spectrum of MMACHC in Chinese pediatric patients with cobalamin C disease: A case series and literature review. Eur J Med Genet. 2019;62:103713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 4. | Yu YF, Li F, Ma HW. [Relationship of genotypes with clinical phenotypes and outcomes in children with cobalamin C type combined methylmalonic aciduria and homocystinuria]. Chinese J Contemporary Pediatr. 2015;17:769-774. [DOI] [Full Text] |
| 5. | Zhang C, Wang X, Hao S, Zhang Q, Zheng L, Zhou B, Liu F, Feng X, Chen X, Ma P, Chen C, Cao Z, Ma X. Mutation analysis, treatment and prenatal diagnosis of Chinese cases of methylmalonic acidemia. Sci Rep. 2020;10:12509. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Morel CF, Lerner-Ellis JP, Rosenblatt DS. Combined methylmalonic aciduria and homocystinuria (cblC): phenotype-genotype correlations and ethnic-specific observations. Mol Genet Metab. 2006;88:315-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 101] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 7. | Jia LY, Feng JZ, Wang X, Ma CX, Feng LL. [Analysis of Gene Mutation in Children with Methylmalonic Acidemia Complicated with Homocysteinemia]. J Int Repord Health/Fam Plan. 2019;38:362-366. [DOI] [Full Text] |
| 8. | Totoskovic D, Dopsaj V, Martinovic J. Methylmalonic acid and neutrophil morphometric index in laboratory diagnosis of cobalamin deficiency without macrocytosis. Int J Lab Hematol. 2016;38:265-272. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 9. | Bailey RL, Carmel R, Green R, Pfeiffer CM, Cogswell ME, Osterloh JD, Sempos CT, Yetley EA. Monitoring of vitamin B-12 nutritional status in the United States by using plasma methylmalonic acid and serum vitamin B-12. Am J Clin Nutr. 2011;94:552-561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 113] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 10. | Remacha AF, Sardà MP, Canals C, Queraltò JM, Zapico E, Remacha J, Carrascosa C. Role of serum holotranscobalamin (holoTC) in the diagnosis of patients with low serum cobalamin. Comparison with methylmalonic acid and homocysteine. Ann Hematol. 2014;93:565-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Tang XL, Yang HM, Liu H, Xu H, Zhou CJ, Li HM, Zhao SY, Liu JR. [Clinical analysis of meylmalonic aemia with hyperhomocysteinemia with diffuse pulmonary disease as prominent or first manifestation]. Chinese J Pediat. 2019;620-624. [DOI] [Full Text] |
| 12. | Han B, Cao Z, Tian L, Zou H, Yang L, Zhu W, Liu Y. Clinical presentation, gene analysis and outcomes in young patients with early-treated combined methylmalonic acidemia and homocysteinemia (cblC type) in Shandong province, China. Brain Dev. 2016;38:491-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 13. | Li Y, Zhang D, Wang BY, Kang LM, Hu XM, Li C. [Clinical analysis of neonatal methylmalonic acemia with cytopenia]. Chinese J Med. 2019;54:989-992. [DOI] [Full Text] |
| 14. | Wang F, Han LS, Hu YH, Yang YL, Ye J, Qiu WJ, Zhang YF, Gao XL, Wang Y, Gu XF. [Analysis of gene mutations in Chinese patients with methylmalonic acidemia and homocysteinemia]. Zhonghua Er Ke Za Zhi. 2009;47:189-193. [PubMed] |
| 15. | Huemer M, Scholl-Bürgi S, Hadaya K, Kern I, Beer R, Seppi K, Fowler B, Baumgartner MR, Karall D. Three new cases of late-onset cblC defect and review of the literature illustrating when to consider inborn errors of metabolism beyond infancy. Orphanet J Rare Dis. 2014;9:161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 90] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 16. | Liu MY, Yang YL, Chang YC, Chiang SH, Lin SP, Han LS, Qi Y, Hsiao KJ, Liu TT. Mutation spectrum of MMACHC in Chinese patients with combined methylmalonic aciduria and homocystinuria. J Hum Genet. 2010;55:621-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 17. | Gündüz M, Ekici F, Özaydın E, Ceylaner S, Perez B. Reversible pulmonary arterial hypertension in cobalamin-dependent cobalamin C disease due to a novel mutation in the MMACHC gene. Eur J Pediatr. 2014;173:1707-1710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |