Published online Nov 26, 2023. doi: 10.12998/wjcc.v11.i33.8050
Peer-review started: August 25, 2023
First decision: October 10, 2023
Revised: October 11, 2023
Accepted: November 14, 2023
Article in press: November 14, 2023
Published online: November 26, 2023
Processing time: 90 Days and 20.1 Hours
Fibrous dysplasia is a congenital disorder in which normal bone is replaced by fibro-osseous tissue or irregular trabeculae of woven bone intermixed with mature collagenous tissue. A single or multiple bones are affected. This rare bone disorder has three clinical patterns including monostotic, polyostotic, and that associated with McCune–Albright syndrome. Most studies report primary fibrous dysplasia. However, a few cases of recurrent monostotic fibular fibrous dysplasia have been reported. Here, we report a therapeutic strategy for recurrent fibular fibrous dysplasia.
A 4-year-old boy was admitted for persistent pain in the left lower limb and abnormal gait over the previous 9 mo. He had no history of present or past illness. Preoperative imaging data showed erosion-like changes with bone expansion of the left middle and lower fibular segment. Tumor tissue in the fibular bone marrow cavity was removed by curettage, and rapid intraoperative pathological examination suggested fibular fibrous dysplasia. An allograft was implanted into the fibular medullary cavity. However, he was readmitted with clinical symptoms including persistent pain, abnormal gait, and local swelling at the age of 6 years. He was diagnosed with recurrent fibular fibrous dysplasia based on the second medical examination. He underwent fibular bone tumor radical resection and longus fibular allograft transplantation combined with fibular bone locking plate and screws. Good host bone to allogenic bone graft fusion was observed by the physician on postoperative regular follow-up.
Radical resection of fibrous dysplasia and longus fibula allograft combined with internal fixation for reconstruction are suitable for the treatment of recurrent monostotic fibular fibrous dysplasia.
Core Tip: The incidence of recurrent monostotic fibular fibrous dysplasia is low. For recurrent fibrous dysplasia, radical resection combined with allograft bone for biodynamic reconstruction is a suitable therapy. We report a case of recurrent fibrous dysplasia in the left fibular bone treated by longus fibula allograft transplantation combined with fibula bone locking plate and screws.
- Citation: Xie LL, Yuan X, Zhu HX, Fu L, Pu D. Fibula allograft transplantation combined with locking plate for treatment of recurrent monostotic fibular fibrous dysplasia: A case report. World J Clin Cases 2023; 11(33): 8050-8057
- URL: https://www.wjgnet.com/2307-8960/full/v11/i33/8050.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i33.8050
As a benign fibrous-osseous lesion, fibrous dysplasia was first reported by Lichtenstein and Jaffe[1] in 1947. Usually, fibrous dysplasia is characterized by bone developmental failure due to abnormal bone proliferation, including fibro-osseous tissue, irregular trabeculae of woven bone, and mature collagenous tissue. Some symptoms such as pain, deformity, claudication, and pathological fractures are caused by insufficient mineralization with substantial loss of mechanical strength[2]. One or several bones can be affected by fibrous dysplasia. GNAS gene mutations have been shown to participate in the pathogenesis of fibrous dysplasia and have been confirmed to have diagnostic significance[3]. Some authors have reported recurrent monostotic fibrous dysplasia in the mandible, and suggested that conservative surgery may not be suitable for the treatment of this lesion[4]. A few cases of recurrent monostotic fibular fibrous dysplasia have been reported. Here, we present a case of recurrent monostotic fibular fibrous dysplasia, which was studied by imaging, and the pathological results. Radical surgery and longus fibula allograft combined with fibula bone locking plate and screws were performed to improve the patient’s contour and function.
A 4-year-old boy was admitted because of persistent lower limb pain and claudication in the left lower limb over the past 9 mo.
The patient had mild persistent lower limb pain and claudication without any inducement. Claudication worsened in one day. The child had no fever, urinary frequency or urgency, numbness, fatigue, or lameness. For further assessment and treatment, he was admitted to our joint hand surgery department.
The child had no history past illness.
The child had no history of family illness, and his medical history was unremarkable.
Pressing pain and local swelling were present in the left shank. The results of sensation and strengthening test, and tendon reflex test were normal in both lower limbs. No pathological signs were observed upon physical examination.
Laboratory examinations were normal.
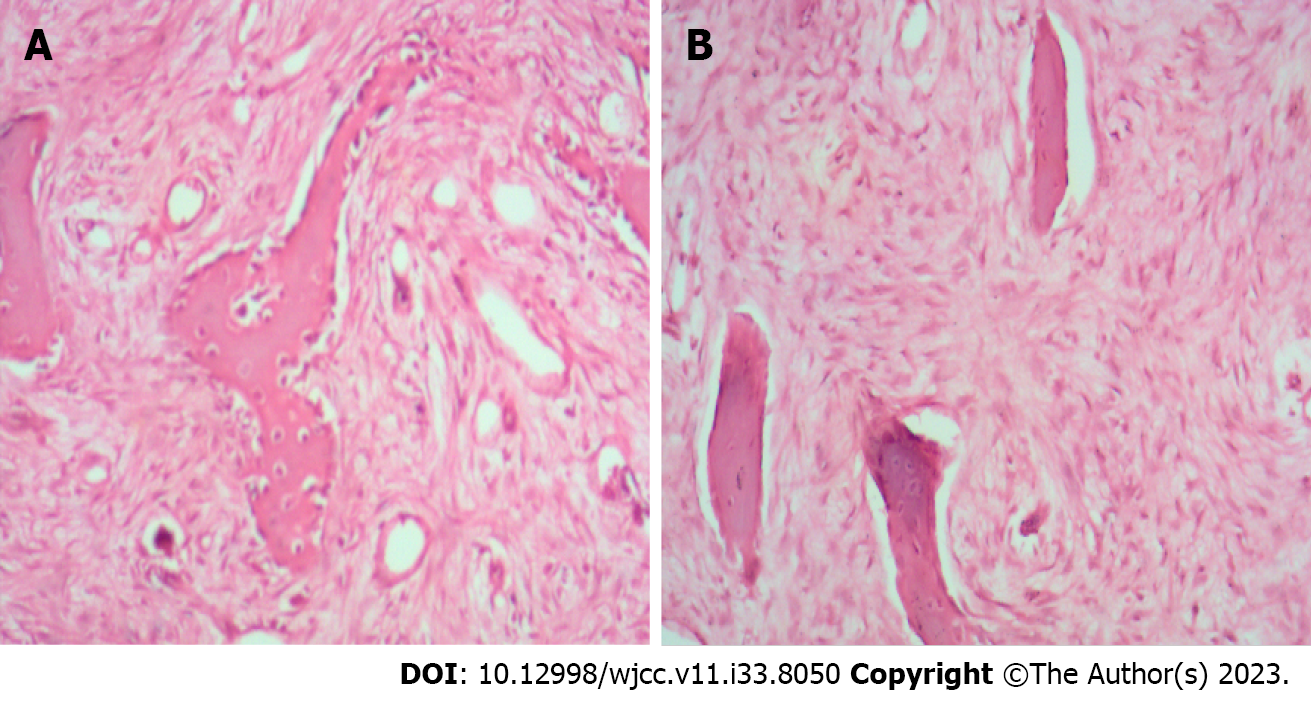
Preoperative imaging examinations, including X-ray photography, computed tomography, and magnetic resonance imaging, showed erosion-like changes with bone expansion of the left middle and lower fibular segment (Figure 1). No invasion of circumferential soft tissue or pathological fracture of the lesion site was observed. Initial pathological examination revealed fibular fibrous dysplasia (Figure 2). Postoperative photography showed that an allograft bone was implanted into the fibular medullary cavity (Figure 3). Recurrent fibular fibrous dysplasia was observed at the age of 6 years (Figure 4).
The second postoperative pathological examination also revealed recurrent fibular fiber dysplasia (Figure 5). The patient was diagnosed with recurrent fibular fibrous dysplasia based on the medical history, physical examination, laboratory and pathological results, and imaging findings.
Treatment strategies were based on multidisciplinary expert consultation and professional discussion of the case data, including clinical symptoms and signs, and imaging and pathological findings. After communication with his guardian, the patient underwent fibular bone tumor resection and longus fibular allograft combined with fibula bone locking plate for treatment of recurrent fibular fibrous dysplasia. A left straight lateral incision was made to expose the site of the fibular bone tumor. All fibular bone tumor tissue was completely excised. Radical resection of the lesion from outside the periosteum to the normal bone and soft tissue was performed. The surgeon soaked the incision with sterilized water for 10 min. A longus fibular allograft bone combined with fibula bone locking plate and screws was used for reconstruction of the left fibula under the guidance of C-arm X-ray (Figure 6). The patient was encouraged to complete postoperative functional exercises in bed. Postoperative routine pathological examination revealed fibular fibrous dysplasia (Figure 5). After 3 mo, he was gradually mobilized and achieved complete weight bearing without crutches after X-ray examination and senior physician assessment. According to postoperative imaging and senior physician assessment, strong autogenous fibular bone fused to allogenic fibular bone was observed, and the internal fixation was removed after 1 year (Figure 6).
During postoperative regular follow-up, strong autogenous fibular bone fused to allogenic fibular bone was observed (Figure 6). No clinical symptoms or signs were observed based on questionnaire survey and visual analog scale.
We report a method of treatment for recurrent fibrous dysplasia of the left fibula. As a rare benign bone disease, fibrous dysplasia presents with abnormal arrest in the woven bone stage during bone maturation, including a monostotic or polyostotic distribution[2]. Previous studies have shown that fibroblastic proliferation replaces normal bone matrix, which leads to irregular trabeculae of partially calcified osteoid[5,6]. Some studies have suggested that fibrous dysplasia has age-related self-limiting characteristics and rare malignant transformation, attributed to the number of mutant cells often decreasing with age[7]. Other studies have found that adequate treatment may lead to a favorable prognosis because of local pain and/or fatigue fracture in adolescence and early adulthood[8]. The therapeutic strategies for fibrous dysplasia include conservative surgery, radical excision, and medical treatment with bisphosphonates. We report radical excision with reconstruction of longus fibula allograft combined with fibula bone locking plate and screws for recurrent monostotic fibular fibrous dysplasia with prolonged symptoms, such as local pain and abnormal gait. Choi et al[9] suggested that such lesion will continue to grow after various treatments in about 20%-25% of the patients. They also revealed that it is impossible to prognosticate whether the fibrous dysplasia will recur or not, even though it is extirpated radically[9,10]. Our patient previously received lesion removal and allograft bone grafting without internal fixation, even if some investigators found no recurrence of monostotic fibrous dysplasia at the femoral neck[2]. Alves et al[4] reported a case of mandibular fibrous dysplasia treated conservatively by excision, and growth of the lesion was observed postoperatively after 1 year. Valentini et al[11] reported no recurrence when radical resection of the lesion was applied. Thus, as the only option for eliminating fibrous dysplasia, radical surgery could prevent recurrence of fibrous dysplasia. Our case underwent radical resection for recurrent fibrous dysplasia of the fibular bone. No signs of recurrence and allograft bone fusion in postoperative X-ray were observed during regular postoperative follow-up. One year after operation, internal fixation was removed by surgeon.
Reconstructive methods for defects in the long bones of the extremities have been developed and significantly improved over the last few years, as a result of development of bone graft materials. However, there is still a lack of consensus or solid evidence for reconstructive methods for fibrous dysplasia, although some recommended guidelines have been advocated by Javaid et al[12]. For craniomaxillofacial fibrous dysplasia, any reconstructive decision should enhance the aesthetics and function of the patient[11]. There are still aesthetic requirements for therapy of long bones of the extremities with fibrous dysplasia. Patankar et al[13] proposed that nonvascularized fibular cortical strut grafting is an effective treatment for fibrous dysplasia of the radius. Majoor et al[14] suggested that cortical strut allograft is a viable treatment option for fibrous dysplasia involving the proximal femur in patients who have not already experienced a fracture. Surgeons should pay particular attention to the proximal fixation point of the allograft to decrease the risk of failure. In our case, fusion of strong autogenous fibular bone to allogenic fibular bone was observed at proximal and distal fibular lesions, and the internal fixation was removed after 1 year.
With regard to clinicopathological presentation of monostotic fibrous dysplasia, Özşen et al[15] reported small differences but mainly similar characteristics to those reported earlier. In their study of 32 cases diagnosed with fibrous dysplasia, four were accompanied by aneurysmal bone cyst, and recurrence occurred in five treated by curettage. We previously reported a case of fibrous dysplasia associated with aneurysmal-bone-cyst-like changes in proximal femur lesion, which was confirmed by pathological examination[16]. Some authors have found that the tumor tissues scatter within fibrous tissue with various degrees of cellularity, and appear narrow and circular, usually shaped as irregular, immature bone trabeculae under the microscope[15]. It is shown that immature bone trabeculae are not surrounded by osteoblasts, and such trabeculae do not evolve into mature bone. The number, distribution, and maturity of bone trabeculae differ geographically and among cases. Our case underwent curettage of the lesions, and recurrent fibular fibrous dysplasia was observed during follow-up. Prior and later pathological examination of our case supported the diagnosis of fibrous dysplasia.
Recurrent fibrous dysplasia of the fibular bone is rare, and appropriate treatment strategies are important. Radical resection of fibrous dysplasia and use of longus fibula allograft combined with fibular bone locking plate with screws for reconstruction are suitable therapeutic methods. After initial treatment of fibrous dysplasia, especially in children, active follow-up and regular reviews should be also carried out for recurrent or malignant tumors.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hoveidaei AH, Iran S-Editor: Lin C L-Editor: Wang TQ P-Editor: Lin C
| 1. | Lichtenstein L, Jaffe HL. Ewing's Sarcoma of Bone. Am J Pathol. 1947;23:43-77. [PubMed] |
| 2. | Nishida Y, Tsukushi S, Hosono K, Nakashima H, Yamada Y, Urakawa H, Ishiguro N. Surgical treatment for fibrous dysplasia of femoral neck with mild but prolonged symptoms: a case series. J Orthop Surg Res. 2015;10:63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 3. | Ruggieri P, Sim FH, Bond JR, Unni KK. Malignancies in fibrous dysplasia. Cancer. 1994;73:1411-1424. [PubMed] [DOI] [Full Text] |
| 4. | Alves N, de Oliveira RJ, Takehana D, Deana NF. Recurrent Monostotic Fibrous Dysplasia in the Mandible. Case Rep Dent. 2016;2016:3920850. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Cohen MM Jr, Howell RE. Etiology of fibrous dysplasia and McCune-Albright syndrome. Int J Oral Maxillofac Surg. 1999;28:366-371. [PubMed] |
| 6. | Levine MA, Modi WS, O'Brien SJ. Mapping of the gene encoding the alpha subunit of the stimulatory G protein of adenylyl cyclase (GNAS1) to 20q13.2----q13.3 in human by in situ hybridization. Genomics. 1991;11:478-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 58] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 7. | Ozek C, Gundogan H, Bilkay U, Tokat C, Gurler T, Songur E. Craniomaxillofacial fibrous dysplasia. J Craniofac Surg. 2002;13:382-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 68] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Zenn MR, Zuniga J. Treatment of fibrous dysplasia of the mandible with radical excision and immediate reconstruction: case report. J Craniofac Surg. 2001;12:259-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Choi JW, Lee SW, Koh KS. Correction of proptosis and zygomaticomaxillary asymmetry using orbital wall decompression and zygoma reduction in craniofacial fibrous dysplasia. J Craniofac Surg. 2009;20:326-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Lee JS, FitzGibbon EJ, Chen YR, Kim HJ, Lustig LR, Akintoye SO, Collins MT, Kaban LB. Clinical guidelines for the management of craniofacial fibrous dysplasia. Orphanet J Rare Dis. 2012;7 Suppl 1:S2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 166] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 11. | Valentini V, Cassoni A, Marianetti TM, Terenzi V, Fadda MT, Iannetti G. Craniomaxillofacial fibrous dysplasia: conservative treatment or radical surgery? A retrospective study on 68 patients. Plast Reconstr Surg. 2009;123:653-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 106] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 12. | Javaid MK, Boyce A, Appelman-Dijkstra N, Ong J, Defabianis P, Offiah A, Arundel P, Shaw N, Pos VD, Underhil A, Portero D, Heral L, Heegaard AM, Masi L, Monsell F, Stanton R, Dijkstra PDS, Brandi ML, Chapurlat R, Hamdy NAT, Collins MT. Best practice management guidelines for fibrous dysplasia/McCune-Albright syndrome: a consensus statement from the FD/MAS international consortium. Orphanet J Rare Dis. 2019;14:139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 149] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 13. | Patankar H, Patankar S, Tandon N, Bairy A. Fibrous Dysplasia of Radius Bone-excision and Fibula Graft: A Case Report. J Orthop Case Rep. 2022;12:31-34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 14. | Majoor BC, Peeters-Boef MJ, van de Sande MA, Appelman-Dijkstra NM, Hamdy NA, Dijkstra PD. What Is the Role of Allogeneic Cortical Strut Grafts in the Treatment of Fibrous Dysplasia of the Proximal Femur? Clin Orthop Relat Res. 2017;475:786-795. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 15. | Özşen M, Yalçinkaya Ü, Bilgen MS, Yazici Z. Fibrous Dysplasia: Clinicopathologic Presentation of 36 Cases. Turk Patoloji Derg. 2018;34:234-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Xie LL, Yuan X, Zhu HX, Pu D. Surgery for fibrous dysplasia associated with aneurysmal-bone-cyst-like changes in right proximal femur: A case report. World J Clin Cases. 2023;11:6170-6175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |