Published online Nov 26, 2023. doi: 10.12998/wjcc.v11.i33.8038
Peer-review started: August 10, 2023
First decision: August 30, 2023
Revised: September 14, 2023
Accepted: November 10, 2023
Article in press: November 10, 2023
Published online: November 26, 2023
Processing time: 105 Days and 15.5 Hours
Kommerell’s diverticulum (KD) with aberrant left subclavian artery is a rare congenital deformity and also has very little research literature about it (35% of case study). There are three types of aortic arch diverticulum. Even literature concerning the treatment options are limited.
We present a case report of a 50-year-old male with KD in the right aortic arch with aberrant left subclavian artery. We conducted a total endovascular repair procedure, which is innovative and will spread more light in the medical world. Our patient has no past medical history and is a non-smoker and non-alcoholic. Patient presented with shortness of breath, chest pain and dizziness for six months. Blood tests were done and computerized tomography (CT) angiogram of the chest confirmed the diagnosis, illustrating showed a 3.9 cm KD. On Day 1, the CT angiogram showed mild dilatation of the thoracic aorta, adjacent esophagus, trachea was compressed and displaced. Surgery was planned as the treatment modality. Carotid-Subclavian artery bypass and endovascular aortic repair was conducted. We used prolene 5-0 C1 sutures to precisely anastomose a 6-mm Dacron graft to the left subclavian artery. Haemostasis was secured and wounds were closed. Protamine was administered and patient was shifted to intensive care unit. Post-operative, patient responded favorably and was discharged. Regular follow-up is done.
The procedure we performed is novel. This will help the cardio-thoracic surgeons a better insight about the full procedures we conducted, thereby bringing more light and better treatment options in managing KD with aberrant subclavian artery.
Core Tip: Kommerell’s diverticulum with aberrant left subclavian artery is an infrequent congenital deformity. Research, and literature about its treatment options is minimal. We present a case report of a 50-year-old male with no comorbidities, with Kommerell’s diverticulum in the right aortic arch with aberrant left subclavian artery. The patient presented with shortness of breath, chest pain and dizziness for six months. Blood tests and a computerized tomography angiogram confirmed the diagnosis. Carotid-subclavian artery bypass and endovascular aortic repair, using a Dacron graft was conducted, which is an innovative procedure, shedding more light on it. Post-operatively, the patient responded favorably.
- Citation: Akilu W, Feng Y, Zhang XX, Li SL, Ma XT, Hu M, Cheng C. Carotid-subclavian bypass and endovascular aortic repair of Kommerell’s diverticulum with aberrant left subclavian artery: A case report. World J Clin Cases 2023; 11(33): 8038-8043
- URL: https://www.wjgnet.com/2307-8960/full/v11/i33/8038.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i33.8038
Originally described in 1936, by a German radiologist, named Kommerell[1], Kommerell’s diverticulum (KD) is also known as “lusoria diverticulum”, “lusoria root” or “remnant diverticulum”[1]. This was seen in a patient with left sided aortic arch, where a pulsatile mass seen to the posterior of the oesophagus, leading to the compression of that part of gut, detected in barium swallow[1]. It is defined as the aneurysmal dilatation of the descending aorta at the origin of an aberrant subclavian artery (ASCA), that can be located in both right and left sided aortic arches[2]. KD is due to persistence remnant of the fourth primitive dorsal arch, which failed to retrogress[3].
As per the classification of Salomonowitz et al[4], there are three types of aortic arch diverticulum namely: (1) Diverticulum in left aortic arch with right ASCA; (2) diverticulum in right aortic arch with left ASCA; and (3) aortic diverticulum without ASCA (at the aortic-ductal junction).We present a case study of patient detected with KD with left ASCA.
A 50-year-old Chinese man was admitted to our hospital, presented with difficulty in breathing, sudden onset chest pain and dizziness on and off for 6 mo.
There was no dysphagia, syncopal attacks, abdominal pain, palpitations or other symptoms.
His past medical history, past surgical history.
Personal history: Smoking-drinking habits are not significant.
Family history: Family history and drug allergy were not major.
The vital signs were: Blood pressure was 120/70 mmHg, temperature was 36.5 °C, pulse was 73 bpm, regular (bilateral radial, brachial sides were normal), no pallor, no cyanosis, no clubbing, no pedal oedema, no peripheral vascular signs. The systemic examinations were normal.
The blood investigations such as the hematological and biochemical investigations were within normal limits.
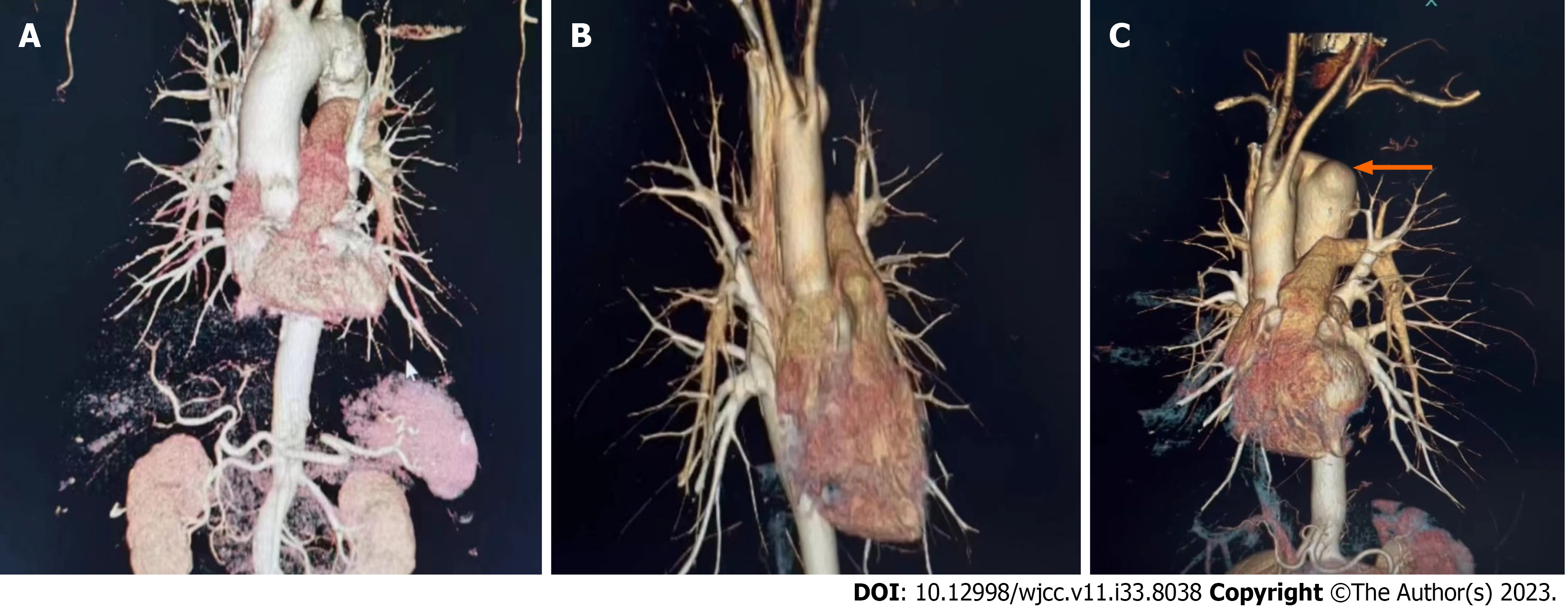
A computed tomography aortography (CTA) was performed on him as diagnostic assessment and findings as shown below (Figure 1).
At the local provincial hospital, on day 1 admission, CTA of the brain and neck region identified aortic arch KD with esophageal compression, left subclavian artery was stenosed (Figure 2) and left subclavian artery steal syndrome was seen. The patient was referred to our Cardiothoracic Surgery Department. On day 5, Another CTA was performed and showed mild dilatation of the thoracic aorta, adjacent esophagus and trachea was compressed and displaced (Figure 1B; Video 1). The right upper lobe tip was streaked, and were mostly inflammatory lesions, several small round low-density foci in the liver.
The treatment options were then discussed with the patient including that of a surgical treatment along with all the risks and benefits involved. The patient agreed for surgical intervention.
On day 5, Another CTA was performed and showed mild dilatation of the thoracic aorta, adjacent esophagus and trachea was compressed and displaced (Figure 1B; Video 1). The right upper lobe tip was streaked, and were mostly inflammatory lesions, several small round low-density foci in the liver.
The treatment options were then discussed with the patient including that of a surgical treatment along with all the risks and benefits involved. The patient agreed for surgical intervention.
Pre-operative CT angiography film of the chest showed a 3.9 cm KD (Figure 1C; Video 1). After placing the patient in supine position, general anaesthesia was administered successfully. The anterior aspect of the chest wall and groin area were routinely disinfected. We began a transverse supraclavicular 5 cm incision (Figure 3A). We separated the platysma muscle and proceeded to the two heads of the sternocleidomastoid muscle.
Firstly, a dissection was done to reach the internal jugular vein. We applied deep wound spreaders to help expose the vessels (Figure 3A). Medial to this vein, we cautiously isolated the vagus nerve, which lies between the internal jugular vein and the left common carotid artery (LCCA) and we opened the carotid sheath. This allowed the chance to do anastomosis proximally on the LCCA at the left lateral side (Figure 3B).
The next procedure was exposing the left subclavian artery (LSA), 1 cm deeper in the neck and its lateral away from the LCC. There was subcutaneous fatty tissue with lymph nodes that obstructs LSA dissection, hence these lymph nodes were resected. We also exposed and looped the left internal thoracic artery using a vessel loop.
We then finally revealed the deep LSA in the neck (Figure 3A) and the associations between the LCCA and LSA were obviously seen. A prescribed heparin dose was administered. We clamped the LSA and incised to make round-shape incision. We used prolene 5-0 C1 sutures to meticulously anastomosed 6-mm Dacron graft (Polymaille C, Perous Medical, France) to the LSA round-shape incised artery and haemostasis was checked afterwards. We made a good size length of our Dacron graft and it was able to stretch from the left subclavian artery to the lateral side of LCCA.
We clamped the LCCA while at the same time monitoring the transcranial Doppler signals. We ensured that there was enough cerebral perfusion pressure by letting the mean blood pressure stable at 90 mmHg. We made a tinny incision on the LCCA at the lateral side and a mild punch to create a round-hole. We then started our anastomosis using 5-0 C1 sutures on a 6-mm Dacron graft (Polymaille C, Perous Medical, France) end to end approach with LCCA. A clamp was used clamping the distal LCCA and then was flushed. Afterwards, we unclamped the LSA and the previously clamped LCCA to de-air them. Also, haemostasis was checked from the vessels after unclamping the vessels.
Preoperative CT angiography revealed morphology of right femoral artery, the LCCA, the right common carotid artery, right subclavian artery, the proximal end of the left subclavian artery was stenosed, and the descending aorta was obviously bulged (33 mm × 25 mm). In accordance to what we have in the patient’s history and CT results, this guided us in our interventional approach for this procedure. The groin femoral region was opened (Figure 3D) and 34200 mm Medtronic Stent graft (Medtronic Inc. United States) (Figure 3C) was placed through the femoral artery, positioned by the right subclavian artery angiographic catheter, and deployed at the proximal end of the right subclavian artery and the areas of the left subclavian artery were covered successfully. There was no leakage of contrast agent after re-angiography. We closed all incisions by way of suturing and systemic Protamine was administered to the Patient, then transferred to the intensive care unit.
The post-operative care was done meticulously, including the daily nursing care. Patient was discharged on Tablet Plavix 75 mg once a day and later shifted to enteric coated Aspirin tablet 100 mg orally once a day and its side-effects (such as bleeding gums, bleeding from any orifices, gastrointestinal bleeding, black stools) have been explained to the patient and to attend hospital immediately once any of it happens.
We suggest the patient for regular monitoring of blood pressure, heart rate, blood routine examination including renal function, liver function tests, cardiac enzymes, electrocardiogram and cardiac echography and to have regular follow-up after 1, 3, 6, 12-mo. The patient’s recovery was normal without any symptoms and he continued to be uneventful one-year post-surgery.
KD in brief, is a rare abnormal congenital condition that occurs in either right or left sided aortic arch and seen to be associated with an ASCA[2]. KD are aneurysmal dilated changes in the aortic walls and have the tendency of dissection[5]. In 1936, initially described in a patient with left sided aortic arch, German radiologist Kommerell saw on barium swallow: a pulsatile mass compressing the esophagus posteriorly[1]. The incidence of KD with a right-sided aortic arch; seen in radiological studies is 0.05-0.1%[6,7].
KD symptoms of oesophageal or tracheal origins, are indicative factors for a surgical treatment. A KD with a size greater than 30 mm in diameter should be considered for operation[5]. It is known that KD has tendency of activating aortic aneurysm, or dissection or even rupture. Open repair and revascularization of the left arm was the surgical approach of preference. Using the techniques of minimally invasive and endovascular techniques for surgical repairs, have recently helped to reduce anguish, pain when compared to open sternotomy[8-10].
We demonstrated rare combined case study of endovascular and open surgical treatment involving KD with aberrant left subclavian artery. Being a rare disease, little literature was found about the surgical treatment. The procedure we conducted is innovative and will bring more understanding and better treatment options in managing KD with ASCA. Again, our technique was able to give a full comprehensive insight on how the stent replacement and left sided revascularization can be performed in an orderly way. Thus, bringing scientific progress in the field.
We would like to thank the patient for giving the consent in writing the paper.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Barik R, India S-Editor: Yan JP L-Editor: A P-Editor: Xu ZH
| 1. | Kommerell B. Verlagerung des oesophagus durch eine abnorm veranfende arteria subclavia dextra (arteria lusoria). Fortschr Geb Roentgenstr. 1936;54:590-595. |
| 2. | Bhatt TC, Muralidharan CG, Singh G, Jain NK. Kommerell's diverticulum: A rare aortic arch anomaly. Med J Armed Forces India. 2016;72:S80-S83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | EDWARDS JE. Anomalies of the derivatives of the aortic arch system. Med Clin North Am. 1948;32:925-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 402] [Cited by in RCA: 352] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 4. | Salomonowitz E, Edwards JE, Hunter DW, Castaneda-Zuniga WR, Lund G, Cragg AH, Amplatz K. The three types of aortic diverticula. AJR Am J Roentgenol. 1984;142:673-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 42] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Cinà CS, Althani H, Pasenau J, Abouzahr L. Kommerell's diverticulum and right-sided aortic arch: a cohort study and review of the literature. J Vasc Surg. 2004;39:131-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 267] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 6. | Tsukube T, Ataka K, Sakata M, Wakita N, Okita Y. Surgical treatment of an aneurysm in the right aortic arch with aberrant left subclavian artery. Ann Thorac Surg. 2001;71:1710-1711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 7. | Svensson LG, Crawford ES. Cardiovascular and vascular disease of the aorta. Philadelphia: W.B. Saunders Company, 1997. |
| 8. | Idrees J, Keshavamurthy S, Subramanian S, Clair DG, Svensson LG, Roselli EE. Hybrid repair of Kommerell diverticulum. J Thorac Cardiovasc Surg. 2014;147:973-976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 101] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 9. | Hayakawa M, Nagano T, Nishijima I, Shinzato K, Ikemura R, Miyagi K, Iha K, Senaha S, Shimoji M, Akasaki M. Successful Endovascular Repair of a Kommerell's Diverticulum and a Right-Sided Aortic Arch. Heart Surg Forum. 2020;23:E860-E862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Ghincea CV, Ikeno Y, Weyant MJ, Mitchell JD, Aftab M, Reece TB. Right Thoracoscopic Aberrant Right Subclavian Artery Division and Subclavian-Carotid Transposition. Ann Thorac Surg. 2020;110:e431-e433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |