Published online Nov 26, 2023. doi: 10.12998/wjcc.v11.i33.8003
Peer-review started: September 25, 2023
First decision: October 17, 2023
Revised: October 25, 2023
Accepted: October 30, 2023
Article in press: October 30, 2023
Published online: November 26, 2023
Processing time: 60 Days and 1.8 Hours
The recovery time of hand wounds is long, which can easily result in chronic and refractory wounds, making the wounds unable to be properly repaired. The treatment cycle is long, the cost is high, and it is prone to recurrence and dis
To investigate the therapeutic efficacy of autologous skin graft transplantation in conjunction with double-layer artificial dermis in treating finger skin wounds that are chronically refractory and soft tissue defects that expose bone and tendon.
Sixty-eight chronic refractory patients with finger skin and soft tissue defects accompanied by bone and tendon exposure who were admitted from July 2021 to June 2022 were included in this study. The observation group was treated with double layer artificial dermis combined with autologous skin graft transplantation (n = 49), while the control group was treated with pedicle skin flap transplan
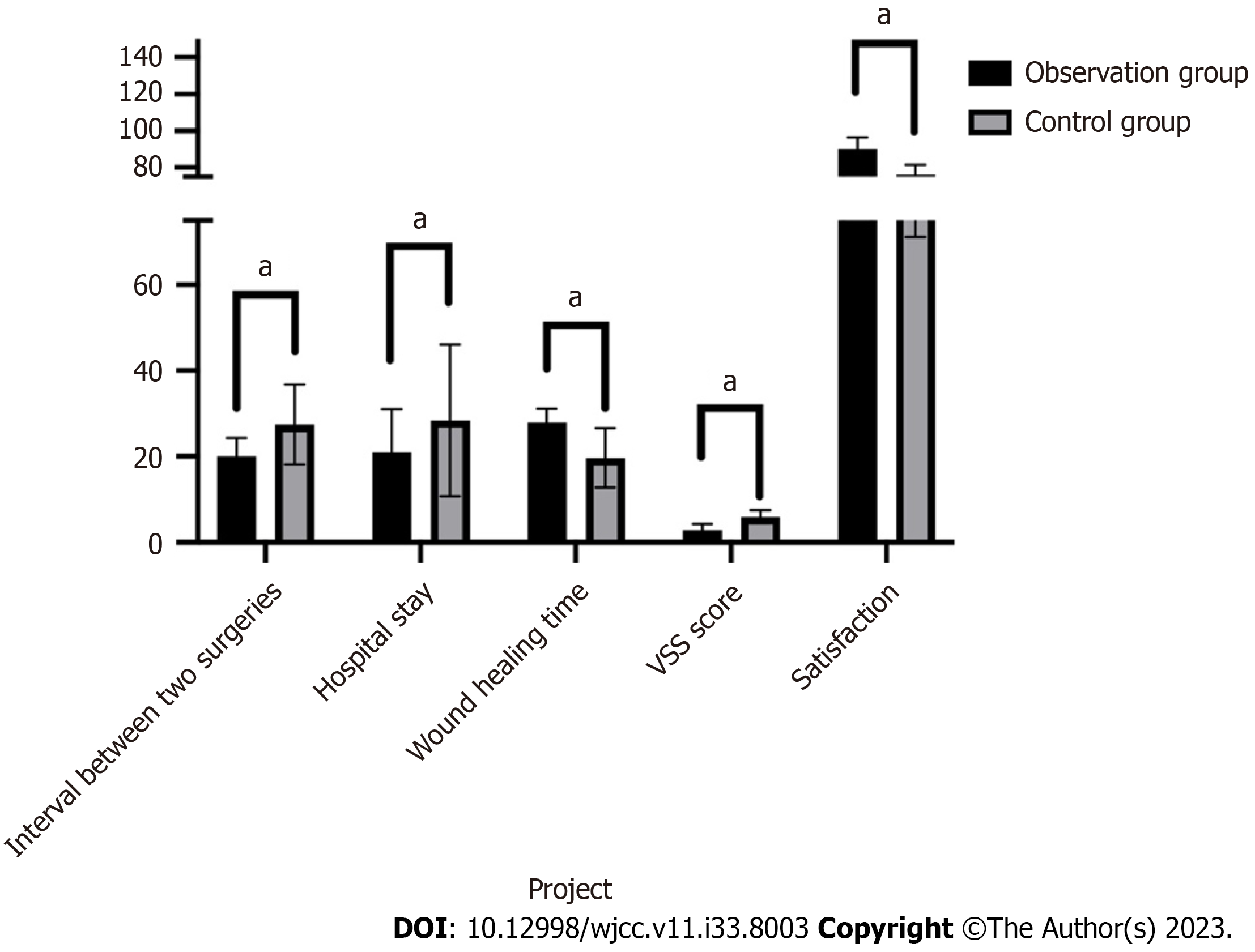
Wound healing time in the observation group was significantly longer than that in the control group (P < 0.05, 27.92 ± 3.25 d vs 19.68 ± 6.91 d); there was no significant difference in the survival rate of skin grafts/flaps between the two patient groups (P > 0.05, 95.1 ± 5.0 vs 96.3 ± 5.6). The interval between two surgeries (20.0 ± 4.3 d) and hospital stay (21.0 ± 10.1 d) in the observation group were both significantly shorter than those in the control group (27.5 ± 9.3 d) and (28.4 ± 17.7 d), respectively (P < 0.05). In comparison to postoperative infection (23.5%) and subcutaneous hematoma (11.8%) in the control group, these were considerably lower at (10.2%) and (6.1%) in the observation group. When comparing the two patient groups at six months post-surgery, the excellent and good rate of sensory recovery (91.8%) was significantly higher in the observation group than in the control group (76.5%) (P < 0.05). There was also no statistically significant difference in two point resolution (P > 0.05). The VSS score in the observation group (2.91 ± 1.36) was significantly lower than that in the control group (5.96 ± 1.51), and group satisfaction was significantly higher (P < 0.05, 90.1 ± 6.3 vs 76.3 ± 5.2).
The combination of artificial dermis and autologous skin grafting for the treatment of hand tendon exposure wounds has a satisfactory therapeutic effect. It is a safe, effective, and easy to operate treatment method, which is worthy of clinical promotion.
Core Tip: In this study, the observation group and the control group were treated with Lando double-layer artificial dermis and pedicled skin flap transplantation to repair hand wounds, respectively. There was no significant difference in the survival rate between the two groups of skin grafts/flaps after surgery. However, compared to the control group, the observation group had shorter surgical intervals and hospital stays, and the appearance of the fingers after surgery was better. The observation group also had better skin contracture and scar formation than the control group, resulting in higher satisfaction in the observation group.
- Citation: Wang W, Chen DS, Guo ZD, Yu D, Cao Q, Zhu XW. Artificial dermis combined with skin grafting for the treatment of hand skin and soft tissue defects and exposure of bone and tendon. World J Clin Cases 2023; 11(33): 8003-8012
- URL: https://www.wjgnet.com/2307-8960/full/v11/i33/8003.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i33.8003
As the primary organs used for labor and work, the hands and arms account for the majority of work-related injuries[1,2]. Hand injuries can have a major impact on a person's social work and everyday activities, causing patients to experience both physical and financial hardship[3,4]. The patient can be greatly impacted by pressure injuries to the hand, and due to the lengthy healing period, chronic and refractory wounds are easily formed, resulting in the wound being inadequately treated. The inability to recover quickly makes it challenging to return to a regular functional state. Patients may continue to have severe problems with the inflammatory response[5-7]. Pressure damage wounds can be chronic and resistant if they are not treated promptly, which will have a major negative impact on the wound's ability to heal[8,9].
The inability of skin and subcutaneous soft tissue to undergo a normal, orderly, and timely repair under the influence of internal and external factors, exhibit a pathological inflammatory response: wound non-healing is the complex formation mechanism of chronic refractory wounds[10,11]. The quality of life is significantly impacted by its intricate pathophysiology, which is frequently incurable, and secondary infections can occur[12]. In addition, the etiology is complicated, the course of therapy is protracted, expensive, and it is likely to reoccur and cause disability. In the medical field, treating refractory wounds is not easy[13]. Tissue engineering advancements have made it possible to treat refractory wounds with a novel approach that combines autologous skin graft with double layer artificial dermis, offering a fresh approach to treating minor finger skin abnormalities. Hypertrophic scars, severe burn wounds, exposed bone and tendon wounds, and postoperative tumor wounds have all been repaired using this approach[14]. This technique can reduce harm to the skin donor area, speed up the healing process, improve the appearance of the healed wound surface, and encourage functional recovery of the hands and feet. It can also lower the chance of scarring and recurrence.
In this study, a prospective analysis of 68 patients with refractory hand wounds admitted to the First People's Hospital of Jiangxia District, Wuhan City from July 2021 to June 2022 was conducted. Double layer artificial dermis combined with autologous skin graft was applied to treat these refractory wounds, which achieved satisfactory results, as described below.
Sixty-eight patients with chronic and refractory hand wounds who were admitted to our hospital from July 2021 to June 2022 were included in this study. The general information and wound status of these patients are shown in Table 1.
| Variable | Observation group | Control group |
| Sex (male/female) | 42/7 | 14/3 |
| Age, yr (mean ± SD) | 45.18 ± 14.04 | 45.94 ± 13.06 |
| BMI | 26.72 ± 6.35 | 27.08 ± 5.07 |
| Wound area, cm2 (mean ± SD) | 3.6 ± 0.47 | 3.7 ± 0.43 |
| Exposed area, cm2 (mean ± SD) | 3.0 ± 0.32 | 3.1 ± 0.35 |
| Leakage type (Tendon/bone) | 5/45 | 1/17 |
Inclusion criteria: (1) Comply with the diagnostic criteria for hand bone and joint injuries in the "Diagnostic Standards for Orthopedic Diseases", with finger skin and soft tissue defects accompanied by tendon or bone exposure, and comply with skin flap transplantation; (2) Receive treatment 12 h after injury; and (3) No severe organ and tissue diseases such as heart, brain, blood vessels, liver, kidney, lung, etc.
Exclusion criteria: (1) Individuals with infections in other parts such as the urinary system, respiratory system, etc.; (2) People with diabetes, heart disease, and arterial occlusive disease of lower limbs; and (3) Individuals with severe mental, neurological, immune, and hematological disorders.
After admission, the patients underwent thorough debridement to remove necrotic and degenerative skin, soft tissue, periosteum, and other tissues attached to the wound surface, tendons, and phalanges. The necrotic tendons were removed where appropriate and the periosteum and aponeurosis were preserved as much as possible. After sufficient hemostasis, the wound surface was repeatedly rinsed with hydrogen peroxide and physiological saline.
The observation group was treated with double-layer artificial dermis combined with autologous skin graft. Artificial dermis implantation: A suitable model of double-layer artificial dermis (Lando, Shenzhen Qikang Medical Equipment Co., Ltd.) was selected based on the size and shape of the wound, the artificial dermis was cut to size, soaked in sterile 0.9% physiological saline, the saline was replaced after 5 min, and this procedure was repeated 3 times. A thick needle was used to puncture the artificial dermis for drainage purposes. The upper and lower layers of the artificial dermis were distinguished, collagen was tightly fitted on the wound surface avoiding wrinkles and gaps, and the edges were sown and fixed. The inner layer was covered with sterile Vaseline gauze, and the outer layer was wrapped with sterile gauze. After 3-5 d, the Vaseline gauze and sterile gauze were replaced, and the wound surface covered by the artificial dermis. If fluid accumulated under the artificial dermis, it was squeezed and discharged appropriately. After treatment, Vaseline gauze and sterile gauze use was continued for wrapping. The dressing was replaced every 2-3 d and the condition of the artificial dermis autologous skin grafting was observed: After 2-3 wk, when the color of the artificial dermis changed to reddish yellow or orange, the outer layer of the artificial dermis was removed. Based on the size of the patient's wound, an autologous medium thickness skin graft approximately 0.4 mm thick was obtained from normal skin (forearm). A thick needle was used to appropriately puncture the autologous skin, it was covered with vascularized artificial dermis, and then sutured and fixed. The inner layer was padded with sterile Vaseline gauze, and the outer layer was padded with sterile gauze for wrapping. The skin supply area was covered with sterile Vaseline gauze, and sterile gauze was used for pressure wrapping. The dressing was changed 7-10 d after surgery, survival of the skin graft was determined, and the dressing was then changed every 2-3 d. According to the survival status of the skin graft, the dressing and sutures were removed 12 to 14 d after surgery.
The control group was treated with pedicle flap transplantation using an abdominal pedicle flap. The skin flap was designed based on the size of the finger wound and the flap extended 20% beyond the area of the finger skin defect. Layered intermittent suturing repair of the flap donor area was performed. The affected finger was placed in the appropriate position of the abdominal skin flap, the skin flap was intermittently sutured and the skin margin of the finger wound the flap has a broken pedicle. Four weeks after surgery, the pedicle of the abdominal pedicle skin flap was performed.
The following treatment outcomes in the two groups of patients were compared: (1) The interval between two surgeries and hospitalization time; (2) Wound healing through complete closure and epithelialization of the wound edge; and (3) The survival rate of skin grafts by assessing the proportion of active skin fragments on the wound surface.
The following postoperative wound infections between the two groups of patients were compared: (1) The amount of exudate using the Falange score of 4, with 1 being the minimum value; 2: Moderate; and 3: Out of control; and (2) Assessment of subcutaneous hematoma.
The postoperative wound recovery in the two groups of patients was compared as follows: (1) Skin sensation recovery after 6 mo using the sensory injury grading method and two-point resolution test. With regard to the sensory injury grading method S5: Completely normal sensation; S4: Some pain and tactile sensation with two-point discrimination; S3: Some pain and tactile sensation; S2: Pain and local touch; S1: Deep pain; S0: No sensation. S0 and S1 were considered poor, S2 and S3 were considered good, and S4 and S5 were considered excellent. The excellent rate and good rate were calculated; The two-point discrimination test, with higher scores and smaller distances indicated better finger recovery; and (2) After 6 mo, the Vancouver Scar Scale (VSS) was used to evaluate scar status in the receptor area, which included four aspects: color, vascular distribution, thickness, and softness, with a score of 0 to 15 points; The higher the score, the greater the scarring, and vice versa.
Satisfaction in the two groups of patients was compared using a questionnaire at the end of the sixth month of follow-up, with the score ranging from 0 to 100. The higher the score, the higher the patient satisfaction.
Data analysis was conducted using SPSS 26.0 statistical software. The chi square test was used to compare differences in categorical variables, such as postoperative wound infection and subcutaneous hematoma. For continuous variables, a normality test was first performed. For variables with a normal distribution, such as exudate volume and VSS, inde
All adult subjects provided written informed consent, and all clinical studies followed the principles of the Helsinki Declaration. Prior to analysis, all patient data were anonymous. The patients agreed in writing to the use of accom
All 68 cases of chronic and refractory wounds of the hand healed. After wound healing all patients were discharged and followed up once a month for 6 mo.
Table 2 and Figure 1 show that the interval between two surgeries (20.0 ± 4.3 d) and hospitalization time (21.0 ± 10.1 d) in the observation group (artificial dermis combined with autologous skin graft transplantation) were significantly shorter than those in the control group (skin flap transplantation) (27.5 ± 9.3 d) and hospitalization time (28.4 ± 17.7 d), (P < 0.05), but the wound healing time in the observation group was significantly longer than that in the control group (P < 0.05, 27.92 ± 3.25 d vs 19.68 ± 6.91 d); There was no significant difference in the survival rate of skin grafts/flaps between the two groups of patients (P > 0.05, 95.1 ± 5.0 vs 96.3 ± 5.6). The rates of postoperative infection (10.2%) and subcutaneous hematoma (6.1%) in the observation group were significantly lower than those in the control group (23.5%) and (11.8%), respectively. At 6 mo after surgery, the excellent and good rate of sensory recovery in the observation group (91.8%) was significantly higher than that in the control group (76.5%) (P < 0.05), and there was no statistically significant difference in two-point resolution between the two groups of patients (P > 0.05); The VSS score in the observation group (2.91 ± 1.36) was significantly lower than that of the control group (5.96 ± 1.51), and satisfaction in the observation group was significantly higher than that in the control group (P < 0.05, 90.1 ± 6.3 vs 76.3 ± 5.2).
| Variable | Observation group | Control group |
| Interval between two surgeries (d) | 20.0 ± 4.3 | 27.5 ± 9.3 |
| Hospital stay (d) | 21.0 ± 10.1 | 28.4 ± 17.7 |
| Wound healing time (d) | 27.92 ± 3.25 | 19.68 ± 6.91 |
| Skin graft/flap survival rate | 95.1 ± 5.0 | 96.3 ± 5.6 |
| Postoperative infection | 5 (10.2) | 4 (23.5) |
| Subcutaneous hematoma | 3 (6.1) | 2 (11.8) |
| Recovery of skin sensation | ||
| Optimal | 25 (51.0) | 5 (29.4) |
| Good | 20 (40.8) | 8 (47.1) |
| Poor | 4 (8.2) | 4 (23.5) |
| Two point resolution | ||
| ≥ 7 mm | 18 (36.7) | 10 (58.5) |
| 4-6 mm | 31 (63.3) | 7 (41.2) |
| VSS score | 2.91 ± 1.36 | 5.96 ± 1.51 |
| Satisfaction | 90.1 ± 6.3 | 76.3 ± 5.2 |
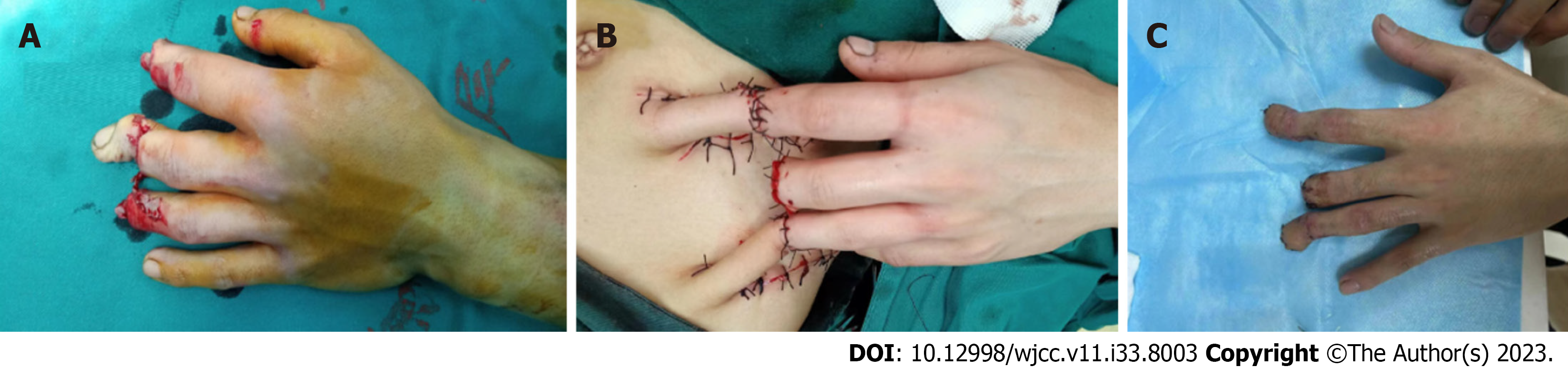
Figures 2 and 3 show representative cases of the natural progress of skin grafts after surgery in the two groups.
Exposed wounds of hand and foot tendons are extremely common in the clinical work of hand and foot surgeons and burn plastic surgeons. Due to the extremely important role of hands and feet in the daily life of patients, priority should be given to the function and aesthetic appearance of the affected limb when deciding on the repair method for such wounds[15]. Free flap transplantation is currently the most widely used treatment method for full-thickness skin defects[16]. However, as people's pursuit of beauty continues to improve, restoration of the appearance of hand and foot wounds after injury is increasingly valued, and the main factor affecting the appearance is excessive scar hyperplasia. Studies have shown[17-19] that the main cause of scar formation is contraction of the wound surface. Inhibiting the contraction of the wound surface helps to promote scar free regeneration, while artificial dermis, as a wound contraction inhibitor, can eliminate contraction and achieve scar minimization when used to treat soft tissue defects. Currently, artificial dermis has been widely used in the repair of burns, severe injuries, and non-healing wounds, and has achieved satisfactory results[20-22]. However, there are few reports on the application of artificial dermis combined with auto
Double layer artificial dermis is a substitute for dermis, with characteristics such as biomimetic and biodegradable properties[23]. The domestically produced double-layer artificial dermis Lando is composed of a medical silicone film on the surface layer and a bovine Achilles tendon collagen and polysaccharide removed from the end peptide on the lower layer. The semi-transparent medical silicone film on its surface has biomimetic functions, which can play a role in breathability, controlling moisture, and blocking bacteria. It has suitable flexibility, can fit the wound surface and has mechanical strength, ensuring the sealing of the wound and reducing the risk of infection. The lower dermis has a degradable function, and its sponge-like scaffold layer guides the inward migration, proliferation, and gradual maturation of vascular endothelial cells and capillaries to form new blood vessels and dermal regeneration, reducing scar formation, contracture, and restoring skin elasticity[24]. Artificial dermis is commonly used to repair burn wounds and has gradually been used in recent years to repair exposed wounds of bones and tendons[25].
Artificial dermis is divided into a silicone membrane on the surface and a collagen sponge on the bottom. The collagen sponge provides a scaffold for the orderly growth of fibroblasts and the formation of capillaries on the wound surface, constructing dermoid tissue with a rich blood supply, covering exposed tendons and bone tissue, thereby providing a good transplant bed for autologous skin graft transplantation and promoting skin graft survival. Therefore, patient satisfaction and the incidence of limb deformities in the observation group in the present study were better than those in the control group. In addition, due to the similar composition and structure of artificial dermis to natural human skin, it can guide the growth of cells and blood vessels, thereby achieving orderly regeneration and permanent reconstruction of dermal tissue[19]. When the artificial dermis is fully vascularized, the silicone membrane is removed and a very thin layer of autologous skin is transplanted onto the newly formed granulation tissue, achieving effects similar to medium or even full thickness skin transplantation. Therefore, in this study, patients with exposed hand and foot tendons were treated with artificial dermis combined with autologous skin grafting. Due to the traditional free skin flap transplantation, the donor site healing time, wound surface, and scar growth in the donor site were repaired.
In this study, Lando double-layer artificial dermis and pedicled skin flap transplantation were used to repair wounds in the observation group and the control group, respectively. There was no significant difference in the survival rate of the skin graft/flap between the two groups after surgery. However, the observation group had shorter surgical intervals and hospital stays compared to the control group, and the postoperative appearance of the fingers was better. Skin contracture and scar formation after repair surgery are also important factors in evaluating the effectiveness of repair. We assessed the VSS score of the patient's wound surface during a follow-up period of 6 mo after surgery, and the results showed that the VSS score in the observation group was significantly lower than that in the control group. This indicated that Lando double-layer artificial dermis combined with autologous skin grafting is an effective method for repairing finger skin and soft tissue defects with bone and tendon exposure. In addition, the results of this study showed that with the prolongation of time after surgery, both groups of patients gradually recovered finger sensation, and the observation group had a significantly higher sensory recovery score at 6 mo after surgery than the control group, indicating that the use of Lando double-layer artificial dermis for wound repair was more effective than pedicle skin flap transplantation. This may be because newly generated fibroblasts and capillaries in the adjacent tissues after implantation of artificial dermis are immersed in the pores of the collagen sponge layer, and degrade to form a dermal-like granulation tissue matrix. On this basis, thin skin grafting was carried out, effectively reducing epidermal contracture and scar hyperplasia, and improving finger sensation recovery and flexibility. The proportion of patients in the observation group who had a 2-point resolution of 4-6 mm was higher than that in the control group at 6 mo after surgery, despite the fact that there was no significant difference in two-point resolution between the two groups. This finding may be related to the bias resulting from the small number of cases.
The results of this study show that compared with traditional skin flap repair methods, the application of artificial dermis in wound repair has the following advantages: (1) It can significantly inhibit scar growth and reduce skin contracture; (2) It can directly cover exposed tendons, reduce tendon adhesion, and create an excellent transplantation bed for blade thick skin grafting; (3) Increase the thickness and quality of soft tissue to achieve thinner autologous epidermal transplantation, minimizing trauma to the donor site; (4) After wound healing, the appearance is close to normal, effectively avoiding secondary scar repair; and (5) The surgical time is short, the surgery is simple and easy to perform, and the risk is low.
The results of this study indicate that thorough debridement of the wound surface is crucial before using double-layer artificial dermis, which is an important foundation for smooth vascularization of the artificial dermis[26,27]. In addition, we found that newly formed dermal tissue slowly grows inward from the outer edge of the wound, gradually covering the exposed bones and tendons until they are completely covered. Therefore, when double-layer artificial dermis is used to cover the wound, it should cover the fresh tissue at the edge of the wound to ensure successful vascularization of the artificial dermis and lay a solid foundation for later skin grafting. Infection and hematoma on the wound surface are the most common causes of failure in artificial dermis transplantation; thus, thorough debridement, hemostasis, and postoperative pressure bandages are all important for postoperative efficacy[28]. We chose to change the dressing for the first time 3-5 d after surgery, observe the condition of the wound, and if hematoma or infection was present, they were removed in a timely manner. The remaining artificial dermis can still be successfully vascularized, and measures such as a gauze dressing soaked with physiological saline containing antibiotics such as gentamicin or combined with negative pressure drainage can be applied. During the treatment process, the treatment strategy can be adjusted in a timely manner according to the patient’s actual situation[29].
However, as reported in some studies[30,31], there are also some problems with the combination of artificial dermis and autologous skin graft repair: (1) This technology inevitably involves secondary surgery; in our study, patients in the observation group had longer wound healing time than those in the control group, and some patients in the observation group were dissatisfied with the long treatment cycle; (2) Although artificial dermis is relatively expensive, due to the obvious scars, transfer flap surgery often requires secondary scar repair treatment; thus, the overall cost increase is not significant. Therefore, in order to compare the hospital stay, cost, and surgical frequency of artificial dermis combined with autologous skin flap repair with traditional skin flap repair, a cost-benefit analysis is still needed; (3) The anti-infection ability of artificial dermis is poor, and the combination of artificial dermis and autologous blade thickness skin grafting requires strict aseptic procedures; and (4) The formation of hematoma is a common complication of artificial dermis and can easily lead to interruption of healing and loss of artificial dermis. Therefore, the combination of artificial dermis and autologous skin graft requires careful hemostasis and appropriate fixation.
The limitation of our research is that firstly, this graduate student center is small; therefore, our sample size was limited and larger studies are needed to determine the clinical efficacy of treatment with artificial dermis combined with skin grafting. Secondly, this study did not take into account factors that may affect wound recovery, such as the patient's job type, and whether they smoke or drink alcohol.
In summary, it is preliminarily believed that the combination of artificial dermis and autologous skin graft has a satisfactory therapeutic effect on hand tendon exposure wounds. It provides a safe, effective, and easy to operate treatment method, which is worthy of clinical promotion.
When paired with autologous skin transplantation, double layer artificial dermis can promote functional recovery of hands and feet, limit damage to the skin donor area, improve the appearance of the healed wound surface, speed up the healing process, and lower the chance of scar formation and recurrence.
Treatment of chronic and refractory skin and soft tissue defects of the fingers with exposed bones and tendons.
To explore the clinical effect of double layer artificial dermis combined with autologous skin graft in repairing chronic refractory skin and soft tissue defects of the fingers with exposed bone and tendon.
Sixty-eight chronic refractory patients with finger skin and soft tissue defects accompanied by bone and tendon exposure admitted to our hospital were selected and divided into the observation group (double layer artificial dermis combined with autologous skin grafting) and the control group (pedicle skin flap transplantation). The treatment status of the two groups of patients was compared, as well as the survival rate, scar formation, recovery, and patient satisfaction with skin grafts/flaps at 6 mo after surgery.
Recovery, postoperative infection, treatment, and patient satisfaction were better in the observation group than in the control group. Skin sensation recovery and skin graft/flap survival rate did not significantly differ between the control and observation groups.
The combination of artificial dermis and autologous skin graft has a satisfactory therapeutic effect on hand tendon exposure wounds.
The combination of artificial dermis and autologous skin grafting can be an effective method for treating hand tendon exposed wounds.
I would like to express my gratitude to all those helped me during the writing of this thesis. I acknowledge the help of my colleagues, they have offered me suggestions in academic studies.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Surgery
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Grabiec AM, Poland S-Editor: Liu JH L-Editor: Webster JR P-Editor: Xu ZH
| 1. | Sozbilen MC, Dastan AE, Gunay H, Kucuk L. One-year prospective analysis of hand and forearm injuries in children. J Pediatr Orthop B. 2021;30:364-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 2. | Luria S, Talmud D, Volk I, Liebergall M, Calderon-Margalit R. The epidemiology of wrist and hand injury in two hospitals in Jerusalem: substantial differences between population subgroups. Isr J Health Policy Res. 2019;8:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 3. | Zhang J, Ren J, Liu Y, Huang D, Lu L. Resveratrol regulates the recovery of rat sciatic nerve crush injury by promoting the autophagy of Schwann cells. Life Sci. 2020;256:117959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 4. | Lee JK, Hwang D, Han SH, Lee Y. Complete Occlusion of Radial and Ulnar Arteries Following Hand Crush Injury with Multiple Carpometacarpal Joint Fracture-Dislocations. J Hand Surg Asian Pac Vol. 2022;27:376-380. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 5. | Güntürk ÖB, Kaplan İ, Yıldırım T, Gürbüz Y, Ademoğlu Y, Ada S. Reconstruction of mutilating hand injuries by microsurgical free tissue transfers from the foot. Injury. 2021;52:3646-3652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Shrestha S, Tamrakar S, Banskota AK. Outline of Hand and Wrist Injuries Presenting to an Emergency of a Tertiary Care Centre in Nepal. J Nepal Health Res Counc. 2019;17:340-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Kerschhagl M, Larcher L, Mattiassich G, Prantl L. Replantation of a circumferentially degloved thumb in an occupational crush injury - A case report and review of the literature. Clin Hemorheol Microcirc. 2019;71:403-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Messana F, Faccio D, Rizzato S, Marchica P, Bassetto F, Vindigni V, Tiengo C. Combining traditional and microsurgical reconstruction after a complex hand trauma with multiple tissue defects. A case report. Ann Ital Chir. 2021;10. [PubMed] |
| 9. | Zhou H, Yu T. Effect of Comprehensive Rehabilitation Training Program in Orthopedic Nursing of Patients with Residual Limb Injury Caused by Crush. J Healthc Eng. 2022;2022:6769572. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Wilkinson HN, Hardman MJ. Wound healing: cellular mechanisms and pathological outcomes. Open Biol. 2020;10:200223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 847] [Article Influence: 169.4] [Reference Citation Analysis (0)] |
| 11. | Cheng B, Jiang Y, Fu X, Hao D, Liu H, Liu Y, Huang Z, Tan Q, Wang L, Hu D, Yang Y, Han C, Cheng Z, Ran X, Li Y. Epidemiological characteristics and clinical analyses of chronic cutaneous wounds of inpatients in China: Prevention and control. Wound Repair Regen. 2020;28:623-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 12. | Raziyeva K, Kim Y, Zharkinbekov Z, Kassymbek K, Jimi S, Saparov A. Immunology of Acute and Chronic Wound Healing. Biomolecules. 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 272] [Cited by in RCA: 468] [Article Influence: 117.0] [Reference Citation Analysis (0)] |
| 13. | Falanga V, Saap LJ, Ozonoff A. Wound bed score and its correlation with healing of chronic wounds. Dermatol Ther. 2006;19:383-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 84] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 14. | Boyce ST, Lalley AL. Tissue engineering of skin and regenerative medicine for wound care. Burns Trauma. 2018;6:4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 128] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 15. | Lv Z, Yu L, Wang Q, Jia R, Ding W, Shen Y. The use of dermal regeneration template for treatment of complex wound with bone/tendon exposed at the forearm and hand, a prospective cohort study. Medicine (Baltimore). 2019;98:e17726. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Wang Y, Xu R, Luo G, Lei Q, Shu Q, Yao Z, Li H, Zhou J, Tan J, Yang S, Zhan R, He W, Wu J. Biomimetic fibroblast-loaded artificial dermis with "sandwich" structure and designed gradient pore sizes promotes wound healing by favoring granulation tissue formation and wound re-epithelialization. Acta Biomater. 2016;30:246-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 17. | Rippa AL, Kalabusheva EP, Vorotelyak EA. Regeneration of Dermis: Scarring and Cells Involved. Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 183] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 18. | Yannas IV, Tzeranis DS, So PTC. Regeneration of injured skin and peripheral nerves requires control of wound contraction, not scar formation. Wound Repair Regen. 2017;25:177-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 67] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 19. | Yannas IV, Tzeranis DS, So PTC. Regeneration mechanism for skin and peripheral nerves clarified at the organ and molecular scales. Curr Opin Biomed Eng. 2018;6:1-7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Verbelen J, Hoeksema H, Pirayesh A, Van Landuyt K, Monstrey S. Exposed tibial bone after burns: Flap reconstruction versus dermal substitute. Burns. 2016;42:e31-e37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 21. | Chen X, Chen H, Zhang G. Management of wounds with exposed bone structures using an artificial dermis and skin grafting technique. Clin Plast Surg. 2012;39:69-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Seavey JG, Masters ZA, Balazs GC, Tintle SM, Sabino J, Fleming ME, Valerio IL. Use of a bioartificial dermal regeneration template for skin restoration in combat casualty injuries. Regen Med. 2016;11:81-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 23. | Qiu X, Wang J, Wang G, Wen H. Vascularization of Lando(®) dermal scaffold in an acute full-thickness skin-defect porcine model. J Plast Surg Hand Surg. 2018;52:204-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Wang W, Zhang L, Sun L, She ZD, Tan RW, Niu XF. Biocompatibility and Immunotoxicology of the Preclinical Implantation of a Collagen-based Artificial Dermal Regeneration Matrix. Biomed Environ Sci. 2018;31:829-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 25. | Karami A, Tebyanian H, Sayyad Soufdoost R, Motavallian E, Barkhordari A, Nourani MR. Extraction and Characterization of Collagen with Cost-Effective Method from Human Placenta for Biomedical Applications. World J Plast Surg. 2019;8:352-358. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 26. | Widjaja W, Tan J, Maitz PKM. Efficacy of dermal substitute on deep dermal to full thickness burn injury: a systematic review. ANZ J Surg. 2017;87:446-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 27. | Nilforoushzadeh MA, Sisakht MM, Amirkhani MA, Seifalian AM, Banafshe HR, Verdi J, Nouradini M. Engineered skin graft with stromal vascular fraction cells encapsulated in fibrin-collagen hydrogel: A clinical study for diabetic wound healing. J Tissue Eng Regen Med. 2020;14:424-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 64] [Article Influence: 12.8] [Reference Citation Analysis (1)] |
| 28. | Hicks KE, Huynh MN, Jeschke M, Malic C. Dermal regenerative matrix use in burn patients: A systematic review. J Plast Reconstr Aesthet Surg. 2019;72:1741-1751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 29. | Ribeiro LM, Guerra AS. [Hidradenitis Suppurativa: Combined Treatment with Dermal Template, Skin Graft and Negative Pressure Wound Therapy, a Case Study]. Acta Med Port. 2018;31:59-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 30. | Yeong EK, Yu YC, Chan ZH, Roan TL. Is artificial dermis an effective tool in the treatment of tendon-exposed wounds? J Burn Care Res. 2013;34:161-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 31. | Yeong EK, Chen SH, Tang YB. The treatment of bone exposure in burns by using artificial dermis. Ann Plast Surg. 2012;69:607-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |