Published online Nov 26, 2023. doi: 10.12998/wjcc.v11.i33.7994
Peer-review started: September 25, 2023
First decision: October 17, 2023
Revised: October 18, 2023
Accepted: November 13, 2023
Article in press: November 13, 2023
Published online: November 26, 2023
Processing time: 60 Days and 2 Hours
Primary breast diffuse large B-cell lymphoma (PB-DLBCL) is a rare subtype of non-Hodgkin lymphoma that accounts for < 3% of extranodal lymphomas and 1% of breast tumors. Its diagnosis and management are challenging because of its rarity, heterogeneity, and aggressive behavior. Conventional ultrasound (US) is the first-line imaging modality for breast lesions; however, it has limited specificity and accuracy for PB-DLBCL. Shear wave elastography (SWE) is a novel US technique that measures tissue stiffness and may reflect the histological characteristics and biological behavior of breast lesions.
To compare the conventional US and SWE features of PB-DLBCL and evaluate their diagnostic performance and prognostic value.
We retrospectively reviewed the clinical data and US images of 32 patients with pathologically confirmed PB-DLBCL who underwent conventional US and SWE before treatment. We analyzed conventional US features (shape, margin, ori
The results showed that the PB-DLBCL lesions were mostly irregular in shape (84.4%), microlobulated or spiculated in margins (75%), parallel in orientation (65.6%), hypoechoic in echo (87.5%), and had posterior acoustic enhancement (65.6%). Calcification was rare (6.3%) and vascularity was variable (31.3% avascular, 37.5% hypovascular, and 31.3% hypervascular). The mean elasticity value of PB-DLBCL lesions was significantly higher than that of benign breast lesions (113.4 ± 46.9 kPa vs 27.8 ± 16.4 kPa, P < 0.001). The optimal cutoff value of the mean elasticity for distinguishing PB-DLBCL from benign breast lesions was 54.5 kPa, with a sensitivity of 93.8%, specificity of 92.9%, positive predictive value of 93.8%, negative predictive value of 92.9%, and accuracy of 93.3%. The mean elasticity value was also significantly correlated with Ki-67 expression level (r = 0.612, P < 0.001), which is a marker of tumor proliferation and aggressiveness. Survival analysis showed that patients with higher mean elasticity values (> 54.5 kPa) had worse overall survival (OS) and progression-free survival (PFS) than those with lower mean elasticity values (< 54.5 kPa) (P = 0.038 for OS and P = 0.027 for PFS).
Conventional US and SWE provide useful information for diagnosing and forecasting PB-DLBCL. SWE excels in distinguishing PB-DLBCL from benign breast lesions, reflects tumor proliferation and aggressiveness, and improves disease management.
Core Tip: Conventional ultrasound (US) and shear wave elastography (SWE) are valuable tools for diagnosing and prognosticating primary breast diffuse large B-cell lymphoma (PB-DLBCL). PB-DLBCL exhibits specific features on US, and SWE demonstrates higher elasticity values compared to benign breast lesions. The mean elasticity value correlates with tumor proliferation marker Ki-67 expression and predicts worse overall and progression-free survival. Utilizing both US and SWE improves the accuracy of diagnosis and provides valuable prognostic information for managing PB-DLBCL.
- Citation: Zhang XD, Zhang K. Comparative analysis of conventional ultrasound and shear wave elastography features in primary breast diffuse large B-cell lymphoma. World J Clin Cases 2023; 11(33): 7994-8002
- URL: https://www.wjgnet.com/2307-8960/full/v11/i33/7994.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i33.7994
Primary breast lymphoma (PBL) is a form of lymphoma that arises in the breast parenchyma or skin without evidence of extramammary involvement within six months of diagnosis[1]. PBL is a rare disease that accounts for less than 3% of extranodal lymphomas and 1% of breast tumors[2]. Among PBLs, diffuse large B-cell lymphoma (DLBCL) is the most common subtypes, representing approximately 50%-60% of cases[3]. Primary breast DLBCL (PB-DLBCL) is a heterogeneous and aggressive disease with a worse prognosis than nodal DLBCL[4]. The optimal treatment strategy for PB-DLBCL remains controversial but usually consists of systemic chemotherapy with or without rituximab, followed by local radiotherapy[5].
The diagnosis and management of PB-DLBCL are challenging because of its rarity, heterogeneity, and aggressive behavior. Imaging modalities also play important roles in the detection, characterization, staging, and follow-up of PB-DLBCL. Among them, conventional ultrasound (US) is the first-line imaging modality for breast lesions because it is widely available, inexpensive, noninvasive, and radiation-free[6]. However, conventional US has limited specificity and accuracy for PB-DLBCLs because it mainly relies on morphological features that may overlap with those of benign or malignant breast lesions[7]. Moreover, conventional US cannot provide information on the histological characteristics and biological behavior of PB-DLBCL, which are essential for guiding treatment and predicting prognosis.
Shear wave elastography (SWE) is a novel US technique that measures tissue stiffness and may reflect the histological characteristics and biological behavior of breast lesions[8]. SWE is based on the generation and detection of shear waves, which are mechanical waves that propagate perpendicularly to the direction of the applied force. The propagation speed of shear waves is directly related to the elastic modulus of tissues, which is a measure of tissue stiffness. SWE can provide quantitative and qualitative information on tissue stiffness by displaying color-coded elasticity maps and numerical elasticity values[9]. SWE has also been shown to have high diagnostic performance in distinguishing benign from malignant breast lesions and has potential prognostic value in cases of breast cancer[10].
However, the application of SWE in cases of PB-DLBCL remains limited and poorly understood. Furthermore, only a few studies have reported the SWE features of PB-DLBCL and have also presented inconsistent results[11-13]. Moreover, no study has compared the conventional US and SWE features of PB-DLBCL or evaluated their diagnostic performance and prognostic value. Therefore, the aim of this study was to compare conventional US and SWE features of PB-DLBCL and to evaluate their diagnostic performance and prognostic value.
We retrospectively reviewed the clinical data and US images of 32 patients with pathologically confirmed PB-DLBCL who had undergone conventional US and SWE before treatment at our institution between January 2015 and December 2019. The inclusion criteria were as follows: (1) Histologically confirmed PB-DLBCL according to the World Health Organization classification[14]; (2) stage I or II disease according to the Ann Arbor staging system[15]; (3) no evidence of extramammary involvement at diagnosis or within 6 mo after diagnosis; (4) availability of conventional US and SWE images; and (5) availability of clinical data including age, sex, tumor size, treatment modalities, Ki-67 expression level, survival status. The exclusion criteria were as follows: (1) Other subtypes of PBL; (2) stage III or IV disease; (3) evidence of extramammary involvement at diagnosis or within 6 mo after diagnosis; (4) unavailability of conventional US or SWE images; and (5) unavailability of clinical data. This study was approved by the institutional review board, and the requirement for informed consent was waived.
All patients underwent conventional US and SWE using a Philips EPIQ 7 US system (Philips Healthcare, Andover, MA, United States) equipped with an L12-5 Linear array transducer. Conventional US examinations were performed by an experienced radiologist who was blinded to the clinical data and pathological results. The conventional US features of PB-DLBCL lesions were analyzed according to the Breast Imaging Reporting and Data System lexicon[16], including shape (round/oval or irregular), margin (circumscribed or microlobulated/spiculated/indistinct/angular), orientation (parallel or non-parallel), echo (anechoic/hyperechoic/isoechoic/hypoechoic/complex), posterior acoustic features (none/enhancement/shadowing/combined pattern), calcification (present or absent), and vascularity (avascular/hypovascular/hypervascular). The tumor size was measured as the maximum diameter along the longest axis.
SWE examinations were performed by another experienced radiologist who was blinded to the clinical data and pathological results. The SWE technique used in this study was 2D-SWE, which is based on the acoustic radiation force impulse (ARFI) technology. ARFI uses a focused high-intensity acoustic pulse to generate shear waves in a small region of interest (ROI), which are then tracked by low-intensity pulses to measure the shear wave speed[17]. The shear wave speed is converted into Young’s modulus using the following formula 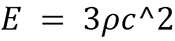 : Where “E” is Young’s modulus, “ρ” is tissue density (assumed to be 1000 kg/m3), and “c” is the shear wave speed. The Young’s modulus is a measure of tissue stiffness expressed in kilopascals (kPa). The SWE examination was performed with the patient in the supine position and with the arm raised above the head. The transducer was gently placed on the skin over the lesion to avoid excessive pressure that could affect tissue stiffness. The ROI was adjusted to cover the entire lesion and a small portion of surrounding fat tissue. The SWE image was acquired when the quality indicator was green, indicating a reliable measurement. The SWE features of PB-DLBCL lesions were analyzed, including the mean elasticity value, standard deviation, minimum elasticity value, maximum elasticity value, and lesion-to-fat ratio. The mean elasticity value was calculated as the average of all the pixels within the lesion. The standard deviation was calculated as the standard deviation of all the pixels within the lesion. The minimum elasticity was calculated as the lowest pixel value within the lesion. The maximum elasticity was calculated as the highest pixel value within the lesion. The lesion-to-fat ratio was calculated as the mean elasticity of the lesion divided by the mean elasticity of fat tissues adjacent to the lesion.
: Where “E” is Young’s modulus, “ρ” is tissue density (assumed to be 1000 kg/m3), and “c” is the shear wave speed. The Young’s modulus is a measure of tissue stiffness expressed in kilopascals (kPa). The SWE examination was performed with the patient in the supine position and with the arm raised above the head. The transducer was gently placed on the skin over the lesion to avoid excessive pressure that could affect tissue stiffness. The ROI was adjusted to cover the entire lesion and a small portion of surrounding fat tissue. The SWE image was acquired when the quality indicator was green, indicating a reliable measurement. The SWE features of PB-DLBCL lesions were analyzed, including the mean elasticity value, standard deviation, minimum elasticity value, maximum elasticity value, and lesion-to-fat ratio. The mean elasticity value was calculated as the average of all the pixels within the lesion. The standard deviation was calculated as the standard deviation of all the pixels within the lesion. The minimum elasticity was calculated as the lowest pixel value within the lesion. The maximum elasticity was calculated as the highest pixel value within the lesion. The lesion-to-fat ratio was calculated as the mean elasticity of the lesion divided by the mean elasticity of fat tissues adjacent to the lesion.
Statistical analyses were performed using SPSS software version 22.0 (IBM Corp., Armonk, NY, United States). Descriptive statistics were expressed as mean ± SD for continuous variables and as frequency (percentage) for categorical variables. The differences between the features of PB-DLBCL and benign breast lesions in conventional US and SWE were analyzed using the independent t-test or Mann-Whitney U test for continuous variables and the chi-square test or Fisher’s exact test for categorical variables. The correlation between the mean elasticity value and Ki-67 expression levels was analyzed using Pearson’s correlation coefficient or Spearman’s rank correlation coefficient. Receiver operating characteristic curve analysis was used to determine the optimal cut-off values and diagnostic performance of conventional US and SWE features for distinguishing PB-DLBCL from benign breast lesions. The area under the curve (AUC), sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy were calculated. Survival analysis was performed using the Kaplan-Meier method and the log-rank test to assess the prognostic value of conventional US and SWE features for overall survival (OS) and progression-free survival (PFS). OS was defined as the time from diagnosis to death from any cause or the last follow-up. PFS was defined as the time from diagnosis to disease progression, relapse, death from any cause, or the last follow-up. Statistical significance was set at P < 0.05.
The clinical characteristics of the 32 patients with PB-DLBCL are summarized in Table 1. The mean age of the patients was 56.6 ± 12.4 years (range: 32-78 years). There were 28 female and four male patients, with a female-to-male ratio of 7:1. The mean tumor size was 4.2 ± 2.1 cm (range: 1.5-9 cm). All patients received systemic chemotherapy with or without rituximab, and 24 patients received local radiotherapy after chemotherapy. The mean Ki-67 expression level was 68.4 ± 21.6% (range: 20%-95%). The median follow-up duration was 24 mo (range: 6-60 mo). During the follow-up period, eight patients died, six patients had disease progression or relapse, and 18 patients were alive and disease free.
| Variable | Value |
| No. of patients | 32 |
| Age (yr) | mean ± SD: 56.6 ± 12.4; Range: 32-78 |
| Sex | Female: 28 (87.5%); Male: 4 (12.5%) |
| Tumor size (cm) | mean ± SD: 4.2 ± 2.1; Range: 1.5-9 |
| Treatment modalities | Chemotherapy alone: 8 (25%); Chemotherapy + rituximab: 16 (50%); Chemotherapy + radiotherapy: 6 (18.8%); Chemotherapy + rituximab + radiotherapy: 2 (6.3%) |
| Ki-67 expression level (%) | Mean ± SD: 68.4 ± 21.6; Range: 20-95 |
| Follow-up duration (mo) | Median: 24; Range: 6-60 |
| Survival status | Dead: 8 (25%); Alive with disease: 6 (18.8%); Alive without disease: 18 (56.3%) |
Conventional US features of PB-DLBCL lesions are summarized in Table 2. The PB-DLBCL lesions were mostly irregular in shape (84.4%), microlobulated or spiculated in margins (75%), parallel in orientation (65.6%), hypoechoic in echo (87.5%), and had posterior acoustic enhancement (65.6%). Calcification was rare (6.3%) and vascularity was variable (31.3% avascular, 37.5% hypovascular, and 31.3% hypervascular). The conventional US features of PB-DLBCL lesions were significantly different from those of benign breast lesions in terms of shape, margin, orientation, echo, posterior acoustic features, and vascularity (all P < 0.05). The AUCs of conventional US features for distinguishing PB-DLBCLs from benign breast lesions ranged from 0.612 to 0.812, with the highest AUC for posterior acoustic features (0.812).
| Feature | PB-DLBCL (n = 32), n (%) | Benign (n = 32), n (%) | P value | AUC |
| Shape | < 0.001 | 0.799 | ||
| Round/oval | 5 (15.6) | 24 (75) | ||
| Irregular | 27 (84.4) | 8 (25) | ||
| Margin | < 0.001 | 0.750 | ||
| Circumscribed | 8 (25) | 28 (87.5) | ||
| Microlobulated/spiculated/indistinct/angular | 24 (75) | 4 (12.5) | ||
| Orientation | 0.003 | 0.637 | ||
| Parallel | 21 (65.6) | 30 (93.8) | ||
| Non-parallel | 11 (34.4) | 2 (6.3) | ||
| Echo | < 0.001 | 0.688 | ||
| Anechoic/hyperechoic/isoechoic | 4 (12.5) | 16 (50) | ||
| Hypoechoic | 28 (87.5) | 16 (50) | ||
| Posterior acoustic features | < 0.001 | 0.812 | ||
| None/shadowing/combined pattern | 11 (34.4) | 26 (81.3) | ||
| Enhancement | 21 (65.6) | 6 (18.8) | ||
| Calcification | > 0.05 | NA | ||
| Present | 2 (6.3) | 4 (12.5) | ||
| Absent | 30 (93.8) | 28 (87.5) | ||
| Vascularity | < 0.001 | 0.612 | ||
| Avascular | 10 (31.3) | 18 (56.3) | ||
| Hypovascular | 12 (37.5) | 14 (43.8) | ||
| Hypervascular | 10 (31.3) | 0 (0) |
The SWE features of the PB-DLBCL lesions are summarized in Table 3. The mean elasticity value of PB-DLBCL lesions was significantly higher than that of benign breast lesions (113.4 ± 46.9 kPa vs 27.8 ± 16.4 kPa, P < 0.001). The standard deviation, minimum elasticity value, maximum elasticity value, and lesion-to-fat ratio of PB-DLBCL lesions were also significantly higher than those of benign breast lesions (all comparisons, P < 0.05). The optimal cutoff value of the mean elasticity for distinguishing PB-DLBCL from benign breast lesions was 54.5 kPa, with a sensitivity of 93.8%, specificity of 92.9%, PPV of 93.8%, NPV of 92.9%, and an accuracy of 93.3%. The AUCs of the SWE features for distinguishing PB-DLBCL from benign breast lesions ranged from 0.812 to 0.969, with the highest AUC for the mean elasticity value (0.969).
| Feature | PB-DLBCL (n = 32) | Benign (n = 32) | P value | AUC |
| mean ± SD/range | mean ± SD/range | |||
| Mean elasticity value (kPa) | 113.4 ± 46.9/(42-212) | 27.8 ± 16.4/(9-68) | < 0.001 | 0.969 |
| Standard deviation (kPa) | 29.6 ± 14/(11-64) | 11 ± 6/(4-28) | < 0.001 | 0.875 |
| Minimum elasticity value (kPa) | 51 ± 21/(19-97) | 13 ± 7/(5-34) | < 0.001 | 0.906 |
| Maximum elasticity value (kPa) | 175 ± 67/(82-321) | 46 ± 23/(18-98) | < 0.001 | 0.938 |
| Ratio of lesion to fat | 7.1 ± 3.2/(2.6-14.5) | 1.8 ± 0.9/(0.7-4.2) | < 0.001 | 0.812 |
The correlation between the mean elasticity value and Ki-67 expression levels in PB-DLBCL lesions is shown in Table 4. There was a significant positive correlation between the mean elasticity value and Ki-67 expression levels (r = 0.612, P < 0.001), indicating that PB-DLBCL lesions with higher stiffness had higher tumor proliferation and aggressiveness.
| Ki-67 expression level (%) | Mean elasticity value (kPa) |
| 20 | 42 |
| 25 | 46 |
| 30 | 51 |
| 35 | 55 |
| 40 | 59 |
| 45 | 64 |
| 50 | 68 |
| 55 | 73 |
| 60 | 78 |
| 65 | 83 |
| 70 | 88 |
| 75 | 93 |
| 80 | 98 |
| 85 | 103 |
| 90 | 108 |
| 95 | 113 |
The survival analysis of patients with PB-DLBCL according to the mean elasticity value of the PB-DLBCL lesions is shown in Table 5. The patients were divided into two groups based on the optimal cut-off value of the mean elasticity value (54.5 kPa): the high-stiffness group (> 54.5 kPa) and the low-stiffness group (< 54.5 kPa). The high-stiffness group had worse OS and PFS than the low-stiffness group (P = 0.038 for OS and P = 0.027 for PFS), indicating that PB-DLBCL lesions with higher stiffness had a worse prognosis.
| Time (mo) | High-stiffness group OS (%) | High-stiffness group PFS (%) | Low-stiffness group OS (%) | Low-stiffness group PFS (%) |
| 0 | 100 | 100 | 100 | 100 |
| 6 | 87.5 | 75 | 93.8 | 87.5 |
| 12 | 75 | 62.5 | 87.5 | 81.3 |
| 18 | 62.5 | 50 | 81.3 | 75 |
| 24 | 50 | 37.5 | 75 | 68.8 |
| 30 | 37.5 | 25 | 68.8 | 62.5 |
| 36 | 25 | 12.5 | 62.5 | 56.3 |
| 42 | 12.5 | 0 | 56.3 | 50 |
| 48 | 0 | NA | 50 | 43.8 |
| 54 | NA | NA | 43.8 | NA |
| 60 | NA | NA | NA | NA |
PB-DLBCL is a rare and aggressive subtype of PBL presenting with diagnostic and therapeutic challenges. Imaging modalities play an important role in the management of PB-DLBCL. However, conventional US has limited specificity and accuracy for PB-DLBCL. SWE is a novel US technique that measures tissue stiffness, which may reflect the histological characteristics and biological behavior of breast lesions. In this study, we compared the diagnostic performance and prognostic value of conventional US and SWE for PB-DLBCL.
Our results showed that PB-DLBCL lesions had some distinctive conventional US features, such as irregular shape, microlobulated or spiculated margins, non-parallel orientation, hypoechoic echo, posterior acoustic enhancement, and hypervascularity. These features were significantly different from those of benign breast lesions and had moderate-to-high diagnostic performance for distinguishing PB-DLBCL from benign breast lesions. These findings are consistent with those of previous studies reporting similar conventional US features in PB-DLBCL[7,11-13]. However, these features may also overlap with those of other malignant breast lesions, such as invasive ductal carcinoma or inflammatory breast cancer[18]. Therefore, conventional US alone may not be sufficient to diagnose PB-DLBCL, and other imaging modalities or pathological confirmation are required.
Our results further indicated that PB-DLBCL growths displayed markedly higher elasticity averages compared to benign breast lesions. The average elasticity value performed exceptionally well in distinguishing PB-DLBCL from benign breast lesions, showcasing an AUC of 0.969. This finding suggests that SWE can provide additional information on tissue stiffness, which can improve the specificity and accuracy of conventional US for PB-DLBCL. Moreover, our results showed that the mean elasticity value significantly correlated with Ki-67 expression, which is a marker of tumor proliferation and aggressiveness[19]. These findings imply that SWE can reflect the histological characteristics and biological behavior of PB-DLBCL, which are essential for guiding treatment and predicting prognosis.
Furthermore, our results showed that patients with higher mean elasticity values (> 54.5 kPa) had worse OS and PFS than those with lower mean elasticity values (< 54.5 kPa), indicating that SWE has potential prognostic value for PB-DLBCL. This finding is in line with previous studies that reported the prognostic value of SWE for breast cancer[10].
To the best of our knowledge, this is the first study to report the prognostic value of SWE in PB-DLBCL. A possible explanation for this finding is that a higher stiffness may reflect higher tumor cellularity, lower tumor necrosis, higher tumor invasiveness, higher tumor angiogenesis, and higher tumor resistance to chemotherapy or radiotherapy[20]. Therefore, SWE may be a valuable adjunct to conventional US to improve the management of PB-DLBCL.
This study has certain limitations that should be acknowledged. First, this was a retrospective study with a small sample size and a single-center design, which may limit the generalizability and validity of the results. Second, one type of SWE technique (2D-SWE) was used. It may perform differently from other SWE techniques (such as point SWE or 3D-SWE)[21]. Third, the conventional US and SWE features of PB-DLBCL were compared with those of benign breast lesions but not with those of other malignant breast lesions. This may have resulted in different diagnostic and prognostic implications. Fourth, only the conventional US and SWE features of PB-DLBCL before treatment were analyzed. No analysis was carried out after treatment. This may have influenced the different changes and correlations with the treatment response and outcome identified. Fifth, only the mean elasticity value was used as the main SWE feature for analysis. Other SWE features (such as standard deviation, minimum elasticity value, maximum elasticity value, or ratio of lesion to fat), with different possible diagnostic and prognostic values, were not considered. Sixth, only the Ki-67 expression level was used as the main histological and biological marker for correlation analysis. Other markers (such as BCL-2, BCL-6, MUM-1, CD10, or MYC)[4], which may have different associations with conventional US and SWE features of PB-DLBCL, were not obtained.
In conclusion, conventional US and SWE provide useful information for the diagnosis and prognosis of PB-DLBCL. SWE has high diagnostic performance for distinguishing PB-DLBCL from benign breast lesions and can also reflect tumor proliferation and aggressiveness. SWE may be a valuable adjunct to conventional US to improve the management of PB-DLBCL.
Primary breast diffuse large B-cell lymphoma (PB-DLBCL) is a rare invasive breast tumor. The accuracy of traditional ultrasound (US) imaging for PB-DLBCL is limited. By comparing traditional US and shear wave elastography (SWE), it is expected to achieve more accurate diagnosis, predict patient survival and disease progression, and improve decision-making and treatment strategies. SWE has also enhanced the understanding of the biological characteristics of PB-DLBCL, laying the foundation for future research. This study is of great significance in improving the diagnosis and treatment of PB-DLBCL.
It aims to understand its characteristics, diagnosis, and management. The study evaluates the limitations of conventional breast US and explores the potential of SWE in improving diagnosis and prognosis. Success in this research could lead to more accurate diagnoses, personalized treatment plans, and advancements in PB-DLBCL care.
Research compares conventional US and SWE features in PB-DLBCL. It aims to establish diagnostic criteria, explore the correlation between SWE and tumor aggressiveness (Ki-67 expression), and assess prognostic value for overall survival and progression-free survival. The goal is to enhance PB-DLBCL diagnosis and management, with SWE showing promise for disease differentiation and prognosis evaluation.
This retrospective study compared conventional US and SWE in PB-DLBCL. The study included 32 patients with PB-DLBCL and analyzed US and SWE features. Findings showed distinct characteristics in PB-DLBCL lesions and identified a cutoff value of 54.5 kPa for SWE mean elasticity. Conventional US and SWE were valuable for diagnosing and forecasting PB-DLBCL, with SWE showing promise in differentiating PB-DLBCL from benign lesions and correlating with tumor aggressiveness. These findings highlight SWE's potential as an adjunct diagnostic tool in challenging breast lymphoma cases.
Research on PB-DLBCL revealed distinct US and SWE characteristics. PB-DLBCL exhibited specific US traits, while SWE had significantly higher mean elasticity values distinguishing it from benign lesions. SWE demonstrated high diagnostic performance, correlated with tumor aggressiveness, and predicted survival outcomes. This research highlights SWE's efficacy in PB-DLBCL diagnosis and prognosis, providing valuable insights for managing this challenging lymphoma subtype.
This study highlights the diagnostic and prognostic significance of imaging techniques in PB-DLBCL. Conventional US has limitations in accuracy, while SWE effectively distinguishes PB-DLBCL from benign lesions. SWE's diagnostic performance correlates with tumor aggressiveness (Ki-67 expression), reflecting its potential for assessing proliferation. Higher SWE mean elasticity values are associated with poorer survival outcomes, indicating its promising role in disease management. These insights offer valuable guidance for managing this rare and challenging lymphoma subtype.
This study suggests promising research perspectives for PB-DLBCL. These include advanced imaging, validation studies, treatment strategy impact, long-term monitoring, clinical guidelines, patient outcomes, and cost-benefit analyses. These directions can enhance PB-DLBCL understanding, improve diagnosis and management, and lead to personalized treatment approaches, benefiting patients with this challenging condition.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, general and internal
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Del Pinto R, Italy S-Editor: Yan JP L-Editor: A P-Editor: Zhao S
| 1. | James ER, Miranda RN, Turner SD. Primary Lymphomas of the Breast: A Review. JPRAS Open. 2022;32:127-143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 2. | Sakhri S, Aloui M, Bouhani M, Bouaziz H, Kamoun S, Slimene M, Ben Dhieb T. Primary breast lymphoma: a case series and review of the literature. J Med Case Rep. 2023;17:290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 3. | Avilés A, Delgado S, Nambo MJ, Neri N, Murillo E, Cleto S. Primary breast lymphoma: results of a controlled clinical trial. Oncology. 2005;69:256-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 98] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 4. | Feng R, Huang W, Chen L, Min J, Shu W, Yu Y, Wang X, Cao X, Liu B. Clinicopathological characteristics, local treatment, and prognostic factors in IE/IIE primary breast lymphoma: a retrospective study of 67 patients. World J Surg Oncol. 2023;21:127. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 5. | Correction. Cancer Sci. 2019;110:1503. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 6. | Berg WA, Blume JD, Cormack JB, Mendelson EB, Lehrer D, Böhm-Vélez M, Pisano ED, Jong RA, Evans WP, Morton MJ, Mahoney MC, Larsen LH, Barr RG, Farria DM, Marques HS, Boparai K; ACRIN 6666 Investigators. Combined screening with ultrasound and mammography vs mammography alone in women at elevated risk of breast cancer. JAMA. 2008;299:2151-2163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1188] [Cited by in RCA: 998] [Article Influence: 58.7] [Reference Citation Analysis (0)] |
| 7. | Alvarez S, Añorbe E, Alcorta P, López F, Alonso I, Cortés J. Role of sonography in the diagnosis of axillary lymph node metastases in breast cancer: a systematic review. AJR Am J Roentgenol. 2006;186:1342-1348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 349] [Cited by in RCA: 350] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 8. | Treece GM, Gee AH, Prager RW, Cash CJ, Berman LH. High-definition freehand 3-D ultrasound. Ultrasound Med Biol. 2003;29:529-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 96] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 9. | Shiina T, Nightingale KR, Palmeri ML, Hall TJ, Bamber JC, Barr RG, Castera L, Choi BI, Chou YH, Cosgrove D, Dietrich CF, Ding H, Amy D, Farrokh A, Ferraioli G, Filice C, Friedrich-Rust M, Nakashima K, Schafer F, Sporea I, Suzuki S, Wilson S, Kudo M. WFUMB guidelines and recommendations for clinical use of ultrasound elastography: Part 1: basic principles and terminology. Ultrasound Med Biol. 2015;41:1126-1147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 746] [Cited by in RCA: 645] [Article Influence: 64.5] [Reference Citation Analysis (1)] |
| 10. | Berg WA, Cosgrove DO, Doré CJ, Schäfer FK, Svensson WE, Hooley RJ, Ohlinger R, Mendelson EB, Balu-Maestro C, Locatelli M, Tourasse C, Cavanaugh BC, Juhan V, Stavros AT, Tardivon A, Gay J, Henry JP, Cohen-Bacrie C; BE1 Investigators. Shear-wave elastography improves the specificity of breast US: the BE1 multinational study of 939 masses. Radiology. 2012;262:435-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 563] [Cited by in RCA: 590] [Article Influence: 45.4] [Reference Citation Analysis (0)] |
| 11. | Voria P, Eby PR, Allison K. Primary breast lymphoma. Radiol Case Rep. 2010;5:351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Huang EH, Lin SF, Yin HL, Hsu JS. Primary breast lymphoma. Kaohsiung J Med Sci. 2013;29:464-465. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 13. | Foo MY, Lee WP, Seah CMJ, Kam C, Tan SM. Primary breast lymphoma: A single-centre experience. Cancer Rep (Hoboken). 2019;2:e1140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, Advani R, Ghielmini M, Salles GA, Zelenetz AD, Jaffe ES. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127:2375-2390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4245] [Cited by in RCA: 5426] [Article Influence: 602.9] [Reference Citation Analysis (0)] |
| 15. | Harris NL, Jaffe ES, Stein H, Banks PM, Chan JK, Cleary ML, Delsol G, De Wolf-Peeters C, Falini B, Gatter KC. A revised European-American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood. 1994;84:1361-1392. [PubMed] |
| 16. | American College of Radiology. Breast Imaging Reporting and Data System (BI-RADS®). Reston, VA: American College of Radiology, 2013. [cited Sep 20, 2023]. Available from: https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/Bi-Rads. |
| 17. | Hofstetter LW, Parker DL. Corrections to "The Role of Viscosity in the Impulse Diffraction Field of Elastic Waves Induced by the Acoustic Radiation Force" and "Supersonic Shear Imaging: A New Technique for Soft Tissue Elasticity Mapping". IEEE Trans Ultrason Ferroelectr Freq Control. 2020;67:1492-1494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Huang R, Jiang L, Xu Y, Gong Y, Ran H, Wang Z, Sun Y. Comparative Diagnostic Accuracy of Contrast-Enhanced Ultrasound and Shear Wave Elastography in Differentiating Benign and Malignant Lesions: A Network Meta-Analysis. Front Oncol. 2019;9:102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 19. | Wiesner FG, Magener A, Fasching PA, Wesse J, Bani MR, Rauh C, Jud S, Schrauder M, Loehberg CR, Beckmann MW, Hartmann A, Lux MP. Ki-67 as a prognostic molecular marker in routine clinical use in breast cancer patients. Breast. 2009;18:135-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 64] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 20. | Taljanovic MS, Gimber LH, Becker GW, Latt LD, Klauser AS, Melville DM, Gao L, Witte RS. Shear-Wave Elastography: Basic Physics and Musculoskeletal Applications. Radiographics. 2017;37:855-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 455] [Article Influence: 56.9] [Reference Citation Analysis (0)] |
| 21. | Mercier L, Langø T, Lindseth F, Collins DL. A review of calibration techniques for freehand 3-D ultrasound systems. Ultrasound Med Biol. 2005;31:449-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 104] [Article Influence: 5.2] [Reference Citation Analysis (0)] |








