Published online Nov 16, 2023. doi: 10.12998/wjcc.v11.i32.7905
Peer-review started: September 22, 2023
First decision: October 17, 2023
Revised: November 2, 2023
Accepted: November 9, 2023
Article in press: November 9, 2023
Published online: November 16, 2023
Processing time: 54 Days and 20.6 Hours
Gastric duplication cysts are very rare disease that are mainly diagnosed by endoscopic ultrasonographic fine-needle aspiration biopsy. In the past, this disease was usually treated with traditional surgery and rarely with minimally invasive endoscopic surgery. However, minimally invasive endoscopic therapy has many advantages, such as no skin wound, organ preservation, postoperative pain reduction, early food intake, fewer postoperative complications, and shorter post-procedure hospitalization.
We report a case of endoscopic ultrasonography-guided fine-needle aspiration (EUS-FNA) combined with lauromacrogol sclerotherapy for pyloric obstruction due to gastric duplication cysts.
EUS-FNA combined with lauromacrogol sclerotherapy provides a new option for the treatment of gastrointestinal duplication cysts.
Core Tip: Endoscopic ultrasonography-guided ablation, as a minimally invasive treatment, has received increasing attention in the past few years. This case report introduces the treatment experience of a 29-year-old male patient. For this patient, we successfully treated a gastric duplication cyst with endoscopic ultrasonography-guided fine-needle aspiration combined with lauromacrogol sclerotherapy for the first time. The patient’s symptoms were relieved with minimal trauma, and there were no complications during the 3-year follow-up.
- Citation: Bu YW, Han RQ, Ma WQ, Wang GN, Er LM. New treatment for gastric duplication cyst: Endoscopic ultrasonography-guided fine-needle aspiration combined with lauromacrogol sclerotherapy: A case report. World J Clin Cases 2023; 11(32): 7905-7910
- URL: https://www.wjgnet.com/2307-8960/full/v11/i32/7905.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i32.7905
Gastric duplication cysts are rare congenital abnormalities of the gastrointestinal tract and are extremely rare, accounting for only 2%-9% of all gastrointestinal duplication cysts[1]. They mainly occur in children and rarely in adults. According to the relevant literature reports[2,3], the main treatment method for gastric duplication cysts is surgical treatment, including laparotomy and laparoscopic surgery. Cases of endoscopic treatment are very rare. Only a few cases have reported endoscopic submucosal dissection surgery or endoscopic submucosal tunnelling techniques to resect gastric duplication cysts[4,5]. Currently, endoscopic ultrasonographic fine-needle aspiration biopsy is recognized as the first-line diagnostic method for gastric duplication cysts[6]. To the best of our knowledge, there is no previous literature report on the treatment of gastric duplication cysts by endoscopic ultrasonography-guided fine-needle aspiration (EUS) (EUS-FNA) combined with lauromacrogol ablation. In this paper, we report a case of gastric duplication cysts treated by EUS-FNA combined with lauromacrogol sclerotherapy.
A 29-year-old man came to our hospital for treatment because of abdominal dis-tension and abdominal pain for three months and vomiting for two days.
Three months before the visit, the patient developed intermittent abdominal distension and pain without obvious causes, especially in the upper abdomen, occasionally accompanied by belching and acid reflex. The patient’s symptoms could not be relieved after rest, and the above symptoms were aggravated by eating. During the period, the patient's weight was reduced by 10 kg compared with the previous period, but the patient still did not pay attention to it. The patient developed vomiting 2 d before the visit, and the vomit was of the stomach contents.
The patient was previously healthy.
No relevant disorders were identified.
Upon admission, the patient's vital signs were stable. Abdominal physical examination: the abdomen was flat and soft, no gastrointestinal peristalsis wave was observed, slight tenderness in the upper abdomen, no obvious rebound pain and muscle tension, and obvious succussion splash could be heard.
His preoperative laboratory findings were normal.
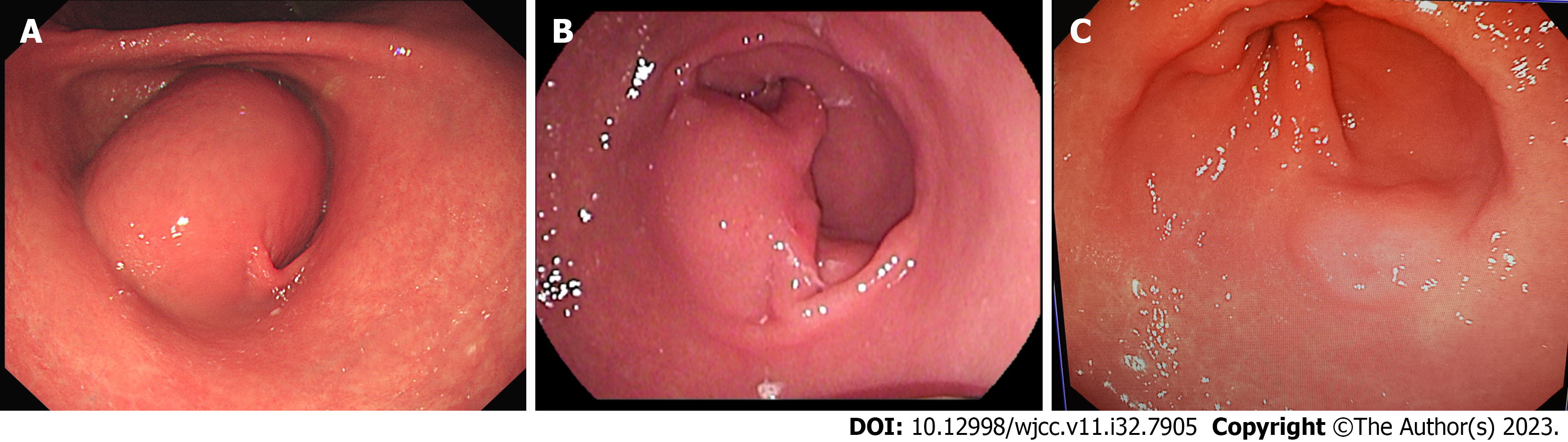
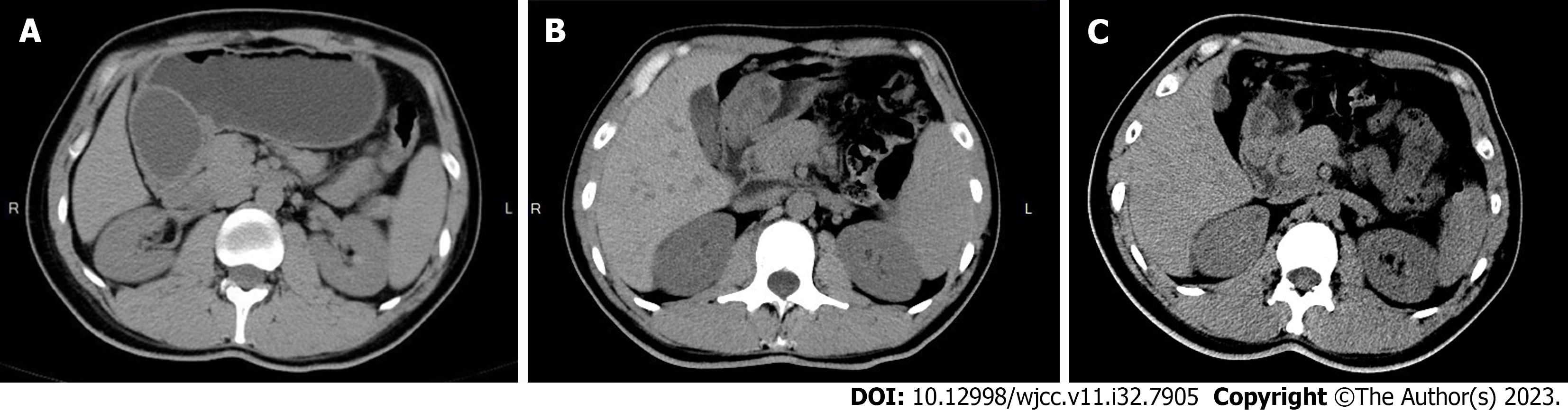
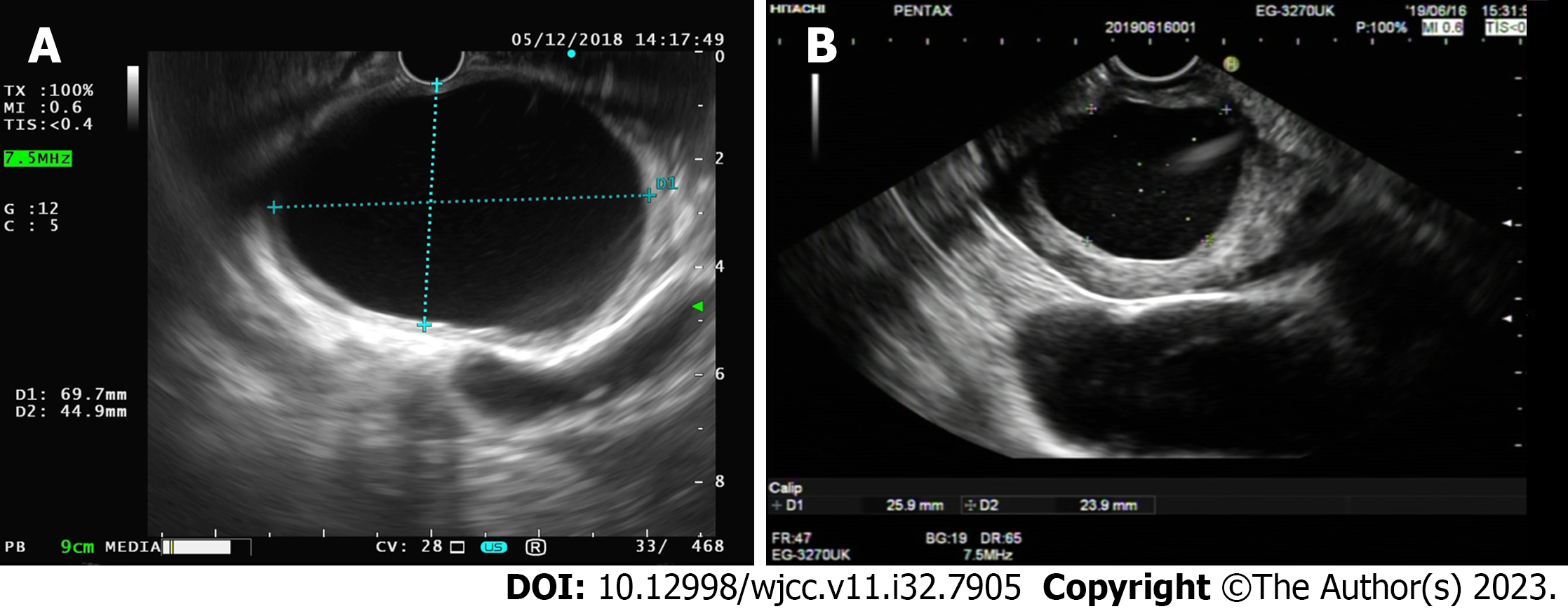
The patient underwent endoscopic gastroscopy in the outpatient department of our hospital, which revealed submucosal masses in the antrum and incomplete pyloric obstruction (Figure 1A). The enhanced computed tomography (CT) scan revealed a cystic density shadow within the initial cavity of the duodenum, measuring approximately 6.78 × 4.7578 × 6.94 centimeter in size, exhibiting smooth edges and a slightly thickened cyst wall. Furthermore, the enhanced CT scan demonstrated enhancement of the cyst wall. No enlarged lymph nodes were detected within the abdominal cavity or retroperitoneum (Figure 2A). For further diagnosis and treatment, the patient underwent (EUS). Ultrasound doppler showed that there was an anechoic homogeneous oval lesion (69.7 × 44.9 millimeter) originating from the submucosa in the gastric antrum (Figure 3A). The cyst wall was smooth, and there was no obvious blood flow signal in the cyst wall. The results of imaging examinations provided an important basis for the final diagnosis.
According to the initial gastroscopy imaging findings of the patient, we considered the localization and qualitative aspects. In terms of localization, we were relatively clear, considering it as the source of gastric tissue. Qualitatively, we considered that benign or low-grade malignant lesions were more likely. In clinical work, we often encounter gastric space occupying lesions, we pay more attention to gastric cancer and other malignant lesions, the diagnosis is relatively easy. However, the knowledge of diagnosis and differential diagnosis of benign lesions is relatively lacking. We considered the following diseases: gastric stromal tumor, gastric leiomyoma, gastric lymphoma, and gastric duplication cysts. For further diagnosis and treatment, the patient underwent enhanced abdominal CT and EUS, and we found a cystic mass. Combined with the patient’s clinical manifestations and imaging examination, the final diagnosis was gastric duplication cysts with incomplete pyloric obstruction after surgery and cytological examination.
The patient’s diagnosis, age, surgical risk assessment, potential complications, and postoperative quality of life were comprehensively evaluated. Following a multidisciplinary treatment meeting in our hospital, it was decided that the treatment scheme of this patient was surgical laparoscopic distal subtotal gastrectomy or minimally invasive treatment under endoscopic ultrasound. Subsequently, we explained in detail the condition and the pros and cons of the two treatment methods to the patient. Laparoscopic surgery has rich treatment experience, clear surgical vision, and may be more thorough in treatment. However, the trauma is relatively large, there are many postoperative complications, and the postoperative recovery of gastrointestinal function in postoperative patients with slow effect of comprehensive rehabilitation. It may lead to serious decline in patients' quality of life after surgery. Endoscopic minimally invasive treatment has the advantages of less trauma, fewer postoperative complications, quicker recovery and remaining the organ and the function. But the treatment technology is not yet mature and may be at risk of additional surgical procedures. After careful consideration, the patient finally chose the latter.
The patient was instructed to refrain from eating and drinking water upon admission, but considering the potential presence of gastric juice and residual food in the stomach cavity, general anesthesia may increase the risk of aspiration pneumonia. Therefore, we finally choose local anesthesia. Following oral administration of dyclonine hydrochloride mucilage for laryngeal anesthesia and lubrication, the patient underwent EUS-guided sclerotherapy with polylauryl alcohol. The COOK19G (ultrasound biopsy needle) puncture needle was used to puncture the cyst, avoiding the blood vessel, and approximately 60 mL of light yellow transparent liquid was extracted. We performed cytological examination on the extracted cystic fluid, and the results showed only foam cells. The cystic cavity was rinsed repeatedly with physiological saline until the cyst fluid became clear. Subsequently, the cystic cavity was rinsed repeatedly with approximately 15 mL of lauromacrogol, and finally, approximately 1/3 (5 mL) of lauromacrogol was retained in the cavity. The operation process was smooth. The operation procedure is shown in the Video.
After treatment, the patient’s obstruction symptoms disappeared. The patient received acid suppression and intravenous nutritional support during hospitalization. On the third postoperative day, the patient exhibited satisfactory recovery and was discharged from the hospital. Additionally, a gradual transition from a liquid diet to a regular diet was recommended. Six months after the operation, the patient’s endoscopic gastroscopy and abdominal CT showed that the cyst was significantly reduced and had developed a thick wall (Figures 1B and 2B). Re-examination by the ultrasonic gastroscope showed a cyst in the gastric antrum, with a thick wall and a diameter of approximately 25.9 × 23.9 millimeter (Figure 3B). At 19 mo after the operation, the patient refused ultrasonography. The endoscopic gastroscopy showed that the mucosa of the greater curvature of the gastric antrum was slightly higher than the mucosal surface, and the mucosa was smooth and intact (Figure 1C). Abdominal CT showed that there was a thick-walled cyst of approximately 1.5 centimeter in the gastric antrum with only a small amount of cyst fluid (Figure 2C). At present, the patient has been followed up for 3 years, and there has been no recurrence or any complication. Compared with before the onset of the disease, the quality of life of the patients after operation has not changed significantly. To closely monitor alterations in condition, we still recommend that patients undergo gastroscopy and CT examinations every 2-3 years.
In 1934, WE Ladd took the lead in introducing the concept of gastrointestinal duplication cysts[7]. Gastrointestinal duplication cysts were often described as spherical or tubular. Spherical cysts, which account for 80% of gastrointestinal duplication cysts, are the most common form and they usually do not communicate with the intestinal lumen. Tubular cysts are common in the small intestine and colon and they communicate with the intestinal lumen[1]. Gastrointestinal duplication cyst is a very rare congenital disease in adults, with a higher incidence in women than in men[8]. It can occur anywhere in the digestive tract, most commonly in the ileum, and gastric involvement is the rarest form. There are also some adults with gastrointestinal duplication cysts that are asymptomatic. Therefore, gastrointestinal duplication cysts are extremely rare in adults.
Gastric duplication cysts are most common in the greater curvature of the stomach[9]. The involvement of the gastric antrum and symptoms of obstruction are rarely reported. The clinical symptoms of gastric duplication cysts mainly depend on the age of the patient and the location and size of the lesion. In adults, gastric duplication cysts are mostly asymptomatic and are usually found incidentally by radiology or endoscopy. However, symptoms related to complications may occur, such as pain, obstruction, weight loss, ulcers and bleeding[10]. We reported that this patient was found to have a duplication cyst of the gastric antrum, mainly with obstructive symptoms.
Multiple imaging modalities, such as abdominal ultrasound, EUS, CT and magnetic resonance imaging (MRI), are used for gastrointestinal duplication cysts. Abdominal CT and MRI can identify gastrointestinal duplication cysts. However, due to the complex composition of the cyst fluid, it is difficult to distinguish some cystic fluid images with a high protein content from solid masses, which are easily misdiagnosed as conditions such as gastrointestinal-stromal-tumours[11]. EUS is essential for the diagnosis of submucosal masses. It can accurately distinguish the relationship between the cyst wall and adjacent gastrointestinal structures, thus helping to distinguish solid from cystic lesions. EUS-FNA can obtain histopathology, which is important for a differential diagnosis from other solid lesions and the exclusion of malignant transformation of cysts[6]. Therefore, EUS, especially EUS-FNA, has become a first-line diagnostic method for gastrointestinal duplication cysts.
Currently, there is no accepted treatment for gastrointestinal duplication cysts. Close follow-up is recommended for small or asymptomatic gastric duplication cysts. For symptomatic or giant cysts, surgical resection (including laparoscopic minimally invasive surgery) is usually preferred to remove the cyst and relieve the symptoms[2,3]. However, the functionality injury of normal organs caused by the surgery, as well as a variety of postoperative complications, make many flinch from the treatment of gastric duplication cysts.
Lauromacrogol was first synthesized in 1936. The main component of lauromacrogol is lauryl polyoxyethylene ether, mixed with ethanol and sterilized water. Lauromacrogol can close cysts by destroying the cells in the cyst wall, preventing cyst fluid secretion, adhesion, and fibrosis. Lauromacrogol does not cause any pain, either during tissue or intravenous injection, because it also has an effect of anaesthesia. It can provide the broadest range of safe treatment without exosmosis leading to necrosis. Lauromacrogol is easier and safer to use than anhydrous alcohol, another hardener. Therefore, since the 1860s, lauromacrogol has been used as a sclerotherapy agent in clinical treatment and has been widely used for the treatment of renal cysts, liver cysts and pancreatic cysts[12-16]. Referring to the treatment experience of lauromacrogol in other cystic lesions, we consider the use of lauromacrogol for sclerotherapy of gastric duplication cysts to be safe. In this case report, we successfully treated a gastric duplication cyst with EUS-FNA combined with lauromacrogol for the first time. As a new treatment method, EUS-FNA-guided lauromacrogol sclerotherapy has its own advantages in various aspects, including less trauma, a short hospital stay, less pain, a lower cost, and a reduced risk of postoperative adverse events, and it can be applied in cases of recurrence or new lesions.
Cases of malignant transformation[17,18] caused by gastric duplication cysts have been reported in the past. In our treatment, whether the damaged cyst wall after lauromacrogol sclerotherapy still has a risk of carcinogenesis and the best indication for treatment still require long-term follow-up and research. We also recommend annual CT and endoscopy and close follow-up for patients after sclerotherapy.
As a new treatment method, EUS-FNA guided lauromacrogol sclerotherapy has its own advantages in various aspects, including less trauma, a short hospital stay, less pain, a lower cost, and a reduced risk of postoperative adverse events, and it can be applied in cases of recurrence or new lesions. EUS-FNA combined with lauromacrogol sclerotherapy provides a new option for the treatment of gastrointestinal duplication cysts.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mubarak M, Pakistan; Suresh Kumar VC, United States S-Editor: Qu XL L-Editor: A P-Editor: Cai YX
| 1. | Doepker MP, Ahmad SA. Gastric duplication cyst: a rare entity. J Surg Case Rep. 2016;2016. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 2. | Passos ID, Chatzoulis G, Milias K, Tzoi E, Christoforakis C, Spyridopoulos P. Gastric duplication cyst (gdc) associated with ectopic pancreas: Case report and review of the literature. Int J Surg Case Rep. 2017;31:109-113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 3. | Ren HX, Duan LQ, Wu XX, Zhao BH, Jin YY. Laparoscopic resection of gastric duplication cysts in newborns: a report of five cases. BMC Surg. 2017;17:37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 4. | Fang Y, Gao T, Yang H, Ma S, Li Q, Zhou PH. Removal of an infant's gastric duplication cyst through endoscopic submucosal dissection: A case report. Medicine (Baltimore). 2019;98:e14820. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Chavan R, Nabi Z, Basha J, Sekaran A, Darisetty S, Reddy PM, Reddy DN. Endoscopic resection of a complex gastric duplication cyst using a submucosal tunneling technique. Endoscopy. 2022;54:E168-E169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Seijo Ríos S, Lariño Noia J, Abdulkader Nallib I, Lozano León A, Vieites Pérez-Quintela B, Iglesias García J, Domínguez Muñoz JE. [Adult gastric duplication cyst: diagnosis by endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA)]. Rev Esp Enferm Dig. 2008;100:586-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Balakrishnan K, Fonacier F, Sood S, Bamji N, Bostwick H, Stringel G. Foregut Duplication Cysts in Children. JSLS. 2017;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | Kim DH, Kim JS, Nam ES, Shin HS. Foregut duplication cyst of the stomach. Pathol Int. 2000;50:142-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Gupta S, Sleeman D, Alsumait B, Abrams L. Duplication cyst of the antrum: a case report. Can J Surg. 1998;41:248-250. [PubMed] |
| 10. | Hawkins ML, Lowery CH, Mullen JT. Gastric duplication. South Med J. 1974;67:189 passim. [PubMed] |
| 11. | Eloubeidi MA, Cohn M, Cerfolio RJ, Chhieng DC, Jhala N, Jhala D, Eltoum IA. Endoscopic ultrasound-guided fine-needle aspiration in the diagnosis of foregut duplication cysts: the value of demonstrating detached ciliary tufts in cyst fluid. Cancer. 2004;102:253-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 47] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Duffy DM. Sclerosants: a comparative review. Dermatol Surg. 2010;36 Suppl 2:1010-1025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 75] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 13. | Spârchez Z, Radu P, Zaharie F, Al Hajjar N, Sparchez M. Percutaneous treatment of symptomatic non-parasitic hepatic cysts. Initial experience with single-session sclerotherapy with polidocanol. Med Ultrason. 2014;16:222-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Wijnands TF, Görtjes AP, Gevers TJ, Jenniskens SF, Kool LJ, Potthoff A, Ronot M, Drenth JP. Efficacy and Safety of Aspiration Sclerotherapy of Simple Hepatic Cysts: A Systematic Review. AJR Am J Roentgenol. 2017;208:201-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 59] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 15. | Qin S, Liu Y, Ning H, Tao L, Luo W, Lu D, Luo Z, Qin Y, Zhou J, Chen J, Jiang H. EUS-guided lauromacrogol ablation of insulinomas: a novel treatment. Scand J Gastroenterol. 2018;53:616-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Gao K, Dai W, Wang F, He S. Efficacy Assessment and Analysis of Related Factors of Ultrasound-Guided Percutaneous Lauromacrogol Injection for Cystic Thyroid Nodules. J Ultrasound Med. 2023;42:1093-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 17. | Zheng J, Jing H. Adenocarcinoma arising from a gastric duplication cyst. Surg Oncol. 2012;21:e97-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 18. | Kuraoka K, Nakayama H, Kagawa T, Ichikawa T, Yasui W. Adenocarcinoma arising from a gastric duplication cyst with invasion to the stomach: a case report with literature review. J Clin Pathol. 2004;57:428-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 88] [Article Influence: 4.2] [Reference Citation Analysis (0)] |