Published online Nov 16, 2023. doi: 10.12998/wjcc.v11.i32.7865
Peer-review started: August 1, 2023
First decision: August 30, 2023
Revised: October 21, 2023
Accepted: November 2, 2023
Article in press: November 2, 2023
Published online: November 16, 2023
Processing time: 106 Days and 11.8 Hours
Abernethy malformation, also known as congenital extrahepatic portosystemic shunt, is an uncommon malformation resulting from aberrant development of the portal venous system. Cystic fibrosis (CF) is an autosomal recessive genetic disease caused by mutations in the CFTR gene. It mainly affects the exocrine glands of the respiratory, digestive and reproductive systems. It is considered extremely rare in the Asian population. We present a clinical case involving a pediatric patient of Asian descent who was diagnosed with Abernethy malfor
A 12-year-old girl presented with a medical history of recurring respiratory infections and hemoptysis, and chest computed tomography (CT) showed bronchiectasis. Whole exome sequencing was performed for the patient, yielding findings that revealed a compound heterozygous variant of the CFTR gene: c.233_c.234insT/p.Trp79fsTer3 (maternal origin); c.2909G>A/p.Gly970Asp (paternal origin). CF was diagnosed. The physician’s attention was drawn to the presence of splenomegaly during disease progression. Abdominal enhanced CT revealed splenomegaly, compression of the left kidney, and multiple tortuous dilated vascular shadows were seen at the splenic hilum, which flowed back into the left renal vein and portal vein, suggesting Abernethy malformation type II. Intraoperatively, the abnormal blood flow was seen to merge into the inferior vena cava through the left renal vein without hepatic processing, and the pathology of liver biopsy showed hypoplastic, dilated or absent portal vein branches, both of which supported the diagnosis of Abernethy malformation type II. This represents the initial documented instance of Abernethy malformation accompanied by a CFTR gene mutation in the existing body of literature.
Coexisting Abernethy malformation and CF are rare. Detailed medical history information, abdominal enhanced CT, venography and genetic testing contribute to diagnosis as well as differential diagnosis.
Core Tip: Abernethy malformation, also referred to as congenital extrahepatic portosystemic shunt, is an uncommon malformation resulting from anomalous development of the portal venous system. Cystic fibrosis (CF) is an autosomal recessive disorder caused by mutations in the CFTR gene, which is rare in Asian populations. We present an Asian child with type II Abernethy malformation coexisting with CF and discuss the diagnosis and treatment of Abernethy malformation and CF.
- Citation: Zhang LJ, Liu XY, Chen TF, Xu ZY, Yin HJ. Type II Abernethy malformation with cystic fibrosis in a 12-year-old girl: A case report. World J Clin Cases 2023; 11(32): 7865-7871
- URL: https://www.wjgnet.com/2307-8960/full/v11/i32/7865.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i32.7865
Abernethy malformation, alternatively referred to as congenital extrahepatic portosystemic shunt, is a congenital anomaly that arises from aberrant embryonic development of the umbilical and yolk veins[1,2]. This condition leads to an anomalous connection between the portal vein and vena cava, which exhibits a low occurrence rate, affecting approximately 1 in every 30000 live births[3]. Cystic fibrosis (CF) is a hereditary disorder characterized by autosomal recessive inheritance, resulting from mutations in the CFTR gene located on chromosome 7, which mainly affects the respiratory, digestive and reproductive systems[4,5]. CF exhibits a higher prevalence among individuals of Caucasian descent, while it is extremely rare in Asian populations[6,7]. We present an Asian girl with type II Abernethy malformation coexisting with CF. Compound heterozygous mutations of the CFTR gene were detected. We discuss the key points of diagnosis and treatment of Abernethy malformation and CF.
A 12-year-old girl was admitted to the respiratory department of our hospital on October 3, 2021, presenting with the chief complaints of cough with hemoptysis and dyspnea persisting for 4 d.
Four days previously, the individual presented with symptoms of a cough accompanied by hemoptysis and dyspnea subsequent to exposure to cold temperatures.
The patient has a medical history of recurrent respiratory tract infections dating back to early childhood. The patient had a history of patent foramen ovale and was admitted to our cardiothoracic surgery department 1 year previously. Cardiac ultrasound revealed a 2-mm echogenic interruption in the atrial septum, confirming the presence of a patent foramen ovale. Due to the small size of the defect and low platelet count (89 × 109/L), surgical intervention was not pursued.
The patient was G3P3, born at term with a birth weight of 3.5 kg. The Apgar score at birth was unknown, and there was no reported history of postnatal asphyxia. The patient’s parents were healthy and not blood relatives. The patient had a 25-year-old brother and a 14-year-old sister; neither of whom had any history of similar medical conditions.
Physical examination at admission showed body temperature 36.4°C, pulse rate 98 bpm, respiratory rate 24 breaths/min, and blood pressure 98/65 mmHg. The patient exhibited clear mental status, stable breathing, absence of cyanosis in the lips, no signs of aspiration, coarse breathing sounds in both lungs with audible wet rales, and the absence of clubbing of the fingers. The abdomen was found to be soft with no evidence of pressure or rebound pain. On palpation, the liver was located 3 cm below the rib cage, while the spleen was found to be 8 cm below the rib cage.
Laboratory test results were as follows: white blood cell count 3.28 × 109/L, platelet count 84 × 109/L; fecal occult blood, negative; blood biochemistry: alanine aminotransferase 28.0 U/L, aspartate aminotransferase 38.0 U/L, creatine kinase-MB 15.0 U/L; positive for Mycoplasma antibodies; sputum culture: Pseudomonas aeruginosa; bone marrow smear: normal proportions and morphology of the erythroid, myeloid, megakaryocytic, and lymphoid populations; tumor tests: a-fetoprotein 1.66 ng/mL, carcinoembryonic antigen 1.63 ng/mL, nonspecific enolase 10.87 ng/mL, carbohydrate antigen 19-9 90.95 ng/mL; fiberoptic bronchoscopy alveolar lavage: numerous erythrocytes and inflammatory cells, 74% neutrophils, 6% lymphocytes, and 20% macrophages.
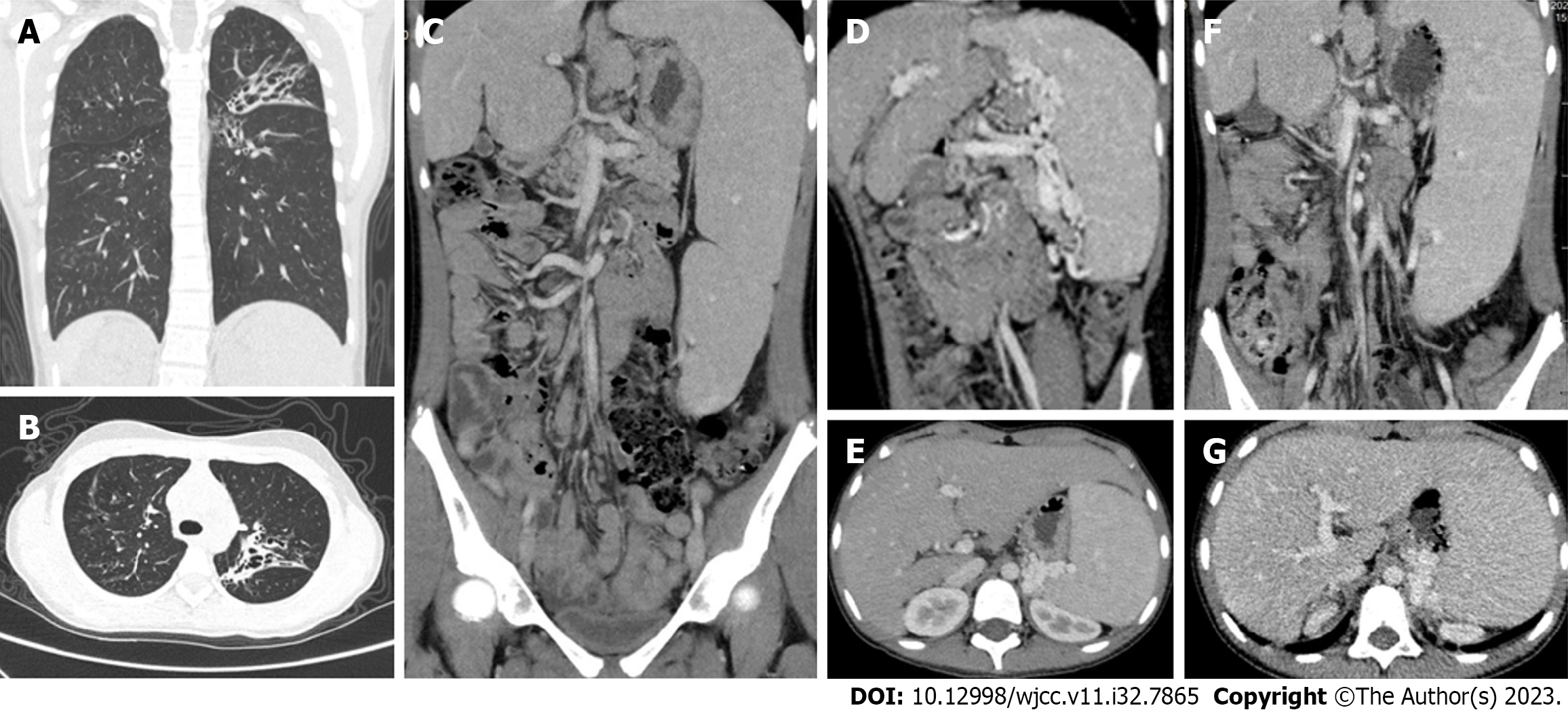
Chest computed tomography (CT) (Figure 1A and B) showed a flocculent shadow with multiple cystic translucent shadows in both lungs, and bronchiectasis with infection was considered. Ultrasound of the portal venous system showed that the internal diameter of the main trunk of the portal vein was 8 mm, with a maximum blood flow velocity of 19.3 cm/s, and a slightly tortuous course. The internal diameter of the splenic vein was 11 mm, with a tortuous course, slowed blood flow velocity, and tortuous vascular echoes around the fundus of the stomach, suggesting that the portal vein had a slightly tortuous course, and the splenic vein was thickened with a tortuous course. The whole abdomen was enhanced on CT imaging (Figure 1C-E). The liver was irregular in shape, with a large caudate lobe and no abnormal density shadows in the parenchyma. The gallbladder was not significantly abnormal in shape or size, and no abnormal density shadows were seen. The spleen was enlarged, the left kidney was compressed, and multiple tortuous dilated vascular shadows were seen at the splenic hilum, which flowed back into the left renal and portal veins. The findings were suggestive of Abernethy malformation type II.
The diagnosis of CF was based on a combination of recurrent respiratory infections, hemoptysis, splenomegaly and bronchiectasis. Prior to conducting full epigenetic testing, the parents were engaged in preoperative communication and provided written informed consent. The testing was carried out at our hospital by the Beijing Full Spectrum Medical Testing Laboratory. Whole-exome sequencing of the patient identified two compound heterozygous mutations, c.233_c.234insT (p.Trp79fsTer32) and c.2909G>A (p.Gly970Asp), in the CFTR gene, associated with CF (OMIM:219700). The c.233_c.234insT and c.2909G>A mutations were inherited from the mother and the father of the patient, respectively, as confirmed by Sanger sequencing (Figure 2). In exons 3 and 18 of the CFTR gene, a frameshift mutation (c.233_c.234insT) and a missense mutation (c.2909G>A) were identified, respectively. The mutation c.233_c.234insT caused a frameshift and premature stop codon 32 amino acids downstream (p.Trp79fsTer32), theoretically leading to the production of a truncated protein. According to the American College of Medical Genetics and Genomics guidelines (2019), both mutation classes are pathogenic. Sanger sequencing confirmed that the father carried a c.2909G>A/p.Gly970Asp missense mutation, while the mother was a carrier of a c.233_c.234insT heterozygous mutation. These findings are consistent with an autosomal compound heterozygous mutation inheritance pattern, known as autosomal stealth inheritance. The compound heterozygous mutation observed in the aforementioned case is the first reported instance in China, following a thorough review of the relevant literature and databases.
The diagnosis of CF was determined through an analysis of medical history, chest CT, and whole exon gene detection. Additionally, the diagnosis of Abernethy malformation type II was established through enhanced abdominal CT, intraoperative portography, and liver biopsy. Ultimately, the patient was diagnosed with Abernethy malformation type II concurrent with CF.
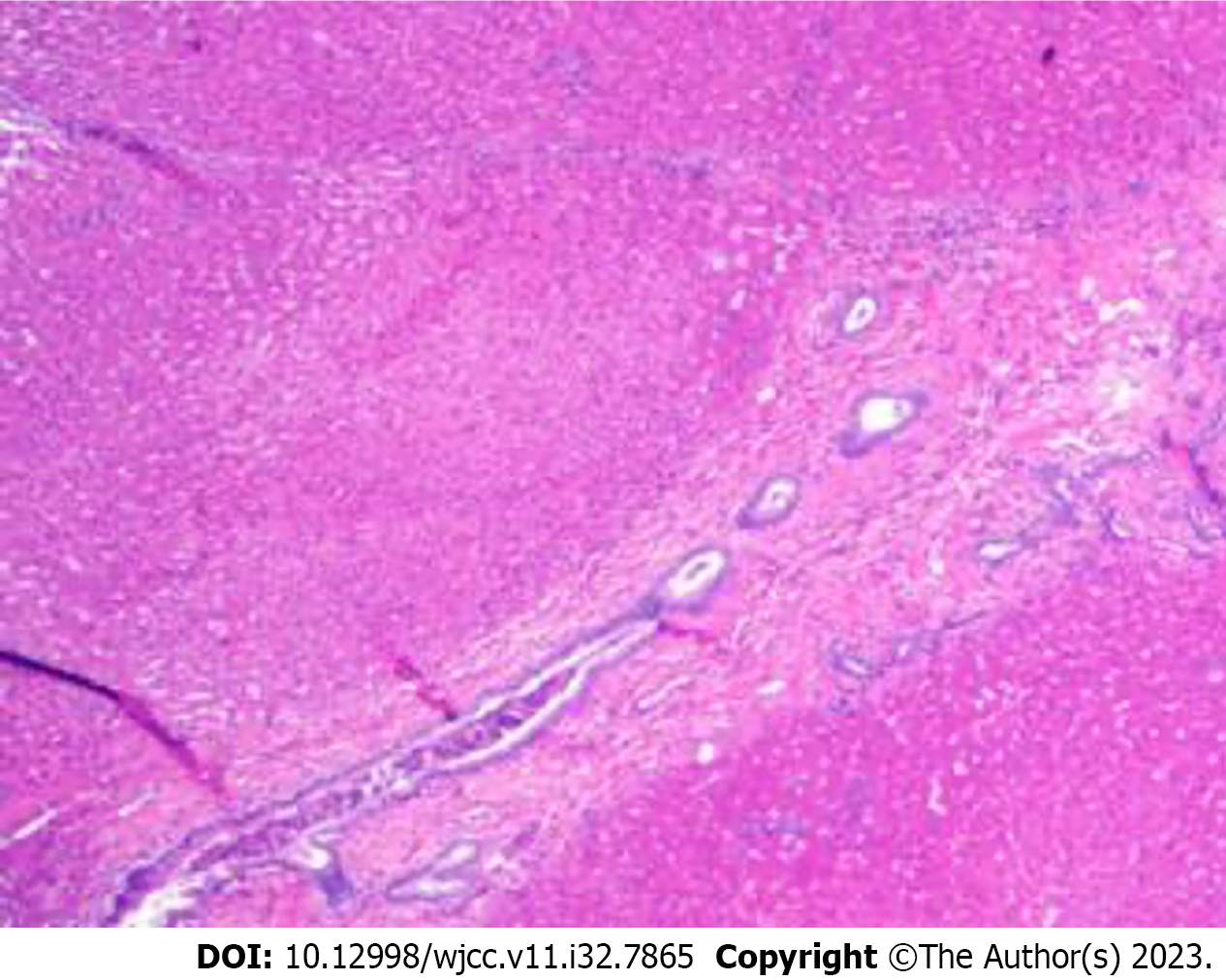
After the pulmonary infection improved, the patient was transferred to the general surgery department and underwent ligation of abnormal branches of the portal vein and liver biopsy on October 20, 2021 after excluding the relevant contraindications. During the intraoperative period, observations revealed hepatic shrinkage, significant splenic enlargement, and tortuous alterations in the splenic vessels. The inferior margin of the spleen exhibited looseness, accompanied by an abnormally thickened vessel measuring ~0.8 cm in diameter, which was observed to be draining into the left renal vein. The central venous catheter remained in situ via the terminal jejunal vein, and portal vein pressure measurements recorded values of 17.1 and 23.1 cmH2O before and after occlusion of the abnormal shunt, respectively. Twenty minutes after blocking, no stasis was seen in the intestinal canal, kidney and spleen, and the branches of the portal vein were seen on portal venography. The abnormal shunt vessels were ligated, and no abdominal organ stasis was seen, and some tissues of the right lobe of the liver were taken for pathological examination. Pathological analysis showed that portal vein branches were dysplastic, dilated or absent, which was consistent with Abernethy malformation type II (Figure 3).
Postoperative anti-infective therapy, rehydration, hemostasis, liver protection, and nutritional support were provided. Subsequently, cardiac enzymes were reassessed: Troponin I 0.005 ng/mL, myoglobin 141.7 ng/mL, creatine kinase isoenzyme 1.3 ng/mL, and B-type natriuretic peptide 33 pg/mL. Additionally, liver function assessed on postoperative days 1, 3 and 7 showed no abnormalities.
The patient had a satisfactory postoperative recovery and was subsequently discharged from the medical facility. However, during routine outpatient follow-up, she was admitted to the respiratory department on two separate occasions in November 2021 and February 2023 for the treatment of recurring cough and hemoptysis, respectively. Sputum bacterial culture revealed the presence of Pseudomonas aeruginosa infection. Subsequent re-evaluation of the abdominal enhanced CT scan revealed irregular liver morphology, splenomegaly, multiple tortuous dilated blood vessels at the splenic hilum, and pancreatic atrophy (Figure 1F and G).
Coexistent Abernethy malformation and CF are infrequent, and a comprehensive examination of the pertinent academic sources yielded no documented occurrences. Intraoperative venography showed multiple tortuous dilated vessels in the splenic hilum, abnormal blood flow into the inferior vena cava through the left renal vein, and portal vein branches and side branches were present, which supported the diagnosis of Abernethy malformation type II[8,9].
There is evidence suggesting a potential association between Abernethy malformation and CF with the occurrence of splenomegaly. Abernethy malformation results in splenomegaly due to obstruction of blood return from the splenic vein as a result of portal vein hypoplasia and abnormal blood shunting. CF is a monogenic disorder resulting from mutations in the CFTR gene, which encodes the epithelial ion channel responsible for the transportation of chloride and bicarbonate ions. These mutations lead to impaired mucus hydration and clearance, resulting in the obstruction of lumens of the respiratory, pancreatic, and biliary tracts, as well as abnormal secretion from exocrine glands[10]. Cystic fibrosis liver disease (CFLD) frequently manifests with hepatic steatosis, cholestasis, and progressive cirrhosis, leading to portal hypertension and subsequent splenomegaly[11,12]. Additionally, non-cirrhotic portal hypertension can arise in CFLD, potentially attributed to inflammatory and fibrotic paracaval portal vein lesions[13]. Therefore, it is hypothesized that the splenomegaly observed in this child was a result of a combination of both diseases.
Splenomegaly may cause secondary hypersplenism, which is clinically manifested by hypoplasia of one or more blood vessels. Complications such as infection, anemia, and hemorrhage can easily arise. In the present case, the child exhibited splenomegaly, reduced peripheral leukocyte count, and thrombocytopenia, indicating the presence of hypersplenism, and bone marrow aspiration was performed to exclude the possibility of hematological disorders. The respiratory system of this child with CF exhibited manifestations such as recurrent respiratory infections and hemoptysis following birth, with imaging indicating the presence of bronchiectasis[14]. In this particular instance, postnatal asphyxia was not observed, and the child had a history of serum transfusion and electrolyte disorders at the age of 2 mo. The diagnostic value of chloride concentration in the sweat test for CF is substantial[15]. Although the child’s serum electrolyte examination before and after hospitalization and surgery did not reveal any significant abnormalities, the sweat electrolyte examination was regrettably not conducted during the hospitalization period. Consequently, the diagnosis of CF was based on clinical manifestations and genetic test results. The genetic analysis indicated that the patient’s father and mother possessed heterozygous alleles for the causative gene. The specific mutations were identified at positions c.233_c.234insT and c.2909G>A, which have not been documented in the pertinent databases. In accordance with Mendelian inheritance patterns, the likelihood of the disease manifesting in the patient’s siblings was 1/4. However, it is important to note that the patient’s brother and sister had no similar history of the disease, and no genetic testing was conducted on them.
Misdiagnosis and underdiagnosis are common occurrences in cases of Abernethy malformation and CF. A comprehensive medical history and meticulous physical examination are invaluable in enhancing diagnostic accuracy. In the presence of splenomegaly, varices of the digestive tract, and hepatic encephalopathy, it is imperative to consider the potential occurrence of Abernethy malformation. Similarly, when encountering a patient with recurrent respiratory tract infections, alongside a history of hemoptysis and abnormal sweating, it is crucial to contemplate the possibility of CF. Patients with Abernethy malformation accompanied by upper gastrointestinal varices may have hematemesis after food stimulation, and CF may also have massive hemoptysis due to bronchiectasis. When inquiring about the history, it is imperative to exercise caution in accurately identifying the two conditions.
The management of Abernethy malformation encompasses both conservative approaches and surgical interventions aimed at rectifying abnormal blood flow[16,17]. Conversely, CF is primarily addressed symptomatically to mitigate respiratory infections, impede disease advancement, and the advent of genetic testing technology has emerged as a valuable tool to enhance diagnosis and treatment precision[10,18]. Consequently, patients exhibiting clinical suspicion of CF necessitate routine genetic testing.
Coexisting Abernethy malformation and CF is rare. In cases where patients present with unexplained thrombocytopenia, splenomegaly, and hypersplenism, it is advisable to perform enhanced abdominal CT to detect Abernethy malformation. In instances where children exhibit symptoms such as hemoptysis, recurrent respiratory infections, and bronchiectasis, it is crucial to raise awareness regarding the possibility of CF, and genetic testing may be conducted to establish a conclusive diagnosis. The co-occurrence of Abernethy malformation and CF is a clinically infrequent phenomenon that necessitates a detailed clinical history, as well as comprehensive laboratory and imaging evaluation to improve diagnostic accuracy.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Pediatrics
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: El-Karaksy H, Egypt S-Editor: Liu JH L-Editor: Webster JR P-Editor: Zhang XD
| 1. | Kumar P, Bhatia M, Garg A, Jain S, Kumar K. Abernethy malformation: A comprehensive review. Diagn Interv Radiol. 2022;28:21-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 2. | Tang H, Song P, Wang Z, Han B, Meng X, Pan Y, Duan W. A basic understanding of congenital extrahepatic portosystemic shunt: incidence, mechanism, complications, diagnosis, and treatment. Intractable Rare Dis Res. 2020;9:64-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 3. | Baiges A, Turon F, Simón-Talero M, Tasayco S, Bueno J, Zekrini K, Plessier A, Franchi-Abella S, Guerin F, Mukund A, Eapen CE, Goel A, Shyamkumar NK, Coenen S, De Gottardi A, Majumdar A, Onali S, Shukla A, Carrilho FJ, Nacif L, Primignani M, Tosetti G, La Mura V, Nevens F, Witters P, Tripathi D, Tellez L, Martínez J, Álvarez-Navascués C, Fraile López ML, Procopet B, Piscaglia F, de Koning B, Llop E, Romero-Cristobal M, Tjwa E, Monescillo-Francia A, Senzolo M, Perez-LaFuente M, Segarra A, Sarin SK, Hernández-Gea V, Patch D, Laleman W, Hartog H, Valla D, Genescà J, García-Pagán JC; REHEVASC, VALDIG an EASL consortium, Abernethy group. Congenital Extrahepatic Portosystemic Shunts (Abernethy Malformation): An International Observational Study. Hepatology. 2020;71:658-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 97] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 4. | Shteinberg M, Haq IJ, Polineni D, Davies JC. Cystic fibrosis. Lancet. 2021;397:2195-2211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 392] [Article Influence: 98.0] [Reference Citation Analysis (0)] |
| 5. | Chen Q, Shen Y, Zheng J. A review of cystic fibrosis: Basic and clinical aspects. Animal Model Exp Med. 2021;4:220-232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 55] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 6. | Wei T, Sui H, Su Y, Cheng W, Liu Y, He Z, Ji Q, Xu C. Research advances in molecular mechanisms underlying the pathogenesis of cystic fibrosis: From technical improvement to clinical applications (Review). Mol Med Rep. 2020;22:4992-5002. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Singh M, Rebordosa C, Bernholz J, Sharma N. Epidemiology and genetics of cystic fibrosis in Asia: In preparation for the next-generation treatments. Respirology. 2015;20:1172-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 8. | Xu L, Zhang H, Liu G, Li Y, Li D, Ma N. Abernethy malformation with unusual cardiac malformation: Case report and literature review. Echocardiography. 2023;40:57-60. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 9. | Bahadori A, Kuhlmann B, Debray D, Franchi-Abella S, Wacker J, Beghetti M, Wildhaber BE, McLin VA, On Behalf Of The Ircpss. Presentation of Congenital Portosystemic Shunts in Children. Children (Basel). 2022;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Ong T, Ramsey BW. Cystic Fibrosis: A Review. JAMA. 2023;329:1859-1871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 110] [Article Influence: 55.0] [Reference Citation Analysis (0)] |
| 11. | Valamparampil JJ, Gupte GL. Cystic fibrosis associated liver disease in children. World J Hepatol. 2021;13:1727-1742. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 12. | Davison S. Assessment of liver disease in cystic fibrosis. Paediatr Respir Rev. 2018;27:24-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Kamal N, Surana P, Koh C. Liver disease in patients with cystic fibrosis. Curr Opin Gastroenterol. 2018;34:146-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 14. | Wainwright CE. Airway inflammation and lung function in cystic fibrosis. Respirology. 2023;28:509-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 15. | Treggiari D, Tridello G, Menin L, Borruso A, Pintani E, Iansa P, Cipolli M, Melotti P. Role of sweat ion ratios in diagnosing cystic fibrosis. Pediatr Pulmonol. 2021;56:2023-2028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Lin Y, Li X, Li S, Ba H, Wang H, Zhu L. Treatment Option for Abernethy Malformation-Two Cases Report and Review of the Literature. Front Pediatr. 2020;8:497447. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Jain V, Sangdup T, Agarwala S, Bishoi AK, Chauhan S, Dhua A, Jana M, Kandasamy D, Malik R, Kothari SS, Patcharu R, Varshney A, Bhatnagar V. Abernethy malformation type 2: varied presentation, management and outcome. J Pediatr Surg. 2019;54:760-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 18. | Maule G, Arosio D, Cereseto A. Gene Therapy for Cystic Fibrosis: Progress and Challenges of Genome Editing. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |