Published online Nov 6, 2023. doi: 10.12998/wjcc.v11.i31.7724
Peer-review started: September 12, 2023
First decision: September 19, 2023
Revised: September 27, 2023
Accepted: October 17, 2023
Article in press: October 17, 2023
Published online: November 6, 2023
Processing time: 55 Days and 6.9 Hours
This report describes a case of intracranial multiple inflammatory pseudotumors (IP) after endoscopic resection of a craniopharyngioma, which is relatively rarely reported in the literature, and neurosurgeons should be aware of its existence.
Herein, we report the case of a 56-year-old man who developed decreased visual acuity and blurred vision without obvious cause or inducement on April 27, 2020. To seek further treatment, he went to the Department of Neurosurgery, Clinical Medical College, Yangzhou University. After falling ill, there was no nausea, vomiting, limb convulsions, obvious disturbance of consciousness, speech disorders, cough, or persistent fever. The neurological examination findings were normal, and pituitary magnetic resonance imaging (MRI) revealed multiple nodules with abnormal signals in the sellar region. The diagnosis was craniopharyngioma. We performed total resection of the tumor via transnasal endoscopy, and the postoperative pathology suggested that the type of tumor was craniopharyngioma. Six months after the operation, the patient experienced sudden hearing loss in the right ear, tinnitus in both ears, and numbness on the right side of the face and head. Meanwhile, cranial MRI showed multiple IP. After steroid hormone and anti-inflammatory therapy, the above symptoms did not significantly improve. Finally, the patient's symptoms were well improved by surgery, and the postoperative pathological diagnosis was multiple IP.
Intracranial inflammatory pseudotumor is a benign disease with slow progression, but the clinical symptoms and imaging findings are not typical, there are no pathological findings, and the diagnosis is relatively difficult. Most of the cases are treated by surgical resection, and the prognosis is good after surgery.
Core Tip: Inflammatory pseudotumor, also known as inflammatory myofibroblastoma, is a nodular lesion with fibrous connective tissue proliferation accompanied by a large number of inflammatory cells. The disease can occur in any part of the body, most often in the lungs, but also in the liver, spleen, lymph nodes, and other parts, and intracranial occurrence is relatively rare, especially after craniopharyngioma resection via an extended nasal endoscopic approach. The purpose of this case report is to remind clinicians of the existence of inflammatory pseudotumors after craniocerebral surgery.
- Citation: Wu H, Ding YW, Yan ZC, Wei M, Wang XD, Zhang HZ. Multiple inflammatory pseudotumor formation after craniopharyngioma resection via an extended nasal endoscopic approach: A case report. World J Clin Cases 2023; 11(31): 7724-7731
- URL: https://www.wjgnet.com/2307-8960/full/v11/i31/7724.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i31.7724
Inflammatory pseudotumors (IP) are localized, isolated, nonneoplastic, space-occupying lesions characterized histopathologically by a mixture of collagenous fibers and inflammatory cells, such as lymphocytes and plasma cells[1]. These tumors have been reported to occur most commonly in the lungs, mesentery, greater omentum, retroperitoneum, genitourinary tract, or upper respiratory tract. However, IP of the central nervous system are rare[2]. Most intracranial IP originate from the dura mater and meningeal structures[3]. There have been few reports of multiple IP (MIP) in the intracranial space after surgery for craniopharyngioma resection.
A 56-year-old man developed decreased visual acuity and blurred vision without obvious cause or inducement that lasted for 15 d.
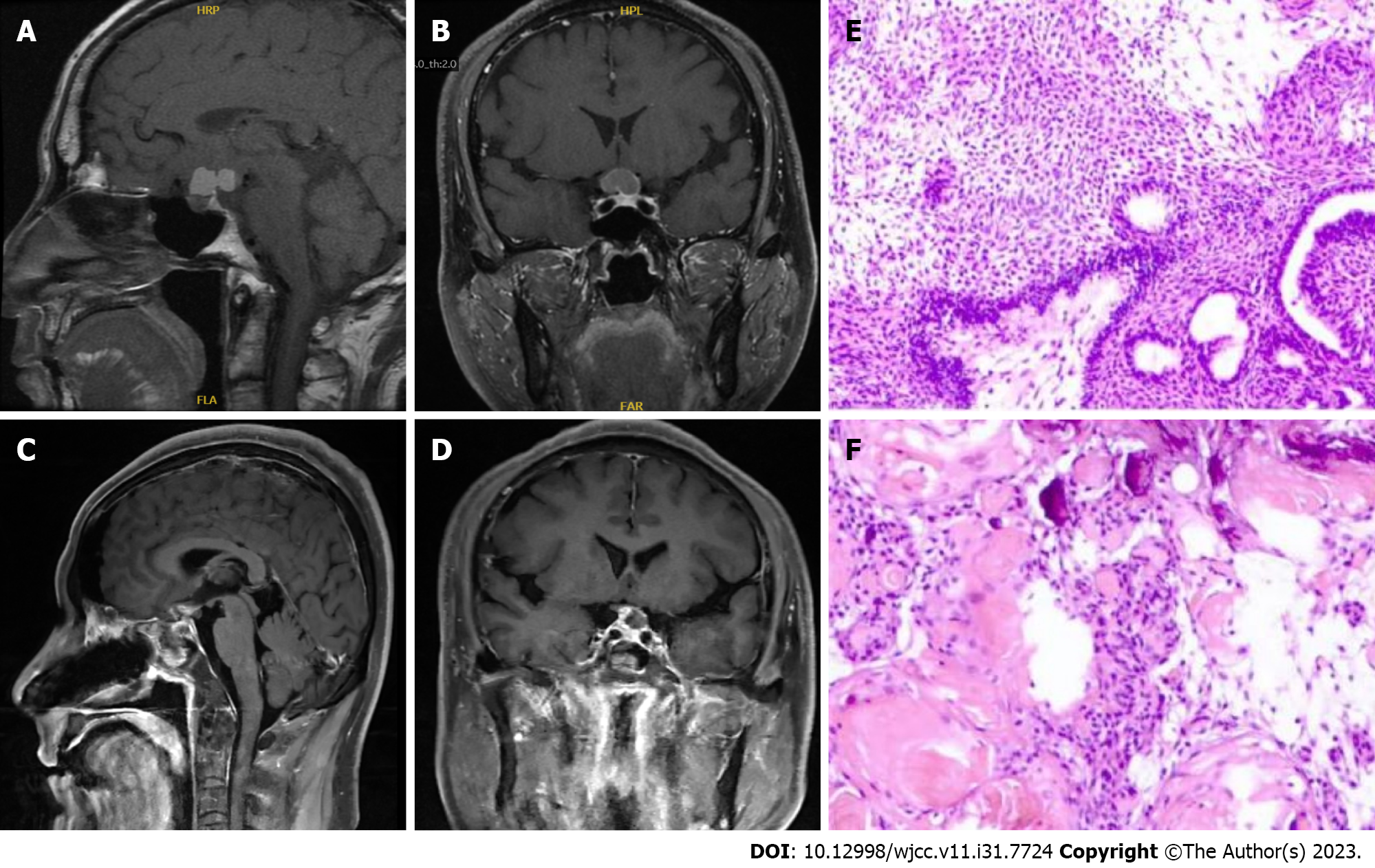
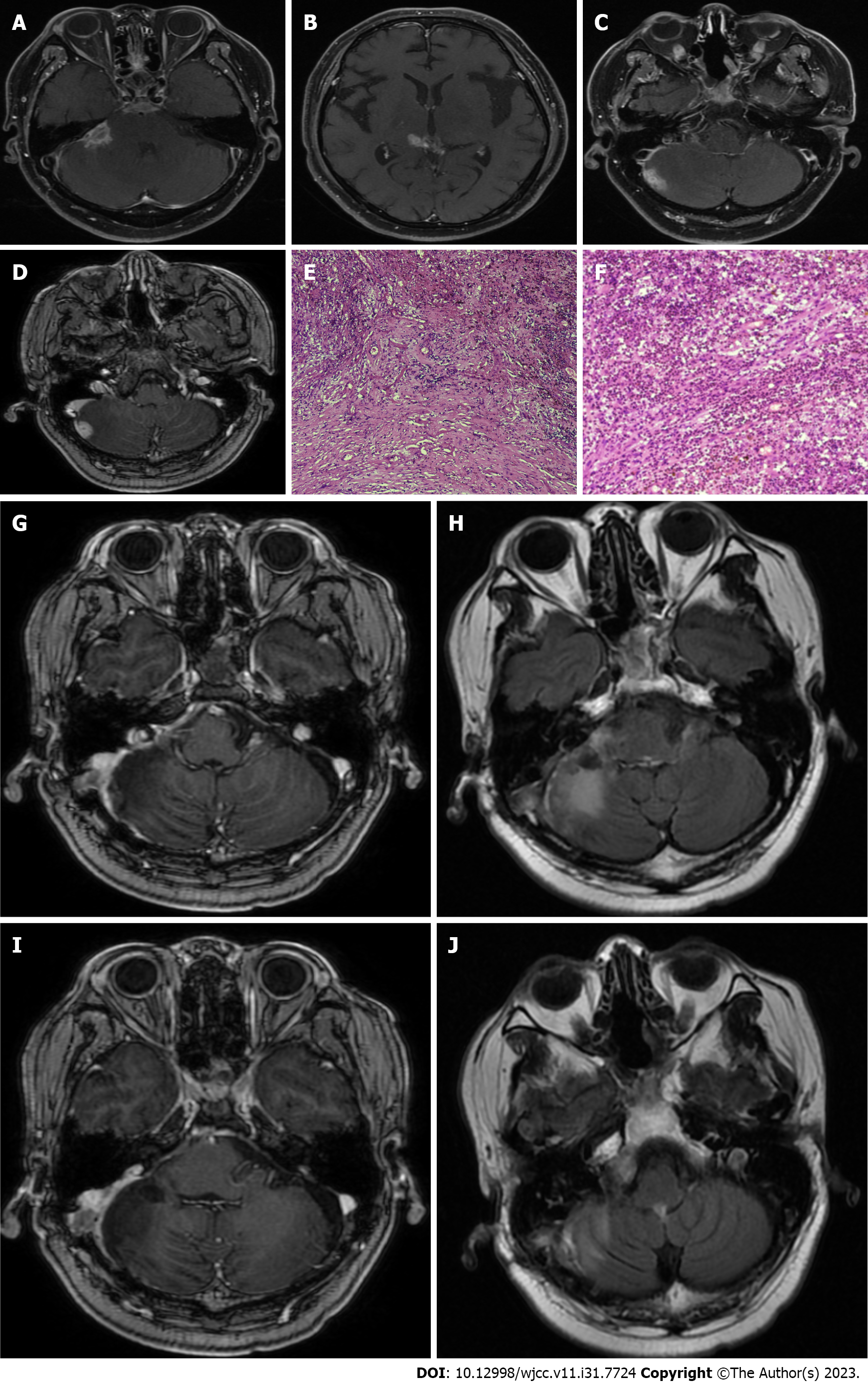
The patient presented to the Department of Neurosurgery, Clinical Medical College, Yangzhou University, with decreased visual acuity and blurred vision that lasted for 15 d. The neurological examination findings were normal, and he had previously been in good health without any infectious diseases or immune disorders. Enhanced pituitary magnetic resonance imaging (MRI) revealed multiple nodules in the suprasellar region with slightly shorter T1 signals, compression of the optic chiasma, and compression of the superior pituitary margin in a region of approximately 19 mm × 12 mm × 21 mm. The preoperative diagnosis was craniopharyngioma (Figure 1A and B). The patient's routine laboratory results were normal. The tumor was removed by an endoscopic transnasal transsellar tubercle sphenoid platform approach. Intraoperatively, the tumor was located in the suprasellar suboptic space with cystic changes and was connected to the posterior part of the pituitary gland. Postoperative MRI showed a patchy mixed signal in the sellar region (Figure 1C and D). Postoperative histopathology revealed hyperplasia, showing nests and a papillary arrangement of epithelial nests with a fenestrated arrangement of marginal cells in some areas; additionally, ghost cells were seen with calcification (Figure 1E and F). The final diagnosis was craniopharyngioma of the sellar region (enamel-forming cell type). Postoperative review MRI showed a patchy mixed signal in the sellar region, and the sellar base was incomplete. A nodal shadow was visible in the sellar region, with significant enhancement; the shadow was approximately 10 mm × 9 mm in extent and poorly demarcated from the visual cross. The patient recovered well after surgery without special discomfort. Six months after the operation, the patient suffered from sudden hearing loss in the right ear, tinnitus in both ears, and numbness of the right side of the face and head, without any signs of infection. Physical examination revealed hearing loss in the right ear. Enhanced MRI of the inner ear showed multiple patchy and nodular, distinctly enhancing abnormal shadows around the right pontocerebellar horn, right cerebellum, and quadrigeminal cistern; the largest lesion was in the right pontocerebellar horn, approximately 17 mm × 14 mm in size, with gross margins and significant thickening of the right rocky meninges. Multiple IP were considered (Figure 2A-D). The patient was treated with steroid pulse therapy, and he received an initial high-dose regimen of 80 mg of methylprednisolone for 4 d, followed by a one-day drug cessation and then a maintenance dose of 40 mg of methylprednisolone for another 4 d. Despite this treatment approach, the patient's primary symptoms remained unresolved, and subsequent cranial MRI indicated no significant change in the size of the pseudotumors. Ultimately, we opted for surgical resection to remove the tumor, and during follow-up and review, there was no recurrence of the IP. A lesion in the right cerebellum and pontocerebellar horn was resected under neuronavigation. Postoperative pathological examination showed fibrocollagenous tissue hyperplasia and extensive infiltration of inflammatory cells, including lymphocytes (Figure 2E and F). Immunohistochemistry suggested the following: KP1 (scattered +), CD3 (scattered +), CD20 (scattered +), S100 (neural +), CD1a (-), GFAP (brain +), Ki67 (scattered 20%), SMA (vascular +), CD34 (vascular +), and MPO (scattered +). Special staining results were PAS (-), antacid (-), and silver stain (-). The postoperative diagnosis was IP. The patient was discharged 7 d after surgery and was reviewed 3 mo after surgery, without recurrence or significant abnormalities on neurological examination. The patient continues to be under close follow-up (Figure 2G-J).
The patient had no previous history of infectious diseases, traumas, or bad habits (such as smoking or drinking).
The patient denied any family history of tumors.
On physical examination, the vital signs were as follows: Body temperature, 36.4 °C; blood pressure, 110/88 mmHg; heart rate, 88 beats per min; and respiratory rate, 19 breaths per min. Annual physical examinations (including various laboratory tests and imaging examinations) indicated that the patient was healthy.
The white blood cell count was 7.78 × 109/L, and neutrophil count was 7.37 × 109/L. Endocrine test results were normal.
Pre-operative pituitary MRI suggested that there were multiple nodules with abnormal signals in the sellar region. The diagnosis was craniopharyngioma.
Pre-operative contrast-enhanced MRI of the inner ear showed multiple patchy and nodular distinctly enhancing abnormal shadows around the right pontocerebellar horn, right cerebellum, and quadrigeminal cistern, with the largest lesion in the right pontocerebellar horn, approximately 17 mm × 14 mm, with gross margins and significant thickening of the right rocky meninges. MIP were considered.
Combined with the patient’s medical history, the final diagnosis was MIP.
The main treatment was resection of intracranial lesions.
Symptoms were relieved gradually after treatment, and complete resolution of symptoms was observed after five days. To date, there has been no recurrence, and the patient is doing well.
The etiology of IP remains uncertain, with three prevailing theories attempting to explain their origin[4]. In terms of their composition, IP exhibit internal fibrosis and infiltration of various inflammatory cells[3], including macrophages, plasma cells, and lymphocytes, suggesting an association with inflammation[5]. Furthermore, minor traumas, surgical interventions, smoking, vasculitis, and malignancies have been proposed as potential triggers[6], indicating an inflammatory response as a causative factor. Infections caused by viruses such as Epstein-Barr virus, cytomegalovirus, and herpes simplex virus have also been linked to the development of IP[3,7]. Moreover, IP have been associated with IgG-4-related diseases, specifically characterized by fibrotic tissue masses infiltrated by IgG-4-positive lymphoplasmacytic cells[8]. In the present case, an intracranial IP emerged subsequent to the transnasal endoscopic resection of a craniopharyngioma, with the patient experiencing an intracranial infection postsurgery. Consequently, it is believed that the inflammatory response triggered by an infection following the initial surgery may be responsible for the subsequent development of the intracranial IP. Neuroendoscopic transsphenoidal craniopharyngotomy has been suggested to enhance the rate of complete tumor resection; however, it also increases the incidence of cerebrospinal fluid leakage and intracranial infection and prolongs the operative time and hospital stay. Thus, for patients undergoing endoscopic transsphenoidal craniopharyngotomy, the administration of adequate antibiotics throughout the postoperative period is recommended to prevent intracranial infection. Elderly patients may require an extended duration of antibiotic treatment, and modifications to the surgical approach may be necessary if deemed appropriate. Additionally, postoperative endoscopic evaluation of the saddle base can facilitate the early detection and treatment of occult cerebrospinal fluid leaks.
Due to the limited number of cases, there is currently no standardized pathological classification for intracranial IP, and most reported cases exhibit a mixed predominance of cell types[9]. Microscopically, these lesions are characterized by the presence of polyclonal plasma cells intermingled with varying numbers of lymphocytes, neutrophils, eosinophils, histiocytes, and myofibroblasts[10]. Immunohistochemical analysis has shown positive expression of smooth muscle actin (SMA) in the majority of intracranial IP[9]. In the present case of MIP, microscopic examination revealed the proliferation of fibrillar tissue with significant infiltration of inflammatory cells, particularly lymphocytes. Immunohistochemistry also confirmed positive SMA expression. These pathological findings were consistent with those typically observed in conventional intracranial IP. Thus, it is essential to consider the possibility of IP in all cases of intracranial tumor-like lesions exhibiting lymphoplasmacytic infiltrates and varying densities of collagenous interstitium[11]. Pathological exam remains the gold standard for the diagnosis of intracranial IP.
Intracranial IP are currently categorized into five distinct types[11]: Intracerebral parenchymal, meningeal, mixed parenchymal-meningeal, intracerebroventricular, and extracranial communicating types. The clinical manifestations of these tumors differ slightly depending on their locations. IP located in the skull base typically manifest as pain on the affected side of the head and face, cranial nerve palsy, or cerebrospinal fluid leakage. IP within the brain ventricles often present with symptoms such as hydrocephalus, headache, nausea, vomiting, and vision loss. IP in the brain parenchyma commonly result in increased intracranial pressure and varying degrees of neurological dysfunction based on the specific location. Lesions involving the meninges can exhibit a range of neurological manifestations, with mild cases being asymptomatic and severe cases leading to dysfunction due to compression of the brain parenchyma[12].
Brain CT scans often reveal a well-defined circumferential shadow with isointense or high-density characteristics, with clear margins and uniform enhancement. MRI of the head demonstrates low signal intensity on both T1- and T2-weighted imaging, with substantial enhancement observed following contrast administration. Delayed enhancement has also been reported in certain cases. It is crucial to note that the diagnosis primarily relies on histopathological examination[13].
Intracranial IP require differentiation from several other conditions[4], including lymphoplasmacyte-rich meningiomas, lymphomas, and plasmacytomas. Given that most intracranial IP originate from dural and meningeal structures[14], distinguishing them from lymphoplasmacyte-rich meningiomas can be challenging, often resulting in misinterpretation as meningiomas with lymphoplasmacytic infiltrates. However, careful pathological examination can differentiate between the two, primarily through the detection of the tumorigenic proliferation of meningeal cells and positive immunohistochemical staining for EMA[11].
Intracranial primary or secondary malignant lymphomas are malignant neoplasms originating from the central or peripheral lymphatic system, and secondary cases are more common, particularly B-cell non-Hodgkin's lymphoma. Immunohistochemical analysis of lymphomas reveals monoclonal expression of B or T lymphocyte antigens along with positive staining for EMA, which significantly distinguishes them from IP[11].
Plasmacytomas can be discerned through immunohistological flow cytometry, allowing for the determination of whether the plasma cells present are polyclonal or monoclonal. Polyclonal plasma cells indicate a plasmacytoid granuloma, while monoclonal plasma cells indicate a plasmacytoma[4].
The optimal treatment approach for intracranial IP remains uncertain, as there is a lack of a general consensus[4]. Existing treatment options include surgical resection, corticosteroid therapy, immunosuppressive therapy, and radiotherapy. Studies have indicated that a significant number of patients exhibit favorable outcomes following surgical resection, with low rates of recurrence or malignant progression[5,15]. Surgery offers the advantage of alleviating the mass effect of the pseudotumors and potentially reducing the risk of distant recurrence[14].
Many experts consider corticosteroids to be the preferred treatment for IP, particularly in the initial stages[10]. Oral corticosteroids have shown positive effects in the majority of patients, effectively improving symptoms and reducing the size of IP. A recommended initial oral steroid dose of 40 mg per day, gradually tapered to 5 mg to 10 mg per day over a month, has demonstrated efficacy. However, it is important to note that studies have revealed a relatively high recurrence rate of IP after the discontinuation of oral corticosteroids[10].
In this particular case, hormone therapy using methylprednisolone was initiated upon the diagnosis of MIP. The patient received an initial high-dose regimen of 80 mg of methylprednisolone for 4 d, followed by a 1-d drug cessation and then a maintenance dose of 40 mg of methylprednisolone for another 4 d. Despite this treatment approach, the patient's primary symptoms remained unresolved, and subsequent cranial MRI indicated no significant change in the size of the pseudotumors. Ultimately, we opted for surgical resection to remove the tumor, and during follow-up and review, there was no recurrence of the IP.
The ineffectiveness of hormone therapy in this case may be attributed to an incorrect hormone dosage and an insufficient treatment duration. The absence of a standardized dose and duration for oral hormone therapy further complicates treatment decisions, highlighting the need for comprehensive case reports to guide future practices and recommendations.
Radiation therapy has been proposed as an alternative treatment option for intracranial IP in cases where the tumors are insensitive to hormone therapy, there are contraindications to high-dose hormone therapy, relapse occurs after hormone therapy, or the tumors are unsuitable for surgery due to the location of the lesion or the possibility of incomplete surgical resection. In such scenarios, low-dose high-potential fractionated therapy is typically recommended, with a total radiation dose ranging from 20 to 40 Gy[16].
In summary, the treatment of intracranial IP remains a complex matter without a definitive treatment approach. Surgical resection and corticosteroid therapy, particularly oral glucocorticosteroid therapy, have shown positive results in improving symptoms and reducing tumor size. However, the risk of recurrence remains a concern, necessitating further research and evaluation of treatment strategies.
Intracranial IP lack specific imaging manifestations and are easily misdiagnosed as other tumors, and pathology is currently the gold standard for the diagnosis of intracranial IP. Due to the small number of cases and the diverse histopathological classification, the best treatment plan is still inconclusive. In our opinion, surgery is preferred for IP of the central nervous system that can be completely resected without damaging important surrounding structures. If the lesion is not suitable for surgery, steroid hormone therapy or hormone therapy combined with radiotherapy can be administered as appropriate. Patients with intracranial IP should be treated individually according to the location of the lesion and the patient's own characteristics.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gica N, Romania S-Editor: Lin C L-Editor: Wang TQ P-Editor: Lin C
| 1. | Coffin CM, Watterson J, Priest JR, Dehner LP. Extrapulmonary inflammatory myofibroblastic tumor (inflammatory pseudotumor). A clinicopathologic and immunohistochemical study of 84 cases. Am J Surg Pathol. 1995;19:859-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1100] [Cited by in RCA: 1030] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 2. | Swain RS, Tihan T, Horvai AE, Di Vizio D, Loda M, Burger PC, Scheithauer BW, Kim GE. Inflammatory myofibroblastic tumor of the central nervous system and its relationship to inflammatory pseudotumor. Hum Pathol. 2008;39:410-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 3. | Jung TY, Jung S, Lee MC, Moon KS, Kim IY, Kang SS, Kim SH. Hemorrhagic intracranial inflammatory pseudotumor originating from the trigeminal nerve: a case report. J Neurooncol. 2006;76:139-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 4. | Häusler M, Schaade L, Ramaekers VT, Doenges M, Heimann G, Sellhaus B. Inflammatory pseudotumors of the central nervous system: report of 3 cases and a literature review. Hum Pathol. 2003;34:253-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 92] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 5. | Lin YJ, Yang TM, Lin JW, Song MZ, Lee TC. Posterior fossa intracranial inflammatory pseudotumor: a case report and literature review. Surg Neurol. 2009;72:712-6; discussion 716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Spinazzi EF, Desai SV, Fang CH, Jyung RW, Liu JK, Baredes S, Eloy JA. Lateral skull base Inflammatory pseudotumor: A systematic review. Laryngoscope. 2015;125:2593-2600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Fukunaga A, Yoshida K, Otani M, Ogawa Y, Horiguchi T, Ishihara M, Toya S, Kawase T. Plasma cell granuloma extending from the extracranial to the intracranial space associated with Epstein-Barr virus infection. Neurol Med Chir (Tokyo). 1998;38:292-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Patnana M, Sevrukov AB, Elsayes KM, Viswanathan C, Lubner M, Menias CO. Inflammatory pseudotumor: the great mimicker. AJR Am J Roentgenol. 2012;198:W217-W227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 230] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 9. | Leclercq D, Trunet S, Bertrand A, Galanaud D, Lehéricy S, Dormont D, Drier A. Cerebral tumor or pseudotumor? Diagn Interv Imaging. 2014;95:906-916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Perkins SJ, Gao R, Glazer TA, Zhao CX, Basura G, McKean EL. Treatment and Prognosis of Inflammatory Pseudotumor of the Skull Base. J Neurol Surg B Skull Base. 2022;83:e555-e563. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 11. | Lui PC, Fan YS, Wong SS, Chan AN, Wong G, Chau TK, Tse GM, Cheng Y, Poon WS, Ng HK. Inflammatory pseudotumors of the central nervous system. Hum Pathol. 2009;40: 1611-1617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 12. | Narla LD, Newman B, Spottswood SS, Narla S, Kolli R. Inflammatory pseudotumor. Radiographics. 2003;23:719-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 299] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 13. | Lee SG, Shin IY, Hwang HS, Choi I. Multimodal Treatment of Skull Base Inflammatory Pseudotumor: Case Report. Brain Tumor Res Treat. 2015;3:122-126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 14. | Derrey S, Charpentier C, Gérardin E, Langlois O, Touchais JY, Lerebours E, Proust F, Laquerrière A. Inflammatory pseudotumor of the cerebellum in a patient with Crohn's disease. World Neurosurg. 2012;77:201.e13-201.e16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Kuang PD, Li QH, Liu ZY, Tang JL, Dong F, Wang Y, Zhu XL. Inflammatory pseudotumor of the pineal region: First reported case. Oncol Lett. 2016;11:2127-2130. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 16. | Desai SV, Spinazzi EF, Fang CH, Huang G, Tomovic S, Liu JK, Baredes S, Eloy JA. Sinonasal and ventral skull base inflammatory pseudotumor: a systematic review. Laryngoscope. 2015;125:813-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |