Published online Nov 6, 2023. doi: 10.12998/wjcc.v11.i31.7718
Peer-review started: September 11, 2023
First decision: September 28, 2023
Revised: October 8, 2023
Accepted: October 16, 2023
Article in press: October 16, 2023
Published online: November 6, 2023
Processing time: 55 Days and 17.9 Hours
We all know that lymph-node metastasis is an important factor for poor clinical outcome in breast cancer prognosis. Tumor deposit refers to a discrete collection of cancer cells that is found in the lymph nodes or other tissues adjacent to the primary tumor site. These tumor deposits are separate from the primary tumor and are often considered as a manifestation of lymph node metastasis. In gastric and colorectal cancer, tumor deposits in the lymph node drainage area have been included as independent prognostic factors. The question arises whether tumor deposits should also be considered as prognostic factors in breast cancer patients. This article aims to provoke some thoughts on this matter through a case study and literature review.
A 70-year-old female patient was found to have a right breast lump for over 2 years. On January 3, 2023, a core needle biopsy of the right breast lump was performed, and the pathology report indicated invasive carcinoma. Subsequently, on January 17, 2023, the patient underwent right breast-conserving surgery, sentinel lymph node biopsy, and right axillary lymph node dissection. The postoperative pathological staging was determined as stage IIB. The patient received chemotherapy, radiotherapy, and endocrine therapy. At present, nearly one year after the surgery, no obvious signs of metastasis have been observed in the follow-up examinations, but the long-term prognosis is still unknown.
There is a need for increased focus on the matter of tumor deposits in the lymph node drainage region, as well as a requirement for further clinical investigation to ascertain the relevance of tumor deposits in the prognosis of individuals with breast carcinoma.
Core Tip: Further investigation is warranted to ascertain the potential underestimation of breast cancer patients' prognosis for survival in the absence of tumor deposits. Additional research is necessary to elucidate the influence of tumor deposits on the survival prognosis of breast cancer patients. The inclusion of tumor deposits in the tumor-node-metastasis staging of the axillary lymph node drainage area necessitates further exploration.
- Citation: Li T, Zhang WH, Liu J, Mao YL, Liu S. Isolated axillary tumor deposit consistent with primary breast carcinoma: A case report. World J Clin Cases 2023; 11(31): 7718-7723
- URL: https://www.wjgnet.com/2307-8960/full/v11/i31/7718.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i31.7718
The prognosis of breast cancer is usually determined using the tumor-node-metastasis (TNM) staging system. The TNM staging system takes into account the size of the tumor (T), involvement of lymph nodes (N), and the presence of distant metastasis (M). In the American Joint Committee on Cancer (AJCC)[1] 8th edition of breast cancer staging, the status of hormone receptors estrogen receptor (ER), progesterone receptor (PR), and HER2 (human epidermal growth factor receptor[2] is also considered to more accurately predict the patient’s prognosis and develop personalized treatment plans. According to the latest research, there is a proposal to include the immunohistochemistry of lymph nodes in the prognostic staging system[2]. Researchers are continuously seeking ways to further enhance the prognostic staging system, it is expected to improve the accuracy of tumor prognosis assessment and provide better individualized treatment for patients. The recent discovery of tumor deposits in the axillary lymph node drainage area, consistent with the origin of the primary tumor, has prompted me to consider whether the inclusion of this region’s tumor deposits (TD) should be incorporated into the prognostic TNM staging. According to the NCCN breast cancer guidelines, tumor deposits are not included in the N staging. However, in this location, it is highly likely that the tumor cells have invaded the lymph nodes. Although lymph node tissue cannot be observed, the location is within the lymph node region. If tumor deposits are not considered as metastatic lymph nodes, it is likely to underestimate the prognosis staging of the tumor, and subsequent treatment plans may be inadequate. Studies on tumor deposits can also be found in gastrointestinal tumors, where tumor deposits have been identified as independent factors for predicting early recurrence and poor prognosis in gastric cancer[3]. The prognostic value of adjuvant chemotherapy in isolated tumor deposits suggested that adjuvant chemotherapy can significantly improve the overall survival and cancer-specific survival of patients with isolated tumor deposits in colon cancer[4]. There is also research indicating that combining tumor deposit counts with positive lymph nodes for N staging in stage III colon cancer patients provides a more accurate prediction of disease-free survival than the current staging system[5]. The contemplation regarding the inclusion of TD in the prognostic staging system for breast cancer patients has been sparked by the case of this patient with a maximum tumor deposit diameter measuring 5cm. It is worthwhile for us to further investigate this matter.
A painless palpable lump in the right breast has been present for a duration exceeding two years. The mass has exhibited a gradual increase in size, devoid of any indications of erythema, edema, calor, thoracic constriction, or thoracic discomfort.
The patient initially detected a lump in the right breast two years ago, but did not pursue medical intervention until her visit to our hospital on January 3, 2023. Utilizing ultrasound guidance, a core needle biopsy was conducted on the aforementioned lump in the right breast. The subsequent postoperative pathology report (pathology number: 230005, Shanghai Baoshan Hospital of Integrated Traditional Chinese and Western Medicine, January 6, 2023) revealed the presence of invasive carcinoma of non-specific subtype. Immunohistochemical staining analysis demonstrated the following results: CK7(+), CK5/6(-), ER (approximately 80% +), PR (approximately 70% +), C-erbB-2(-), P63(-), CD10(-), and Ki-67 (approximately 30%+).
The patient has a documented medical record of hypertension spanning over a decade. Presently, they are undergoing treatment with sustained-release nifedipine tablets, taking one tablet per day. The patient's blood pressure is effectively managed. There is no reported history of additional chronic internal medicine ailments, including coronary heart disease. Furthermore, the patient denies any past occurrences of infectious diseases such as hepatitis or tuberculosis. Nevertheless, they do possess a medical history of fractures in the left hip joint and rib bones, resulting in the presence of residual fixation devices within their body.
The patient had a family history of breast cancer.
On physical examination, a palpable mass is found in the upper outer quadrant of the right breast. The size of the mass is approximately 2.5 cm × 3.0 cm. It is hard in consistency, poorly mobile, and has an indistinct border with the surrounding tissue. There are no signs of skin changes, such as the “orange peel” appearance. No significantly enlarged lymph nodes are palpable in the bilateral axilla.
Liver and kidney function, as well as tumor markers, show no significant abnormalities.
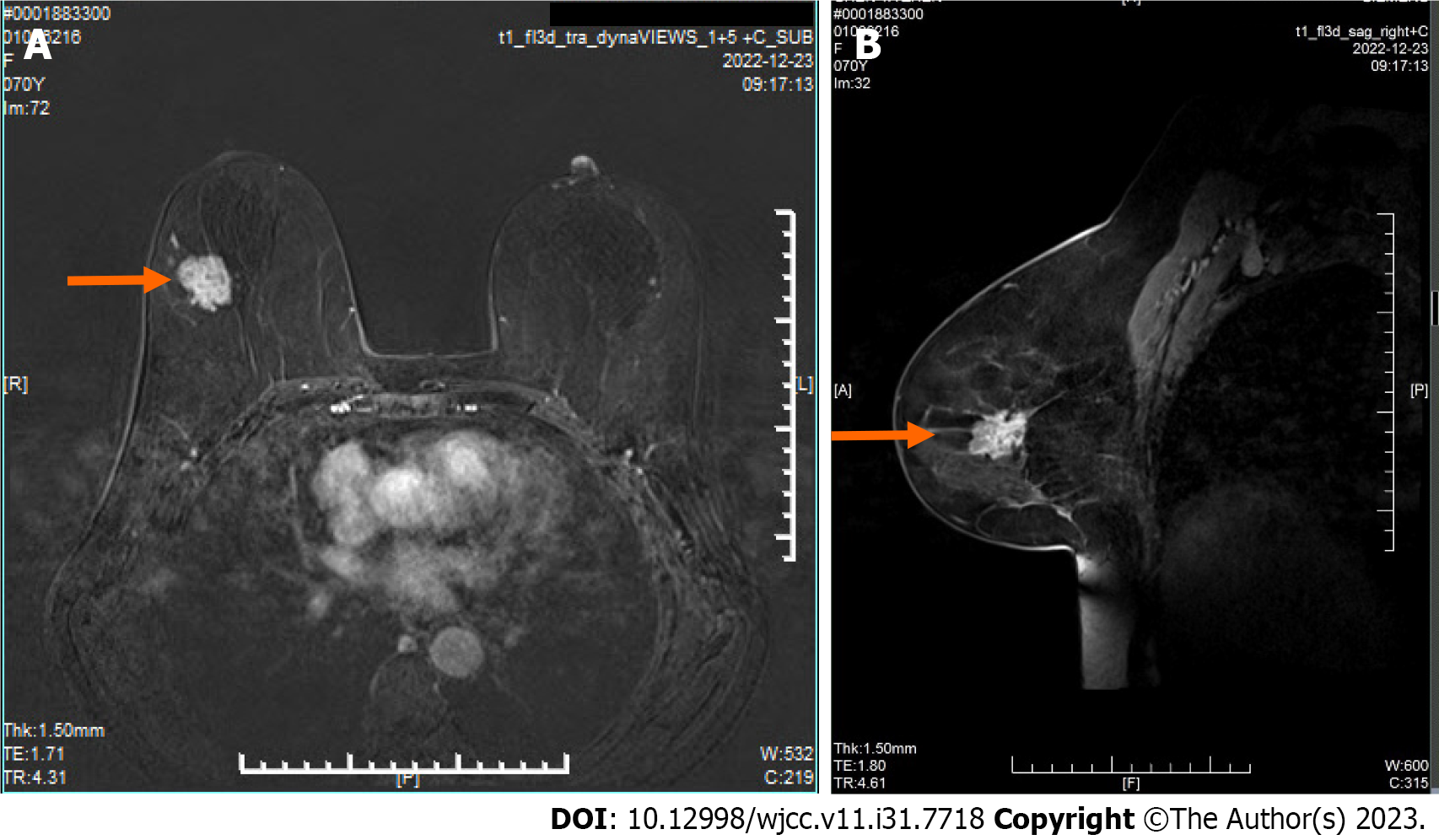
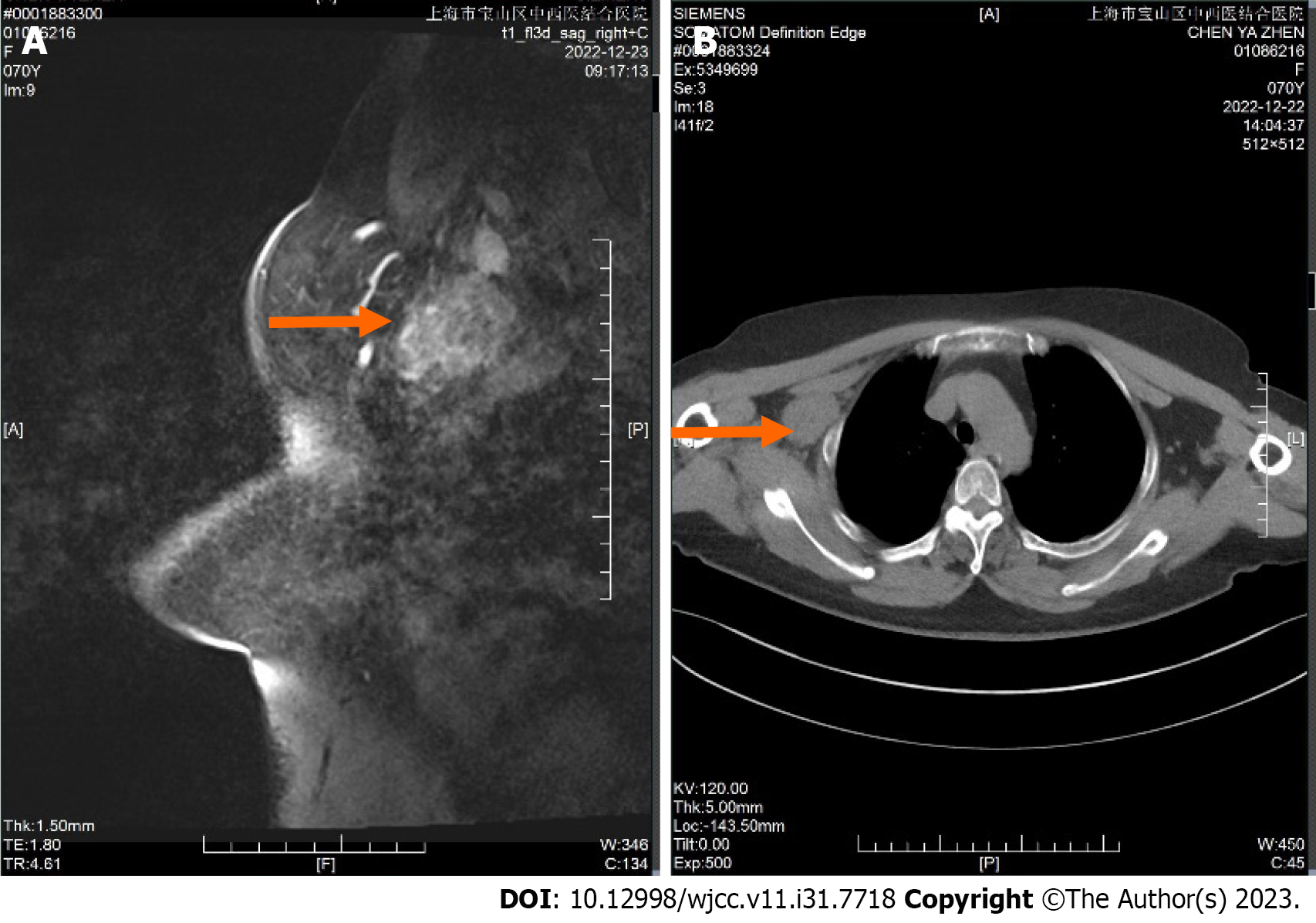
Dynamic contrast enhanced magnetic resonance imaging showed there is a mass in the upper outer quadrant and outer upper quadrant of the right breast is observed (Figure 1A and B), suggesting a possible malignant tumor. The Breast Imaging Reporting and Data System assessment is 4c. A mass-like enhancing lesion is also identified in the right axilla and adjacent to the pectoralis muscle, raising suspicion of metastatic lesions (Figure 2A and B).
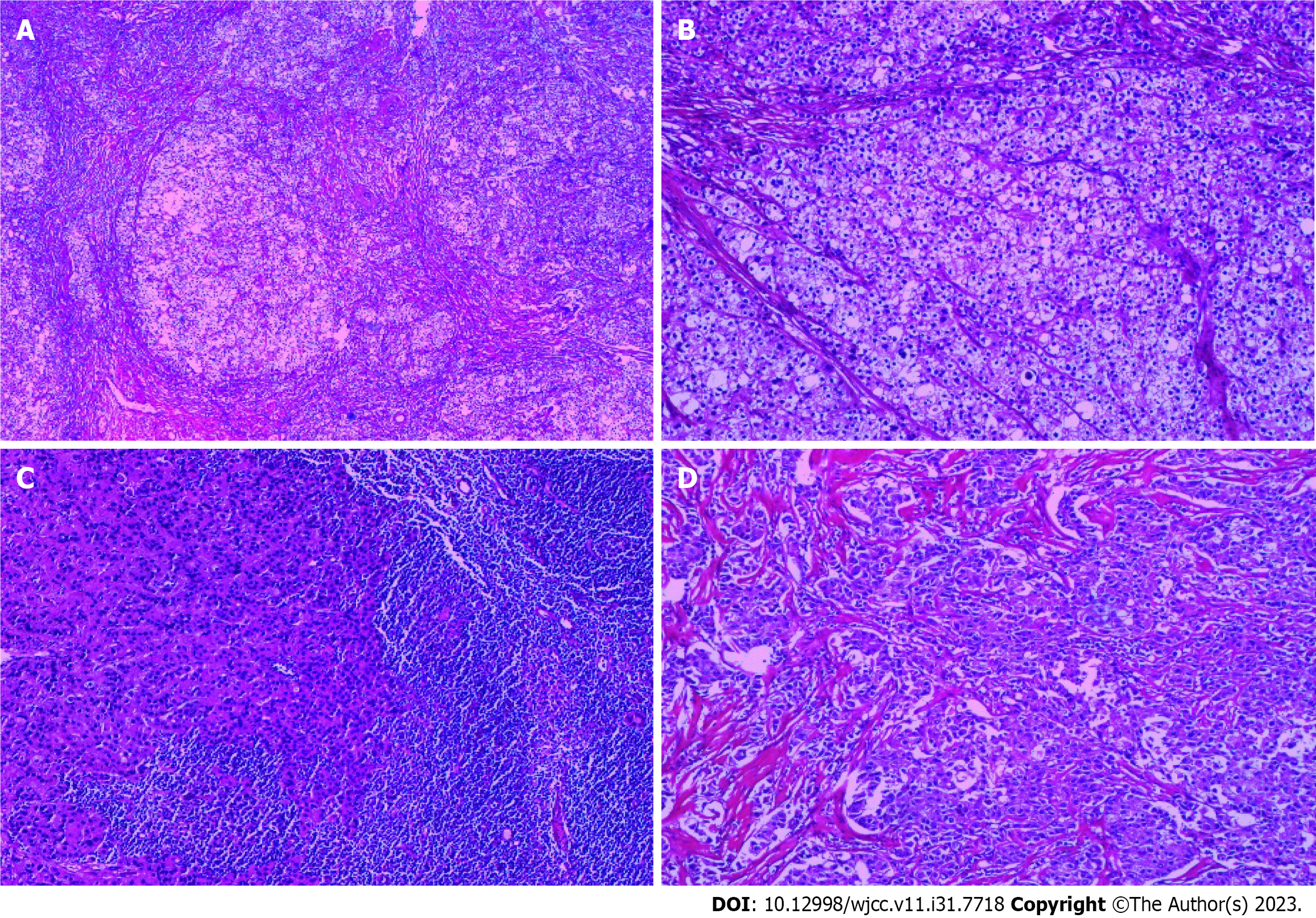
Pathology Report (pathology number: 2300526, Shanghai Baoshan Hospital of Integrated Traditional Chinese and Western Medicine, 2023-01-20): (right breast): Invasive carcinoma, non-special type, Scarff-Richardson grade III (Figure 3A and B). The tumor has infiltrated into the surrounding adipose tissue, with evidence of tumor emboli within blood vessels. No signs of neural invasion are observed. Sentinel lymph node (1/4): Carcinoma metastasis is observed (Figure 3C). One tumor deposit from right axillary area measuring 5 cm × 4 cm × 3 cm is observed (Figure 3D), along with eight additional lymph nodes that show no evidence of cancer metastasis. Immunohistochemical markers results: (Right breast) tumor: CK7(+), CK5/6(-), ER(3+, approximately 90%), PR (3+, approximately 80%), C-erbB-2(-), ECAD(+), P63(-), CD10(-), Ki67 (approximately 50%+). (Right axillary tumor deposit): ER (3+, approximately 90%), PR (3+, approximately 40%), C-erbB-2(-).
The patient has been diagnosed with invasive carcinoma of the right breast, pTNM stage was pT2, N1, M0 (clinical stage: IIB).
The patient underwent right breast-conserving surgery with sentinel lymph node biopsy and right axillary lymph node dissection on January 17, 2023. The patient received 6 cycles of chemotherapy with TC regimen, consisting of Docetaxel 130 mg and Cyclophosphamide 1000 mg. Post-chemotherapy, the patient exhibited normal liver and kidney functions, albeit with a decrease in white blood cell count. To restore the white blood cell count to normal levels, the patient was administered granulocyte colony-stimulating factor. Following chemotherapy, the patient underwent 25 sessions of radiotherapy. At present, the patient is undergoing treatment with oral Letrozole and Abemaciclib for endocrine therapy.
In the past year after the surgery, this patient has been undergoing a comprehensive examination every three months. No signs of recurrence or metastasis have been found.
There is limited research on tumor deposits in breast cancer. However, based on the development and definition of tumor deposits, they are deposits of cancer that have spread through lymphatic channels to distant soft tissues. According to the definition, this indicates regional metastasis. However, these nodules are not included in lymph node staging and prognosis due to the lack of lymph node morphology in pathological HE staining. For this patient, if this 5 cm tumor deposit is included as a metastatic axillary lymph node, the prognosis stage would become pT2N2M0 (clinical stage: IIIA), according to the eighth edition of the TNM staging system which incorporates biomarkers. In this case, the chemotherapy regimen may involve EC*4-T*4 instead of TC*6. Advanced imaging techniques and treatment methods are constantly improving and have an impact on patients’ survival outcomes, the tumor deposits originating from the lymph node drainage area also need to be taken seriously. This is crucial for assessing patients’ prognosis and guiding subsequent treatment.
To conduct research on whether tumor deposits should be included in the staging of breast cancer prognosis, we can consider adopting some methods used in gastric cancer and colorectal cancer studies. There have been numerous studies on tumor deposits in gastric cancer and colorectal cancer. A national cohort study from Sweden found that TDs are considered a risk factor for postoperative recurrence in colon cancer, suggesting the need for adjuvant chemotherapy[6]. A Delphi consensus study also supported the notion that TDs are indeed associated with worse prognosis compared to cases without TDs[7]. Even in the presence of lymph node metastasis, extramural venous invasion, perineural invasion, and lymphatic invasion, the number of TDs should be recorded[8]. Similarly, a retrospective study found that in gastric cancer[3], patients with positive tumor deposits had poorer 5-year disease-free survival rates (32.60% vs 92.45%) and overall survival rates (41.22% vs 89.37%) compared to those with negative tumor deposits. Tumor deposits are a strong and independent predictor of lower recurrence rates and survival rates in gastric tumors. These studies have made us realize the significance of tumor deposits in other types of cancer, and we can also consider these studies in defining whether to include tumor deposits in the prognosis staging and guiding treatment for breast cancer patients.
We should pay more attention to the presence of axillary tumor deposits and there is a need for further clinical research to discuss whether to include axillary tumor deposits in the prognostic criteria for breast cancer.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: D'Orazi V, Italy S-Editor: Liu JH L-Editor: A P-Editor: Zhao S
| 1. | Giuliano AE, Edge SB, Hortobagyi GN. Eighth Edition of the AJCC Cancer Staging Manual: Breast Cancer. Ann Surg Oncol. 2018;25:1783-1785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 236] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 2. | Fischer JR, Jackson HW, de Souza N, Varga Z, Schraml P, Moch H, Bodenmiller B. Multiplex imaging of breast cancer lymph node metastases identifies prognostic single-cell populations independent of clinical classifiers. Cell Rep Med. 2023;4:100977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 3. | Fujikawa K, Omori T, Shinno N, Hara H, Yamamoto M, Yasui M, Matsuda C, Wada H, Nishimura J, Haraguchi N, Akita H, Ohue M, Miyata H. Tumor Deposit Is an Independent Factor Predicting Early Recurrence and Poor Prognosis in Gastric Cancer. J Gastrointest Surg. 2023;27:1336-1344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 4. | Lin Q, Zhou H, Shi S, Lin J, Yan W. The Prognostic Value of Adjuvant Chemotherapy in Colon Cancer With Solitary Tumor Deposit. Front Oncol. 2022;12:916091. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Pyo DH, Kim SH, Ha SY, Yun SH, Cho YB, Huh JW, Park YA, Shin JK, Lee WY, Kim HC. Revised Nodal Staging Integrating Tumor Deposit Counts With Positive Lymph Nodes in Patients With Stage III Colon Cancer. Ann Surg. 2023;277:e825-e831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 19] [Article Influence: 9.5] [Reference Citation Analysis (1)] |
| 6. | Agger E, Jörgren F, Jöud A, Lydrup ML, Buchwald P. Negative Prognostic Impact of Tumor Deposits in Rectal Cancer: A National Study Cohort. Ann Surg. 2023;278:e526-e533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 7.0] [Reference Citation Analysis (1)] |
| 7. | Lord A, Brown G, Abulafi M, Bateman A, Frankel W, Goldin R, Gopal P, Kirsch R, Loughrey MB, Märkl B, Moran B, Puppa G, Rasheed S, Shimada Y, Snaebjornsson P, Svrcek M, Washington K, West N, Wong N, Nagtegaal I. Histopathological diagnosis of tumour deposits in colorectal cancer: a Delphi consensus study. Histopathology. 2021;79:168-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 8. | Pei JP, Zhang CD, Liang Y, Zhang C, Wu KZ, Li YZ, Zhao ZM, Dai DQ. A Modified Pathological N Stage Including Status of Tumor Deposits in Colorectal Cancer With Nodal Metastasis. Front Oncol. 2020;10:548692. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |