Published online Nov 6, 2023. doi: 10.12998/wjcc.v11.i31.7690
Peer-review started: August 30, 2023
First decision: September 20, 2023
Revised: October 6, 2023
Accepted: October 26, 2023
Article in press: October 26, 2023
Published online: November 6, 2023
Processing time: 67 Days and 20.5 Hours
Renal pelvis sarcomatoid carcinoma (RPSC) is a rare and aggressive malignancy whose diagnosis is difficult because radiological imaging results can lead to misclassification as a more common type of renal tumor. In addition, clinical management of patients with RPSC is difficult because of the limited efficacy of available treatments. In this study, we present a comprehensive description of a patient who presented with RPSC and a simultaneous renal vein tumor thrombus.
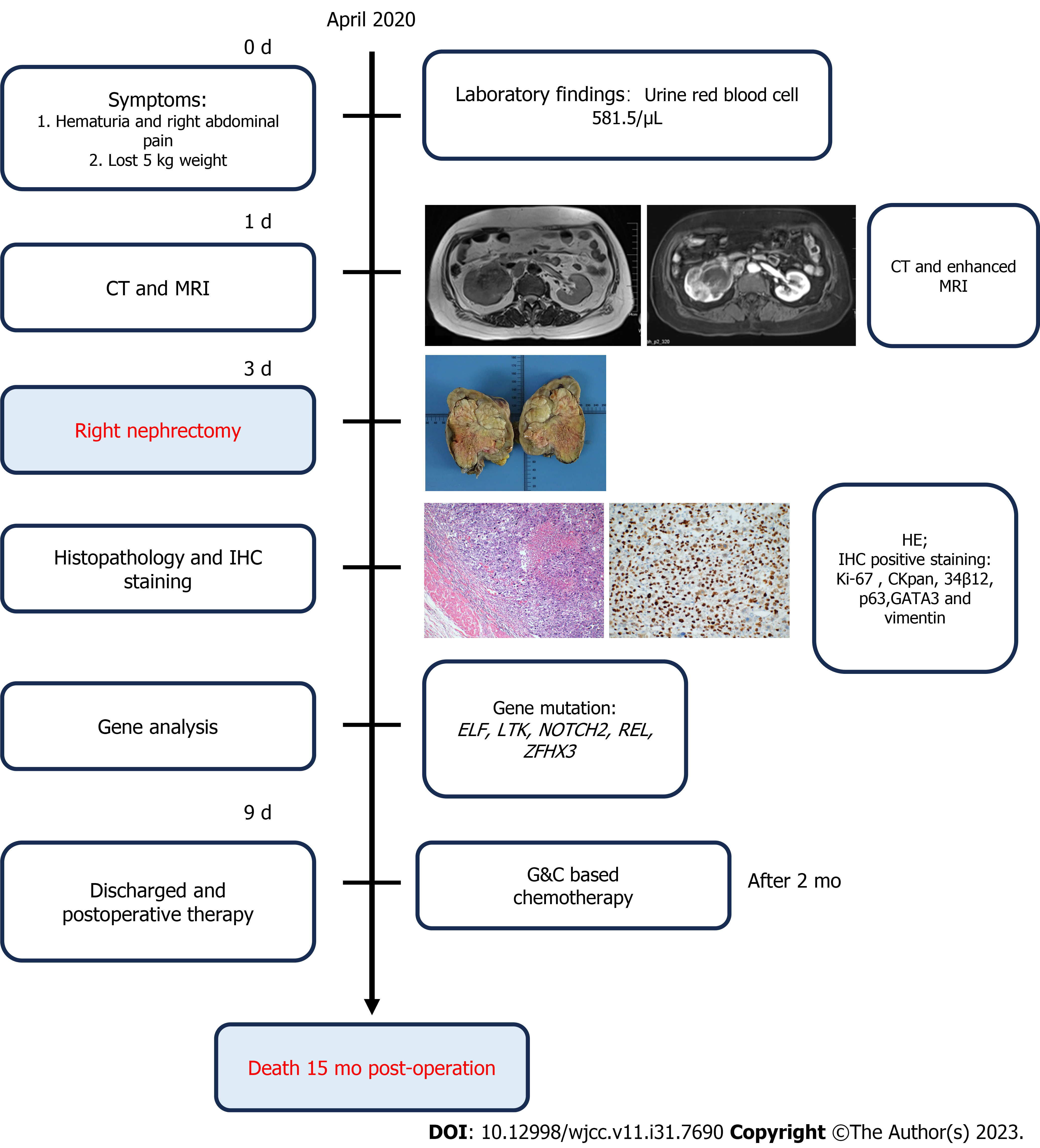
During April, 2020, a 64-year-old female presented with an isolated episode of hematuria accompanied by abdominal pain. Computed tomography (CT) and magnetic resonance imaging (MRI) showed a lesion in the right renal pelvis. We therefore performed a radical nephrectomy of the right kidney. The subsequent histopathological and immunological results verified the diagnosis of RPSC. Despite administration of 6 cycles of a gemcitabine-cisplatin regimen, the patient's condition progressively deteriorated, and she died about 15 mo after the ne
We performed a comprehensive analysis of a patient with RPSC that included CT, MRI, immunohistochemistry, and genetic testing. The insights from our detailed analysis of this patient and our concomitant review of the literature may assist clinicians in their diagnosis and treatment of RPSC.
Core Tip: Renal pelvis sarcomatoid carcinoma (RPSC) is an extremely rare tumor that is associated with a high mortality rate. We present a rare case of right RPSC with renal vein tumor thrombus, in which diagnosis was based on evidence from radiology, pathology, and analysis of tumor mutations. The specific genetic mutations in this patient’s tumor may provide insights into the invasive phenotype and pathogenesis of this cancer.
- Citation: Guan HY, Wang J, Wang JX, Chen QH, Lu J, He L. Renal pelvis sarcomatoid carcinoma with renal vein tumor thrombus: A case report and literature review. World J Clin Cases 2023; 11(31): 7690-7698
- URL: https://www.wjgnet.com/2307-8960/full/v11/i31/7690.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i31.7690
Renal pelvis sarcomatoid carcinoma (RPSC) is a rare type of urinary tract malignancy[1]. This cancer has a low incidence, and it accounts for only about 0.3% of all urothelial carcinomas[2]. Since the initial description of this tumor by Fauci and colleagues in 1961, there have been fewer than 30 reported cases[3]. There are similar predisposing factors for RPSC and renal squamous cell carcinoma, including tobacco consumption, persistent irritation, chronic inflammation, and nephrolithiasis[4]. Surgery is the most efficacious and widely adopted treatment for patients with RPSC[5], and the major approaches are nephroureterectomy or nephrectomy. An accurate diagnosis of RPSC requires a comprehensive clinical assessment with histological and immunohistochemical analyses of the tumor[6].
Herein we present a patient with RPSC and describe the results from imaging, histochemistry, and genetics (Figure 1). We also provide a comprehensive review of the literature on this topic to consolidate all previous clinical findings on this cancer.
A 64-year-old female (weight: 60 kg, height: 165 cm) who presented in April, 2020 was assessed for a recent isolated episode of hematuria that was accompanied by abdominal pain.
During the past five months, the patient reported a decrease in body weight of about 5 kg.
The patient had a medical history of bilateral renal stones. Additionally, she received pharmaceutical management for hypertension for many years, and intermittently utilized nifedipine.
The patient had no specific personal or family history of illnesses.
Upon admission, the patient's body temperature was 36.5°C, heart rate was 75 beats per min, respiratory rate was 19 breaths per min, blood pressure was 145/90 mmHg, and oxygen saturation (while breathing ambient air) was 99%. She also had mild tenderness in the right lumbar and abdominal regions.
A urinalysis indicated the red blood cell count was 581.5/μL and the round epithelial cell count was 2.4/μL. An exfoliative cytology test indicated no atypical epithelial cells.
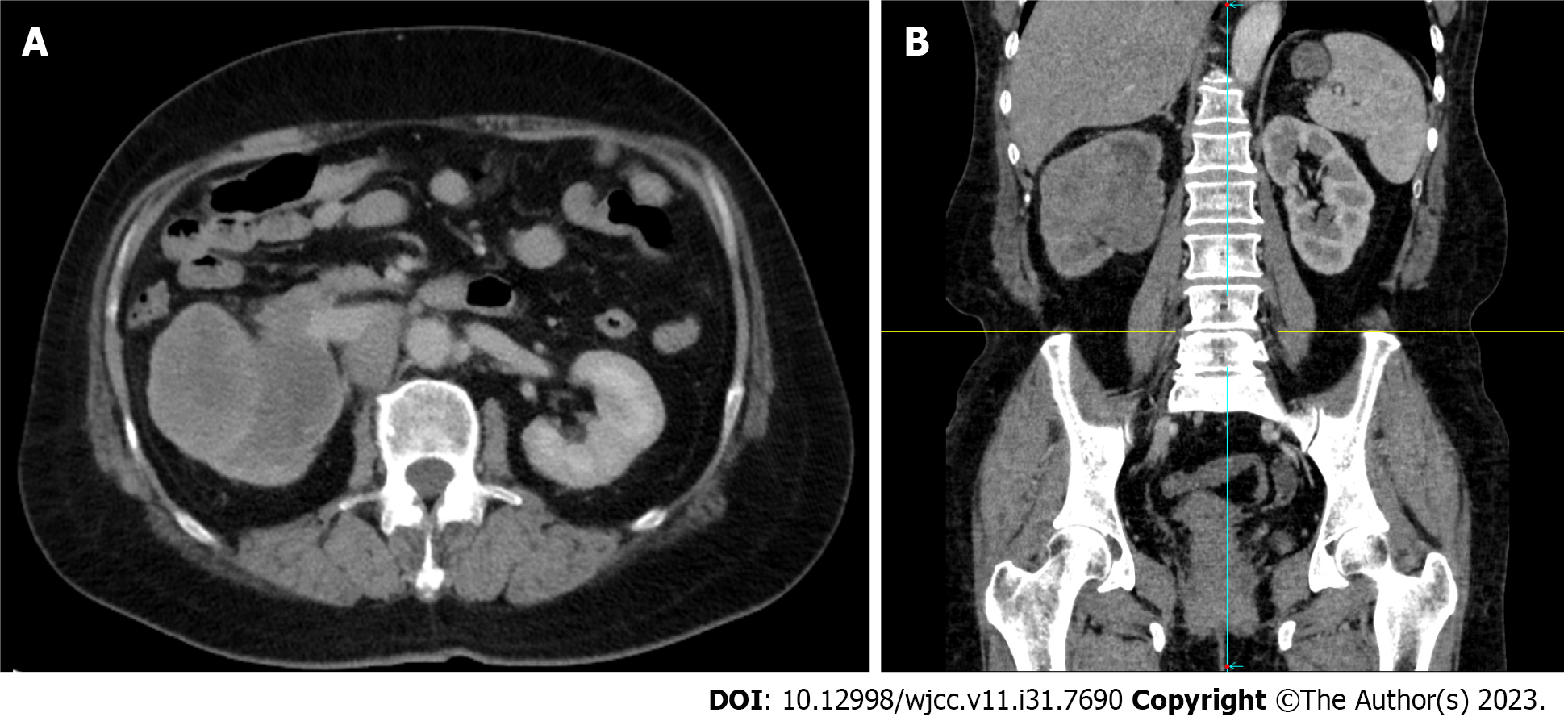
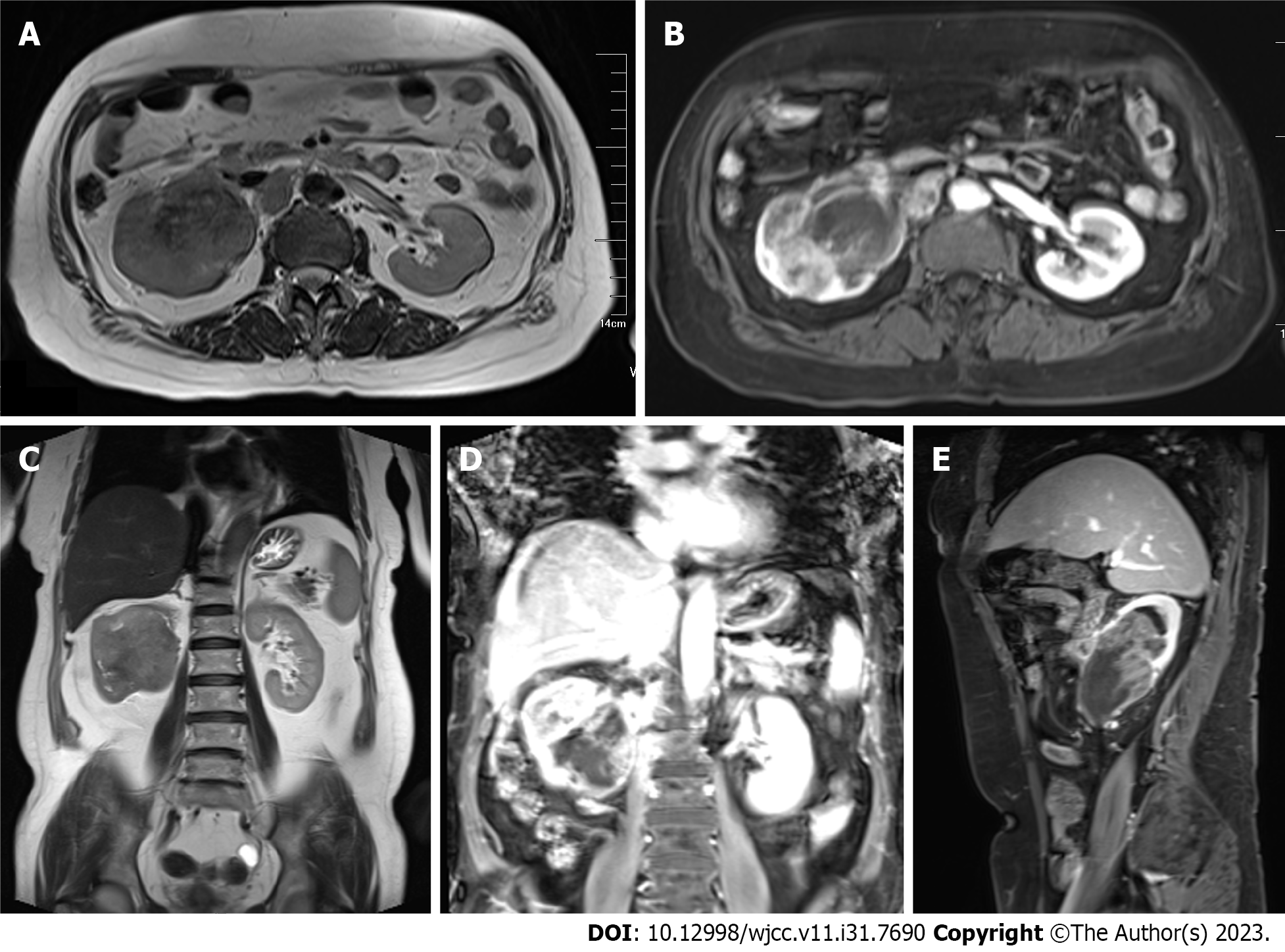
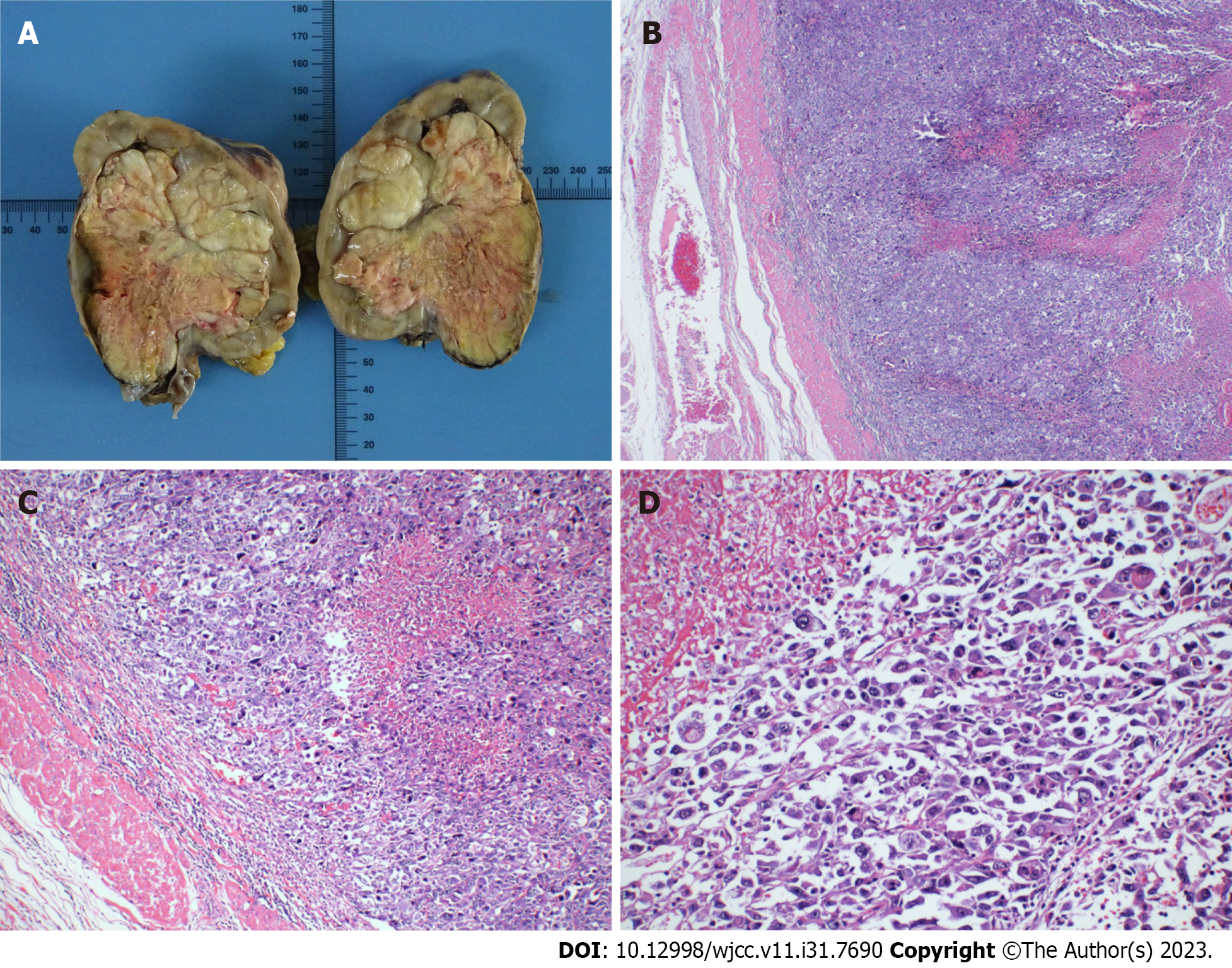
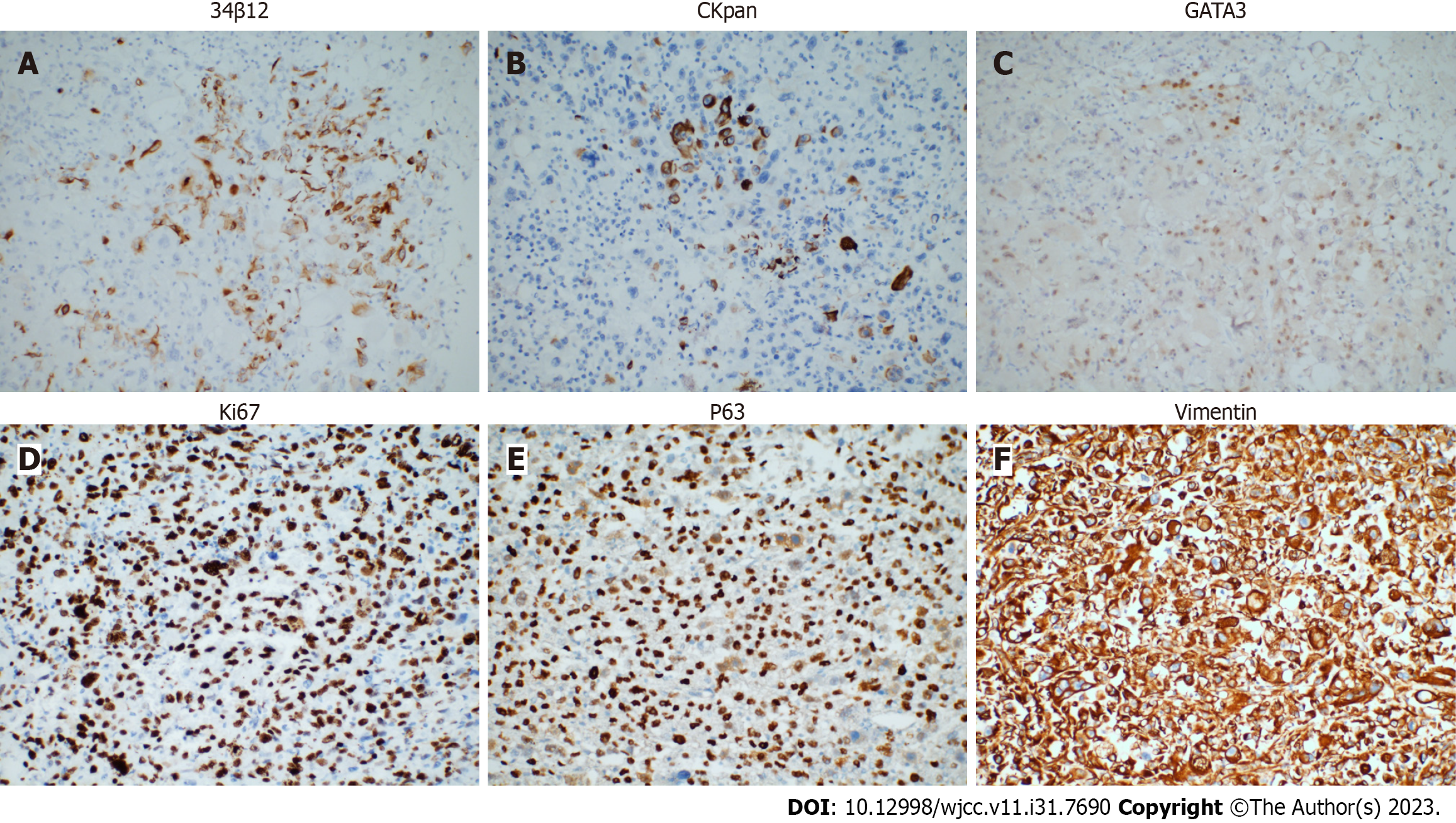
A conventional abdominal computed tomography (CT) scan (Figure 2) demonstrated irregular enlargement of the right kidney, indicative of a space-occupying lesion. The subsequent enhanced magnetic resonance imaging (MRI) results, which used a gadolinium-based contrast agent (Figure 3), revealed a distinctive clumpy and abnormal signal in the right renal pelvis that had dimensions of 7.9 cm × 6.4 cm × 7.4 cm (Figure 4). The tumor had uniformly low signal intensity in the T1-weighted phase, and slightly elevated mixed signal intensity in the T2-weighted phase. We also identified multiple enlarged lymph nodes in the retroperitoneal region, raising a concern of metastasis. After nephrectomy, immunohistochemical analysis of tumor samples indicated positive staining for Ki-67 (70%), CKpan, 34β12, p63, GATA3, and vimentin, but negative staining for CK7 and PAX8 (Figure 5). There was also evidence from the MRI of cancerous infiltration in the renal sinus, renal parenchyma, and vasculature, and a cancerous thrombus in the right renal vein.
A genetic analysis of the tumor tissue showed mutations in the ELF, LTK, NOTCH2, REL, and ZFHX3 genes (Table 1). However, there were no mutations in genes that have known relationships with hereditary tumors. Furthermore, the gene analysis yielded no specific drug targets that could be used for pharmacological intervention.
| Characteristics | Gene | Exon | Nucleic acid variation | Amino acid change | Abundance |
| Gene name | ELF | Exon 4 | c.421G>C | p.E141Q | 9.45% |
| LTK | Exon 10 | c.1321A>G | p.M441V | 7.00% | |
| NOTCH2 | Exon 25 | c.4028A>G | p.Q1343R | 1.34% | |
| REL | Exon 9 | c.947A>G | p.D316G | 7.17% | |
| ZFHX3 | Exon 9 | c.6643C>T | p.P2215S | 2.94% |
A postoperative pathological assessment confirmed that the tumor was RPSC, and had dimensions of 9.0 cm × 8.0 cm × 5.5 cm. Based on standard staging criteria[7], we classified the tumor as T3N1Mx.
Following a thorough preoperative evaluation, the patient underwent right renal nephrectomy. Two months later, she opted for conventional systemic chemotherapy. This treatment commenced in July, 2020, and consisted of 6 cycles (21 d per cycle) of gemcitabine-cisplatin (GC).
The patient experienced a favorable postoperative recovery and was discharged after 6 d. However, the subsequent GC chemotherapy was ineffective and the patient died about 15 mo after the surgery.
Upper-tract urothelial cancer is the most prevalent malignancy affecting the renal pelvis, but only accounts for 5% to 10% of all urothelial cancers. Squamous cell carcinoma and adenocarcinoma are the second and third most common types of renal pelvis malignancies[8]. RPSC is a very rare and aggressive type of urothelial carcinoma, and there have only been descriptions of fewer than 30 cases (Table 2). A retrospective analysis of these previous cases indicated the onset of RPSC typically occurs in patients more than 50-years-old, and the sex ratio is about 4 or 5 males to 12 females[2] (Table 2). The apparent risk factors for RPSC are tobacco smoking, previous genital tract irradiation, inflammation, and nephrolithiasis[9]. Our 64-year-old female patient had no history of smoking or genital tract irradiation, but she did have a history of nephrolithiasis. Renal stones can possibly lead to provocation of squamous cell carcinoma in the renal pelvis[10], so we hypothesize that renal stones could have contributed to the progression of RPSC in our patient. The origin of RPSC remains controversial. The monoclonal theory posits that carcinomatous and sarcomatous tumor cells arise from pluripotent stem cells, whereas the multiclonal theory proposes that sarcomatoid carcinoma is a “collision tumor” that results from derivatives of distinct epithelial and mesenchymal stem cells[11].
| No | Ref. | Age/sex/side | Size | Prognosis | Subsequent treatment |
| 1 | Chu et al[6], 2023 | 71/M/R | 6.3 × 4.6 cm | Survived over 1 yr | Chemotherapy |
| 2 | Rashid et al[11], 2018 | 70/M/R | 5.5 cm × 4.5 cm × 4.5 cm | NA | NA |
| 3 | Mohan et al[22], 2018 | 68/F/L | 10.0 cm × 4.5 cm × 3.0 cm | 10 m no recurrence | Non |
| 4 | Lisa et al[23], 2017 | 47/M/L | 7.0 cm × 5.0 cm × 4.5 cm | 1 mo recurrence; Died < 5 mo | Non |
| 5 | Kivlin et al[4], 2016 | 77/M/R | 10.0 cm × 10.0 cm × 10.0 cm | NA | Non |
| 6 | Cuadra-Urteaga et al[24], 2016 | 44/M/R | 6.0 cm × 6.5 cm × 7.5 cm | T3aNxMx; Died 8 wk after surgery | Non |
| 7 | Tian et al[2], 2014 | 49/F/L | Approximately 2 cm in diameter | T1N0M0; 30-mo follow-up without recurrence | Non |
| 8 | Fernández-Pello et al[25], 2014 | 58/M/R | 15.0 cm × 9.0 cm × 7.0 cm | T4NxMx; 18 mo follow-up without recurrence | Non |
| 9 | Gill-Samra et al[9], 2012 | 76/F/R | NA | 2 mo recurrence after surgery | Non |
| 10 | Chen et al[26], 2011 | 77/M/L | 16.0 cm × 12.0 cm × 9.0 cm | Non-resurrence until the article published | Non |
| 11 | Mimura et al[27], 2010 | 66/M/L | 4.0 cm × 3.5 cm × 3.0 cm | Died 7 mo after surgery | Non |
| 12 | Masue et al[8], 2007 | 58/M/L | 3.5 cm × 2.3 cm × 2.5 cm | T3N0M1; 46 mo disease-free | Non |
| 13 | Thiel et al[28], 2006 | 61/M/L | 4.5 cm in diameter | 16 mo without recurrence or metastasis | Non |
| 14 | Shimasaki et al[29], 2005 | 61/F/R | 6.5 cm × 4.0 cm × 3.0 cm | 16 mo without recurrence or metastasis | Non |
| 15 | Acikalin et al[30], 2005 | 66/M/L | 2.5 cm in diameter | Died 7 mo after operation | Non |
| 16 | Vermeulen et al[31], 2000 | 77/M/R | 4.7 cm in diameter | 8 mo without recurrence or metastasis | Non |
| 17 | Sekido et al[32], 2000 | 72/M/R | 8.4 cm × 6.5 cm | 9 mo without recurrence or metastasis | Non |
| 18 | Kandemir et al[33], 1995 | 26/M/R | 12.0 cm × 8.0 cm × 7.0 cm | Died of septic shock 3 d after the operation | Non |
The clinical manifestations of RPSC are variable. Previous studies reported that hematuria was the most frequently reported symptom. Localized or non-specific pain was reported as the second most prevalent symptom[5], and this can escalate to severe pain. In rare cases, the presence of a renal pelvis abscess could indicate RPSC[6,12]. These patients also frequently experience initial symptoms indicative of an inflammatory infection. In situations where RPSC coincides with a renal abscess, the abscess frequently obscures the symptoms of the tumor, leading to a delayed or missed diagnosis.
Imaging examinations are crucial for the preoperative assessment of tumors in the renal pelvis. CT and MRI scans can be used to determine tumor size, metastasis, and the presence of a tumor thrombus (Figures 2 and 3). Mild to moderate heterogeneous enhancement occurs when using a contrast agent, but distinguishing RPSC from other renal pelvis tumors remains challenging. Immunohistochemistry results are therefore essential for the diagnosis of RPSC. The sarcomatoid component of RPSC can coexist with other tumor types, including adenocarcinoma, small cell carcinoma, or squamous cell carcinoma. HE staining yields similar cellular morphologies for carcinosarcoma and sarcomatoid carcinoma (Figure 4), but RPSC has positive staining for Ki-67 (70%), CKpan, 34β12, p63, GATA3, and vimentin, while negative staining for PAX8 and CK7.
We also performed a comprehensive analysis of genetic alterations in the patient’s tumor, and identified mutations in the ELF, LTK, NOTCH2, REL, and ZFHX3 genes (Table 1). The ELF3 gene is a member of the E26 transformation-specific family of transcription factors, is located on chromosome 1q32.1, and encodes a protein of 371 amino acids[13]. Previous studies have implicated ELF3 in various diseases, including cancers of the bladder, ovary, biliary tract, stomach, cervix, breast, prostate, lung, liver, and colon[14]. Studies of mutations in the ELF3 gene demonstrated this gene functions as a tumor suppressor in certain cancers, although increased ELF3 expression also occurs in other cancers[15]. NOTCH2 is a member of the Notch receptor family that is overexpressed in many cancers, and is linked with distinctive oncogenic mechanisms[16]. LTK is in the anaplastic lymphoma kinase (ALK)/LTK subfamily, and increased expression of this gene is associated with metastasis in certain cancers[17].
Although our genetic testing confirmed that RPSC has a highly invasive and metastatic phenotype, the lack of effective targeted therapies accounts for the grim prognosis for most patients with this cancer. Only a limited number of these patients achieve survival beyond two years[1]. The unique characteristics of this tumor are likely responsible for the ineffectiveness of conventional chemotherapy and radiotherapy regimens. Surgical excision is the preferred initial procedure for treating RPSC[6], and subsequent radiotherapy can enhance local control when combined with cisplatin-based chemotherapy[18]. However, cisplatin-based chemotherapy is unfeasible in some patients due to a low estimated glomerular filtration rate and diminished renal function after nephrectomy[19]. Previous research has indicated heightened expression of the epidermal growth factor receptor (EGFR) on the surface of RPSC cells[20], so therapeutic strategies that target EGFR may have some potential. Other research found that RPSC cells had high expression of PDL1, suggesting that immunotherapy may be beneficial[21]. Our patient only survived 15 mo after surgery, despite use of GC-based chemotherapy. We hope that future studies which identify more effective treatments can prolong the survival times of these patients.
Given the rarity of RPSC, diagnosis is difficult and there are inadequate available treatments. In this study, we presented a new case of RPSC and conducted a comprehensive review of the most recent literature. We also performed immunohistochemical and genetic analyses of our patient. The indicators described herein may be useful in guiding future clinical interventions for the treatment of patients with RPSC.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Urology and nephrology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cassell III AK, Liberia S-Editor: Lin C L-Editor: A P-Editor: Zhao S
| 1. | Chen S, Chen G, Xia D, Li J, Wang S, Shen B, Jin B. Sarcomatoid carcinoma of the renal pelvis: Experience of multiple cases over a ten-year period. Oncol Lett. 2013;6:513-516. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 2. | Tian X, Zhao J, Wang Y, Xing N. Sarcomatoid carcinoma of the renal pelvis: A case report. Oncol Lett. 2014;8:1208-1210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 3. | Fauci Pa Jr, Therhag Hg, Davis Je. Carcinosarcoma of the renal pelvis. J Urol. 1961;85:897-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | Kivlin D, Tong C, Friedlander J, Perosio P, Simhan J. Sarcomatoid Squamous Cell Carcinoma of the Renal Pelvis Masquerading as Emphysematous Pyelonephritis with Staghorn Calculus. J Endourol Case Rep. 2016;2:87-89. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 5. | Mantica G, Benelli A, Ackermann H, Nxumalo Z, Solaiman A, Dotta F, van der Merwe A, Terrone C. Clinical and histopathological features of carcinosarcoma of the renal pelvis: a systematic review of a rare tumor. Minerva Urol Nefrol. 2019;71:121-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Chu Y, Ning H, Yin K, Chen T, Wu H, Wang D, Liu F, Zhao Z, Lv J. Case report: Sarcomatoid urothelial carcinoma of the renal pelvis masquerading as a renal abscess. Front Oncol. 2023;13:1055229. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 7. | Rouprêt M, Seisen T, Birtle AJ, Capoun O, Compérat EM, Dominguez-Escrig JL, Gürses Andersson I, Liedberg F, Mariappan P, Hugh Mostafid A, Pradere B, van Rhijn BWG, Shariat SF, Rai BP, Soria F, Soukup V, Wood RG, Xylinas EN, Masson-Lecomte A, Gontero P. European Association of Urology Guidelines on Upper Urinary Tract Urothelial Carcinoma: 2023 Update. Eur Urol. 2023;84:49-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 266] [Article Influence: 133.0] [Reference Citation Analysis (0)] |
| 8. | Masue N, Hasegawa Y, Moriyama Y, Ikeda Y, Gotoh T, Deguchi T. Spontaneous disappearance of multiple lung metastases after nephroureterectomy from sarcomatoid carcinoma of the renal pelvis: a case report. Int J Urol. 2007;14:75-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Gill-Samra S, Brooks A, P'ng CH. Sarcomatoid carcinoma involving the renal pelvis and ureter with heterologous osteosarcomatous differentiation. Pathology. 2012;44:367-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 10. | Vallejo EP, Tejido Sánchez Á, Miranda Utrera N, Gómez Del Cañizo C. Massive Hydronephrosis, Countless Lithiasis and Upper Urinary Tract Carcinoma. Urology. 2022;165:e7-e8. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 11. | Rashid S, Akhtar M. Sarcomatoid Variant of Urothelial Carcinoma of the Renal Pelvis with Inferior Vena Cava Tumour Thrombus: A Case Report and Literature Review. Case Rep Pathol. 2018;2018:1837510. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Terada T. Multiple cytokeratin-negative malignant tumors composed only of rhabdoid cells in the renal pelvis: a sarcomatoid urothelial carcinoma? Int J Clin Exp Pathol. 2013;6:724-728. [PubMed] |
| 13. | Luk IY, Reehorst CM, Mariadason JM. ELF3, ELF5, EHF and SPDEF Transcription Factors in Tissue Homeostasis and Cancer. Molecules. 2018;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 87] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 14. | Sarmah S, Hawkins MR, Manikandan P, Farrell M, Marrs JA. Elf3 deficiency during zebrafish development alters extracellular matrix organization and disrupts tissue morphogenesis. PLoS One. 2022;17:e0276255. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 15. | Takaoka A, Ishikawa T, Okazaki S, Watanabe S, Miya F, Tsunoda T, Kikuchi A, Yamauchi S, Matsuyama T, Tokunaga M, Uetake H, Kinugasa Y. ELF3 Overexpression as Prognostic Biomarker for Recurrence of Stage II Colorectal Cancer. In Vivo. 2021;35:191-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Xiu MX, Liu YM. The role of oncogenic Notch2 signaling in cancer: a novel therapeutic target. Am J Cancer Res. 2019;9:837-854. [PubMed] |
| 17. | Cooper AJ, Sequist LV, Johnson TW, Lin JJ. LTK fusions: A new target emerges in non-small cell lung cancer. Cancer Cell. 2022;40:23-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 18. | Hall MC, Womack JS, Roehrborn CG, Carmody T, Sagalowsky AI. Advanced transitional cell carcinoma of the upper urinary tract: patterns of failure, survival and impact of postoperative adjuvant radiotherapy. J Urol. 1998;160:703-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 38] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Tafuri A, Marchioni M, Cerrato C, Mari A, Tellini R, Odorizzi K, Veccia A, Amparore D, Shakir A, Carbonara U, Panunzio A, Trovato F, Catellani M, Janello LMI, Bianchi L, Novara G, Dal Moro F, Schiavina R, De Lorenzis E, Parma P, Cimino S, De Cobelli O, Maiorino F, Bove P, Crocerossa F, Cantiello F, D'Andrea D, Di Cosmo F, Porpiglia F, Ditonno P, Montanari E, Soria F, Gontero P, Liguori G, Trombetta C, Mantica G, Borghesi M, Terrone C, Del Giudice F, Sciarra A, Galosi A, Moschini M, Shariat SF, Di Nicola M, Minervini A, Ferro M, Cerruto MA, Schips L, Pagliarulo V, Antonelli A. Changes in renal function after nephroureterectomy for upper urinary tract carcinoma: analysis of a large multicenter cohort (Radical Nephroureterectomy Outcomes (RaNeO) Research Consortium). World J Urol. 2022;40:2771-2779. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 20. | Wang X, MacLennan GT, Zhang S, Montironi R, Lopez-Beltran A, Tan PH, Foster S, Baldridge LA, Cheng L. Sarcomatoid carcinoma of the upper urinary tract: clinical outcome and molecular characterization. Hum Pathol. 2009;40:211-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 21. | Zhu S, Yu C, Wang C, Ding G, Cheng S. Case report: Significant benefits of tislelizumab combined with anlotinib in first-line treatment of metastatic renal pelvic urothelial carcinoma with sarcomatoid carcinoma differentiation. Front Oncol. 2022;12:969106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 22. | Mohan BP, Jayalakshmy PL, Letha V, Bhat S. Sarcomatoid Carcinoma of Renal Pelvis Involving Ureter and Renal Parenchyma with Heterologous Osteosarcomatous Differentiation: A Case Report and Review of Literature. Iran J Pathol. 2018;13:89-93. [PubMed] |
| 23. | Lisa M, Singh GR, Madhawi R, Kumar B, Imam ZS. Sarcomatoid Carcinoma of Renal Pelvis with Abundant Heterologous Osteosarcomatous Element: A Case Report. J Clin Diagn Res. 2017;11:ED31-ED32. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 24. | Cuadra-Urteaga JL, Font A, Tapia G, Areal J, Taron M. Carcinosarcoma of the upper urinary tract with an aggressive angiosarcoma component. Cancer Biol Ther. 2016;17:233-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 25. | Fernández-Pello S, Venta V, González I, Gil R, Menéndez CL. Pyonephrosis as a sign of sarcomatoid carcinoma of the renal pelvis. World J Clin Cases. 2014;2:215-218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 26. | Chen GM, Chen SW, Xia D, Li J, Yan S, Jin BY. Sarcomatoid carcinoma of the renal pelvis in duplex kidney. Chin Med J (Engl). 2011;124:2074-2076. [PubMed] |
| 27. | Mimura A, Sakuma T, Furuta M, Tanigawa N, Takamizu R, Kawano K. Sarcomatoid collecting duct carcinoma of kidney diagnosed with urine and renal pelvic lavage cytology. Diagn Cytopathol. 2010;38:603-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 28. | Thiel DD, Igel TC, Wu KJ. Sarcomatoid carcinoma of transitional cell origin confined to renal pelvis. Urology. 2006;67:622.e9-622.11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 29. | Shimasaki N, Inoue K, Nishigawa H, Kuroda N, Shuin T. Combined small cell carcinoma and sarcomatoid squamous cell carcinoma in the renal pelvis. Int J Urol. 2005;12:686-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 30. | Acikalin MF, Kabukcuoglu S, Can C. Sarcomatoid carcinoma of the renal pelvis with giant cell tumor-like features: case report with immunohistochemical findings. Int J Urol. 2005;12:199-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 31. | Vermeulen P, Hoekx L, Colpaert C, Wyndaele JJ, Van Marck E. Biphasic sarcomatoid carcinoma (carcinosarcoma) of the renal pelvis with heterologous chondrogenic differentiation. Virchows Arch. 2000;437:194-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 32. | Sekido Y, Satoh F, Usui Y, Tsutsumi Y. Sarcomatoid carcinoma of the renal pelvis: a case report. Pathol Int. 2000;50:562-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |