Published online Nov 6, 2023. doi: 10.12998/wjcc.v11.i31.7640
Peer-review started: September 4, 2023
First decision: September 13, 2023
Revised: September 26, 2023
Accepted: October 23, 2023
Article in press: October 23, 2023
Published online: November 6, 2023
Processing time: 62 Days and 19.1 Hours
Severely elevated intracranial pressure due to various reasons, such as decreased cerebral perfusion, can lead to devastating neurological outcomes, such as brain herniation. Decompression craniectomy is a life-saving procedure that is commonly performed for such a critical situation, but the changes in cerebral microvessels after brain herniation and decompression are unclear. Ultrafast power Doppler imaging (uPDI) is a new microvascular imaging technology that utilizes high frame rate plane/diverging wave transmission and advanced clutter filters. uPDI significantly improves Doppler sensitivity and can detect micro
In this report, uPDI was used for the first time to observe the brain blood flow of a hypoperfusion area in a 4-year-old girl who underwent decompression crani
uPDI showed the local blood supplies and anatomical structures of the patient after decompressive craniectomy. uPDI is potentially a more intuitive and noninvasive method for evaluating the effects of severe ICP on cerebral microvessels.
Core Tip: Decompression craniectomy is a life-saving procedure for severe intracranial hypertension (ICP), but its effects on cerebral microvessels are unclear. We used ultrafast power Doppler imaging (uPDI), a new noninvasive and highly sensitive microvascular imaging technology, to observe the brain blood flow of a 4-year-old girl who underwent decompression craniectomy after malignant brain tumor surgery. uPDI showed the local blood supplies and anatomical structures of the patient after decompressive craniectomy. uPDI is potentially a more intuitive and noninvasive method for evaluating the effects of severe ICP on cerebral microvessels.
- Citation: Huang LJ, Jiao JF, He Q, Luo JW, Guo Y. Ultrafast power Doppler imaging for ischemic encephalopathy: A case report. World J Clin Cases 2023; 11(31): 7640-7646
- URL: https://www.wjgnet.com/2307-8960/full/v11/i31/7640.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i31.7640
Refractory intracranial hypertension (ICP) is a potentially devastating complication that can cause neurological damage followed by severe traumatic brain injury, intracranial tumors, intracranial hemorrhage, and brain edema. Reduced cerebral perfusion, cerebral ischemia and herniation from intractable elevated ICP can lead to a poor prognosis in neurosurgical patients[1]. Decompression craniectomy is a life-saving procedure for such a critical situation[2]. Repeated computed tomography (CT) scans are the most widely used anatomical imaging evaluation for such patients. However, there are few fast and noninvasive imaging methods that can evaluate changes in cerebral microvascular structure and hemodynamics.
Recently, thanks to the emergence of ultrahigh-frame-rate plane/diverging wave transmission[3] and advanced clutter filters [e.g., singular value decomposition (SVD)[4]], ultrafast power Doppler imaging (uPDI) was proposed[5] and developed[6,7] to visualize microvessels that are invisible on conventional ultrasound Doppler images. Several studies have been conducted to explore the relationship between the progression of diseases and the changes in microvessels[8-10] because it is well-known that microvessels experience morphological and hemodynamic changes as diseases progress[8,11]. In this study, uPDI was first used to visualize the microvessels in the brain of a patient after decompression craniectomy.
A 4-year-old girl was admitted to our hospital with a 5-d history of intermittent headache and a 2-d history of both drowsiness and vomiting.
Five days ago, the patient experienced intermittent headaches without any apparent cause. Two days later, she developed drowsiness and vomiting. While waiting for a scheduled enhanced magnetic resonance imaging (MRI), she suffered a sudden onset of epilepsy; then, she fell into a coma, showed bilateral pupil dilation and was in critical condition.
The patient’s history of past illness was not significant.
The patient’s personal and family history were not significant.
Physical examination showed that her body temperature was 36.5 °C, pulse rate was 100 beats per min, respiratory rate was 20 breaths per min and blood pressure was 95/48 mmHg, showing bilateral pupil dilation.
Complete blood count: White blood cells 7.67 × 109/L, red blood cells 4.36 × 1012/L, hemoglobin 117.00 g/L, platelets 338.00 × 109/L.
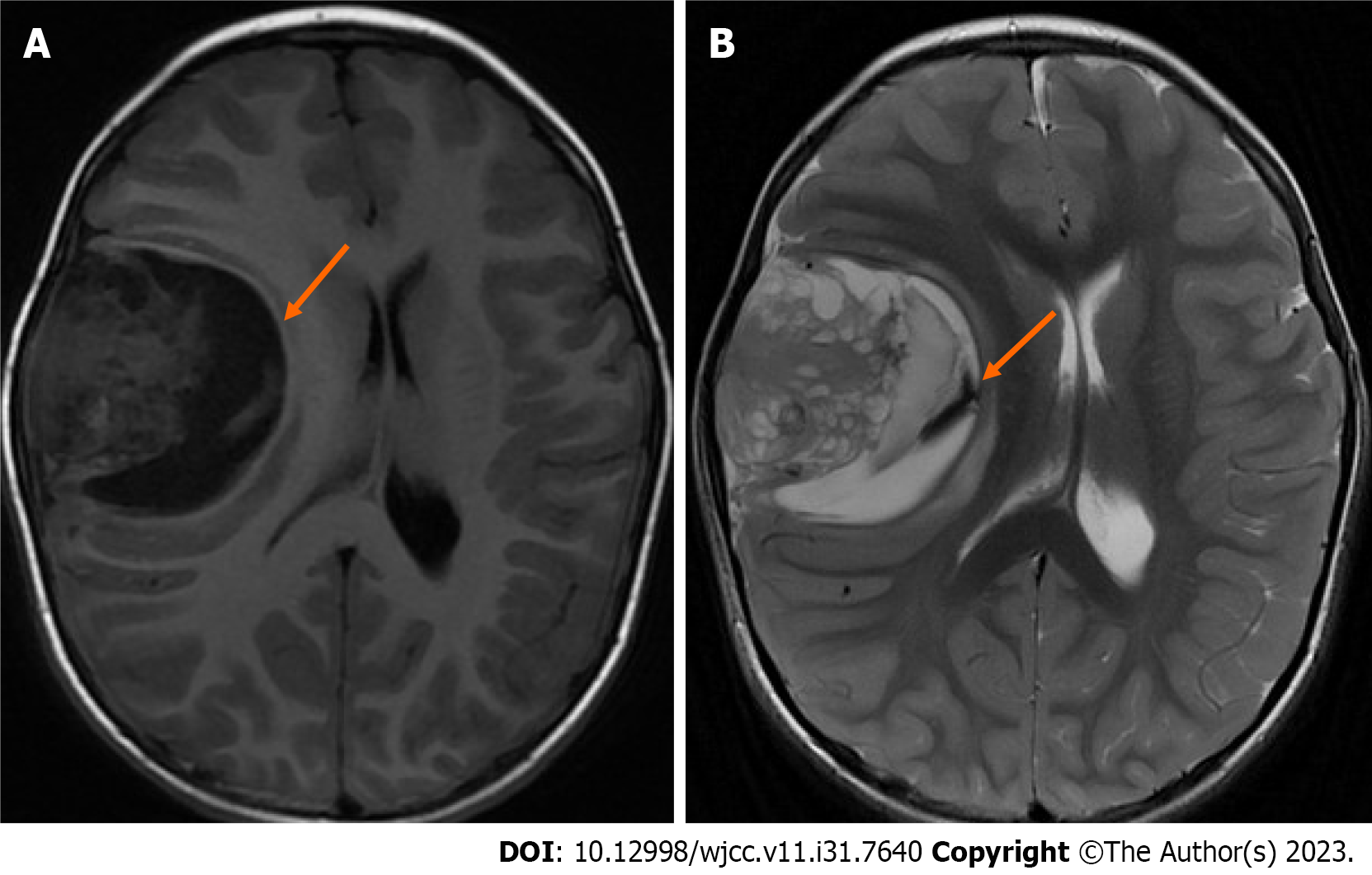
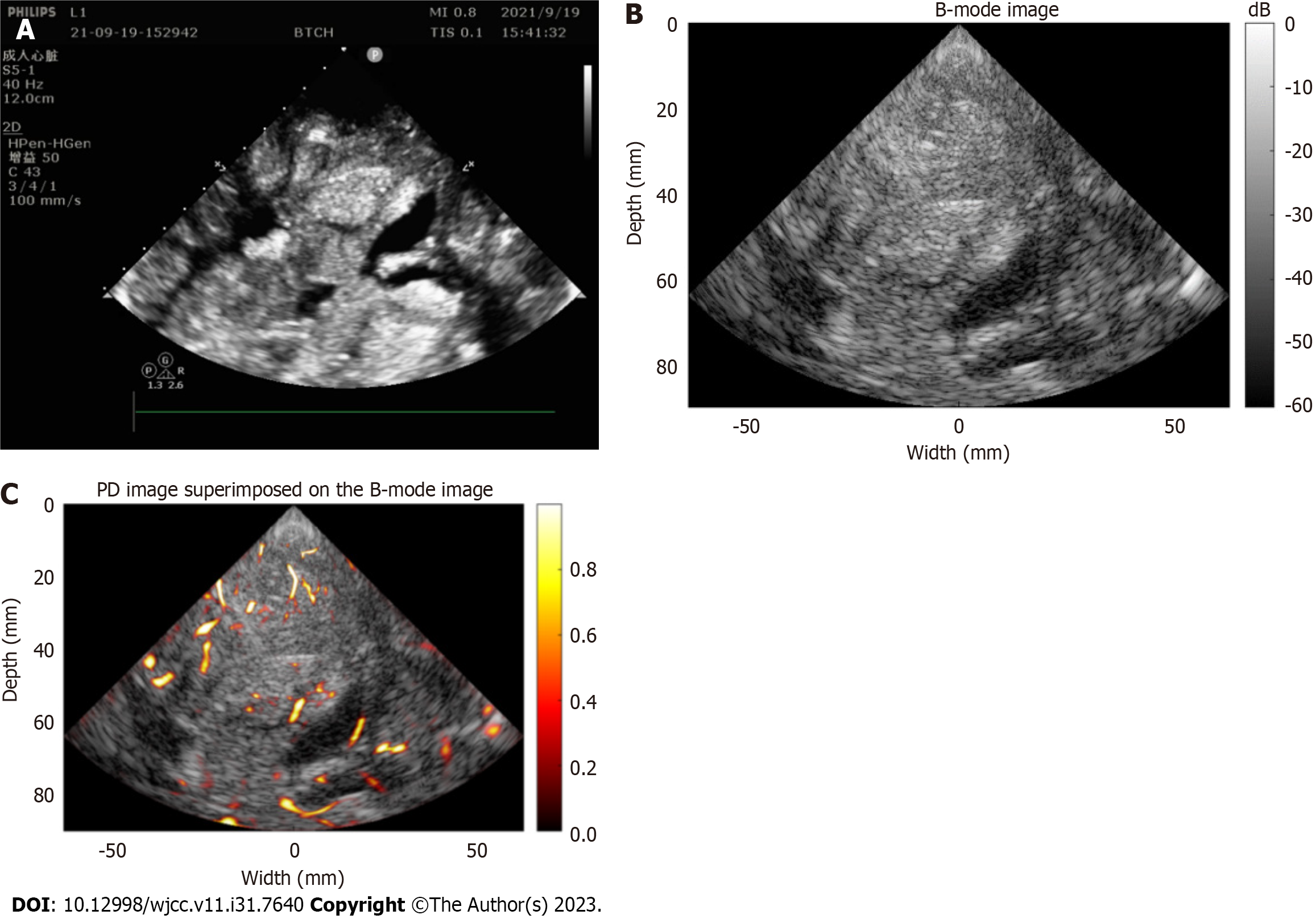
Nonenhanced MRI showed a large space-occupying lesion containing cystic and solid components in her right frontotemporal lobe (Figure 1). A Verasonics Vantage 256 System (Verasonics Inc., Kirkland, WA, United States) equipped with a P4-2v (Verasonics Inc., Kirkland, WA, United States) phased probe was used to acquire 200 frames of ultrasound radio-frequency (RF) data from the decompression window. The center frequency of transmission for imaging of a deep region was 2.72 MHz. The excitation voltage to guarantee safe image acquisition was 13 V. Coherent compounding of diverging waves from 5 angles (-20°, -10°, 0°, 10°, 20°) was used to improve the imaging quality, and the effective frame rate was 1000 Hz. First, the RF channel data were beamformed using the conventional delay-and-sum method. Then, the SVD method was used to reject tissue clutter and extract blood flow signals[4]. Afterward, a Hessian-based vessel enhancement filter was applied to further decrease noise and enhance the visualization of vessels[9]. Finally, the enhanced vessels were superimposed on the B-mode image for a more comprehensive display.
The pathological report showed mesenchymal chondrosarcoma, which is a rare malignant tumor with a poor prognosis.
The patient was taken to the operating room to undergo an emergency craniotomy with tumor resection. Postoperatively, the patient remained critically ill and was admitted to the surgical intensive care unit. Aggressive treatment for ICP was given, including sedatives, antiepileptics, osmotherapy, and steroids.
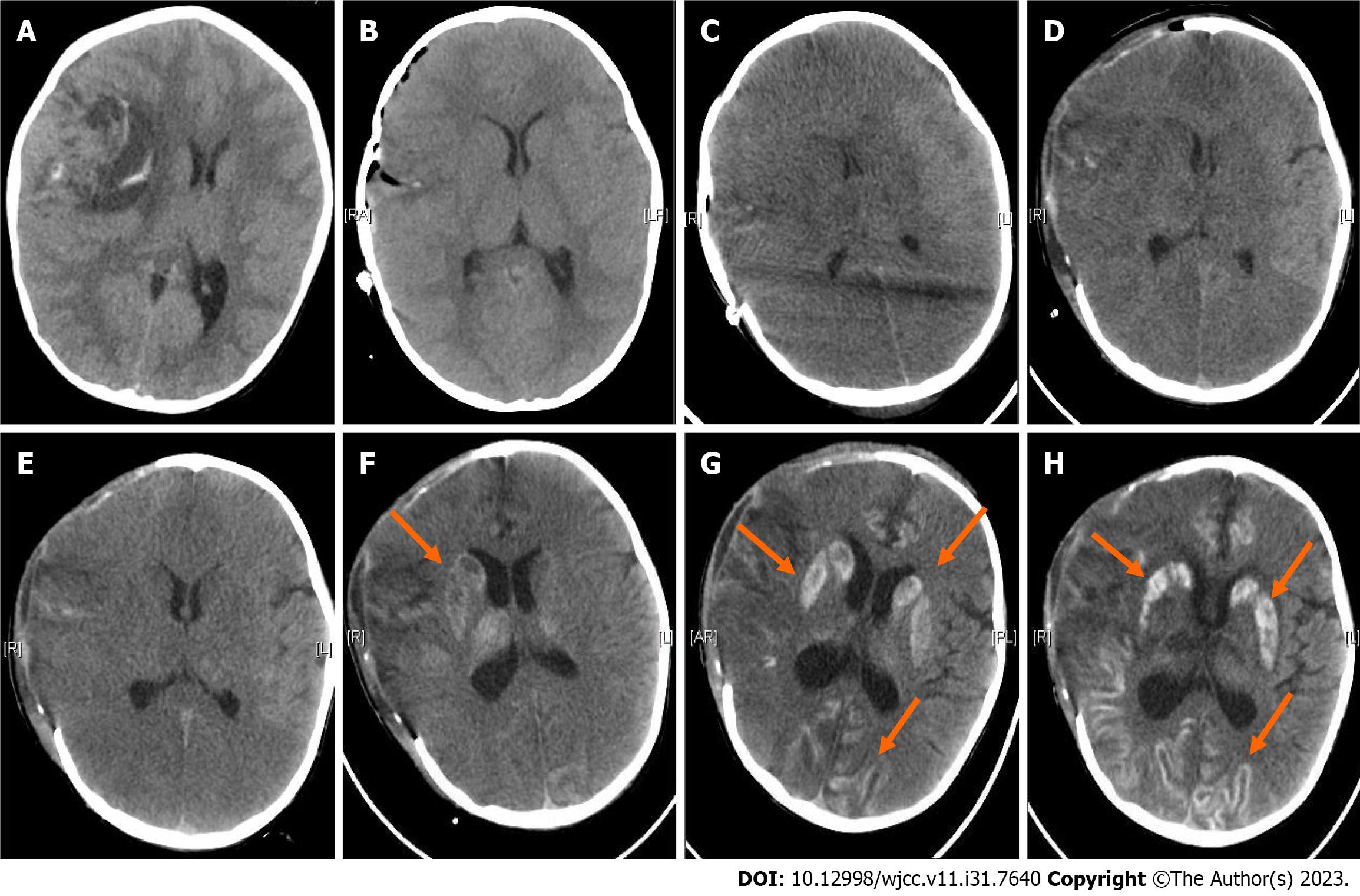
Figure 2 shows the results of preoperative (Figure 2A) and repeated postoperative (Figures 2B-H) CT scans. Six hours after emergency tumor resection, the result of noncontrast CT showed the resection of the large space-occupying lesion as shown in Figure 2B. On the third postoperative day, noncontrast CT showed diffuse brain swelling and low perfusion, and she underwent decompressive craniectomy (Figures 2C and D). Noncontrast CT scans were scheduled on the 6th, 9th, 12th and 15th postoperative days after decompressive craniectomy (Figures 2E-H). Repeated CT imaging showed a gradual decrease in brain edema and a gradual development of hyperdensity in the cerebral cortex, entire putamen, and caudate head. On the 15th postoperative day, we used uPDI to detect the blood flow in the operative area through the decompression window. The B-image showed that both the cerebral cortex and the basal ganglia were hyperechoic, which was highly consistent with the CT images. There were microvessels in the sulci and the subcortical white matter but not in the cortical gray matter or subcortical nuclei, such as the putamen and caudate head (Figure 3). The patient remained in a coma and died of complications 2 mo after the operation.
In this report, we present a case in which uPDI was first used to visualize the microvessels involved in cortical laminar necrosis and luxury perfusion in a pediatric patient who had severe hypoperfusion after brain herniation due to a giant intracranial mesenchymal chondrosarcoma, which is a very rare cancer that mainly affects young people aged 10 to 30 years old.
Nonenhanced repeated postoperative CT scans showed that the density of the cortical gyrus and the bilateral basal ganglia (lenticular nucleus and caudate nucleus) gradually and symmetrically started to increase on the 9th postoperative day, suggesting that it was due to systemic and metabolic mechanisms. The CT imaging features were consistent with cortical layer necrosis (CLN) and luxury perfusion syndrome.
CLN, a special type of cerebral infarction also known as pseudolayer necrosis, can be easily confused with calcification or hemorrhage. Neuropathology defines CLN as focal or diffuse necrosis of one or more cerebral cortex layers. CLN includes glial cells, neurons and blood vessels, and its underlying white matter is relatively or absolutely preserved. Its features on CT are increased cortical gyrus density and a relatively clear boundary[12]. This may be due to extensive anoxia of the cerebral cortex caused by excessive intracranial pressure and protein degradation due to extensive necrosis. The causes that have been reported in the literature are hypoglycemic or hypoxic encephalopathy[13,14], seizures[12,15], and migraine cerebral infarction[16,17].
Luxury perfusion syndrome is defined as excessive blood flow that flushes into brain tissue that has low perfusion and severely impaired autoregulation of reperfused cerebral vessels, so the increased blood flow is nonnutritive and does not actually help to repair damaged brain tissue[18]. Luxury perfusion was observed in the second and third weeks after stroke in both humans who suffered a subacute stroke[19] and in experimental models used in ischemia research[20].
In clinical practice, CT, MRI, and perfusion imaging are often used to evaluate anatomical and perfusion changes. However, there are few fast and noninvasive imaging methods to evaluate changes in cerebral microvascular structure and hemodynamics. Recently, the uPDI technique was developed to visualize microvessels that are undetectable using conventional ultrasound Doppler imaging in experimental animal models. To our knowledge, there is no report on the application of uPDI to detect the intracranial changes in microvessels following severe hypoperfusion after brain herniation, as in this case.
Highly sensitive uPDI enables us to observe the blood supplies of cerebral microvessels. Normally, the greatest density of microvessels is found in the cerebral cortex and basal ganglia[21]. While there were very few blood flow signals detected in the cortex and basal ganglia in this case, most of these vessels were located in the sulcus and internal capsule, which is consistent with CLN and luxury perfusion.
Due to the strong attenuation and aberration effect of ultrasound caused by the skull, the uPDI technique can only be used intraoperatively or for patients who have a decompression window to image the cerebral microvessels, which is a nonnegligible limitation. Notably, uPDI has the potential to be used for adult patients with malignant middle cerebral artery infarction (a devastating type of ischemic stroke) intraoperatively or with a decompression window[22].
uPDI images can accurately identify anatomical and hemodynamic characteristics. It has the advantages of being noninvasive, real-time, convenient, safe, and radiation-free; furthermore, microvessels can be observed clearly. It has the potential to serve as an alternative to postoperative CT scans for such critically ill patients to minimize the possible risks of repeated radiation exposure and patient transfer. It also has the potential value to reveal the sequential changes and mechanisms of brain injury in neurocritical patients in future clinical research.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Arboix A, Spain; Ferraioli G, Italy S-Editor: Wang JJ L-Editor: A P-Editor: Zhao S
| 1. | Mathews L, Barwise J. Refractory Intracranial Hypertension. In: McEvoy MD, Furse CM, McEvoy MD, Furse CM. Advanced Perioperative Crisis Management. United States: Oxford University Press, 2017: 492-498. |
| 2. | Bor-Seng-Shu E, Figueiredo EG, Fonoff ET, Fujimoto Y, Panerai RB, Teixeira MJ. Decompressive craniectomy and head injury: brain morphometry, ICP, cerebral hemodynamics, cerebral microvascular reactivity, and neurochemistry. Neurosurg Rev. 2013;36:361-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 3. | Montaldo G, Tanter M, Bercoff J, Benech N, Fink M. Coherent plane-wave compounding for very high frame rate ultrasonography and transient elastography. IEEE Trans Ultrason Ferroelectr Freq Control. 2009;56:489-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1184] [Cited by in RCA: 865] [Article Influence: 54.1] [Reference Citation Analysis (0)] |
| 4. | Demené C, Deffieux T, Pernot M, Osmanski BF, Biran V, Gennisson JL, Sieu LA, Bergel A, Franqui S, Correas JM, Cohen I, Baud O, Tanter M. Spatiotemporal Clutter Filtering of Ultrafast Ultrasound Data Highly Increases Doppler and fUltrasound Sensitivity. IEEE Trans Med Imaging. 2015;34:2271-2285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 515] [Cited by in RCA: 498] [Article Influence: 49.8] [Reference Citation Analysis (0)] |
| 5. | Macé E, Montaldo G, Cohen I, Baulac M, Fink M, Tanter M. Functional ultrasound imaging of the brain. Nat Methods. 2011;8:662-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 402] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 6. | Huang L, Zhang J, Wei X, Jing L, He Q, Xie X, Wang G, Luo J. Improved Ultrafast Power Doppler Imaging by Using Spatiotemporal Non-Local Means Filtering. IEEE Trans Ultrason Ferroelectr Freq Control. 2022;69:1610-1624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 7. | Huang L, Wang Y, Wang R, Wei X, He Q, Zheng C, Peng H, Luo J. High-Quality Ultrafast Power Doppler Imaging Based on Spatial Angular Coherence Factor. IEEE Trans Ultrason Ferroelectr Freq Control. 2023;70:378-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 8. | Qiu L, Zhang J, Yang Y, Zhang H, Lee FF, He Q, Huang C, Huang L, Qian L, Luo J. In vivo assessment of hypertensive nephrosclerosis using ultrasound localization microscopy. Med Phys. 2022;49:2295-2308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 9. | Huang L, Hao Y, Jing L, Wang Y, He Q, Wang G, Luo J. Contrast-free Ultrasound Microvascular Imaging for Intraoperative Detection of Human Spinal Cord Tumor: An In vivo Feasibility Study. 2021 IEEE IUS. 2021;. [DOI] [Full Text] |
| 10. | Huang L, Liu Y, Wei X, Hou X, He Q, Tong X, Luo J. Ultrafast Power Doppler Imaging of Human Newborn with Periventricular Venous Infarction: A Pilot Study. 2022 IEEE IUS. 2022;. [DOI] [Full Text] |
| 11. | Zhang H, Huang L, Yang Y, Qiu L, He Q, Liu J, Qian L, Luo J. Evaluation of Early Diabetic Kidney Disease Using Ultrasound Localization Microscopy: A Feasibility Study. J Ultrasound Med. 2023;42:2277-2292. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 12. | Donaire A, Carreno M, Gómez B, Fossas P, Bargalló N, Agudo R, Falip M, Setoaín X, Boget T, Raspall T, Obach V, Rumiá J. Cortical laminar necrosis related to prolonged focal status epilepticus. J Neurol Neurosurg Psychiatry. 2006;77:104-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 62] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 13. | Khardenavis V, Karthik DK, Kulkarni S, Deshpande A. Cortical laminar necrosis in a case of migrainous cerebral infarction. BMJ Case Rep. 2018;2018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Rajasekharan C, Jithesh B, Renjith SW. Cortical laminar necrosis due to hypoglycaemic encephalopathy:-images in medicine. BMJ Case Rep. 2013;2013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 15. | La Rocca G, Della Pepa GM, Gaudino S, Sabatino G, Zoia C, Raffa G, Altieri R, Mazzucchi E. Cortical Laminar Necrosis as a Result of Status Epilepticus After Resection of Parafalcal Meningioma. Surg Technol Int. 2020;36:159-177. [PubMed] |
| 16. | Morais R, Sobral F, Cunha G, Brito O, Santana I. Advanced MRI study of migrainous infarction presenting as cortical laminar necrosis - Case report and literature review. Clin Neurol Neurosurg. 2018;167:82-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Arboix A, González-Peris S, Grivé E, Sánchez MJ, Comes E. Cortical laminar necrosis related to migrainous cerebral infarction. World J Clin Cases. 2013;1:256-259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Lassen NA. The luxury-perfusion syndrome and its possible relation to acute metabolic acidosis localised within the brain. Lancet. 1966;2:1113-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 640] [Cited by in RCA: 568] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 19. | Raynaud C, Rancurel G, Tzourio N, Soucy JP, Baron JC, Pappata S, Cambon H, Mazoyer B, Lassen NA, Cabanis E. SPECT analysis of recent cerebral infarction. Stroke. 1989;20:192-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 26] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Wegener S, Artmann J, Luft AR, Buxton RB, Weller M, Wong EC. The time of maximum post-ischemic hyperperfusion indicates infarct growth following transient experimental ischemia. PLoS One. 2013;8:e65322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 21. | Hsieh BY, Kao YJ, Zhou N, Lin YP, Mei YY, Chu SY, Wu DC. Vascular responses of penetrating vessels during cortical spreading depolarization with ultrasound dynamic ultrafast Doppler imaging. Front Neurosci. 2022;16:1015843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 22. | Arboix A, García-Eroles L, Oliveres M, Comes E, Sánchez MJ, Massons J. Malignant middle cerebral artery infarction: a clinical study of 32 patients. Rev Invest Clin. 2015;67:64-70. [PubMed] |