Published online Nov 6, 2023. doi: 10.12998/wjcc.v11.i31.7593
Peer-review started: August 23, 2023
First decision: September 13, 2023
Revised: October 9, 2023
Accepted: October 23, 2023
Article in press: October 23, 2023
Published online: November 6, 2023
Processing time: 74 Days and 23.9 Hours
Although Liu-Wei-Bu-Qi capsule (LBC) inhibits tumor progression by improving the physical condition and immunity of patients with lung cancer (LC), its exact mechanism of action is unknown.
To through compound multi-dimensional network of chemical ingredient-target-disease-target- protein-protein interaction (PPI) network, the principle of action of Chinese medicine prescription was explained from molecular level.
Network pharmacology and molecular docking simulations were used to analyze the relationship among the main components, targets, and signaling pathways of LBC in treatment of LC.
From the analysis, 360 LBC active ingredient-related targets and 908 LC-related targets were identified. PPI network analysis of the LBC and LC overlapping targets identified 16 hub genes. Kyoto Encyclopedia of Genes and Genomes analysis suggested that LBC can target the vascular endothelial growth factor signaling pathway, Toll-like receptor signaling pathway, prolactin signaling pathway, FoxO signaling pathway, PI3K-Akt signaling pathway and HIF-1 signaling pathway in the treatment of LC. Molecular docking simulations showed that quercetin had the best affinity for MAPK3, suggesting that quercetin in LBC may play an important role in the treatment of LC.
The results showed that the active ingredients in LBC can play a crucial role in the treatment of LC by regulating multiple signaling pathways. These results provide insights into further studies on the mechanism of action of LBC in the treatment of LC.
Core Tip: The network pharmacological and molecular docking study of Liu-Wei-Bu-Qi capsule (LBC) on lung cancer revealed that LBC’s active ingredients target multiple signaling pathways, including the endothelial growth factor, toll-like receptor, prolactin, FoxO, PI3K-Akt, and HIF-1 pathways. Quercetin, found in LBC, showed promising affinity for MAPK3, highlighting its potential role in lung cancer treatment. These findings provide valuable insights into the mechanisms of action of LBC and pave the way for further investigations into its therapeutic effects on lung cancer.
- Citation: Yang Q, Li LY. Network pharmacological and molecular docking study of the effect of Liu-Wei-Bu-Qi capsule on lung cancer. World J Clin Cases 2023; 11(31): 7593-7609
- URL: https://www.wjgnet.com/2307-8960/full/v11/i31/7593.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i31.7593
Lung cancer (LC) was the leading cause of cancer-related deaths and had the second highest incidence rate worldwide in 2020[1]. Since most of the patients are usually diagnosed when they are in the middle or advanced stage of disease, surgery is usually not an option, which necessitates the use of chemotherapy or radiotherapy. However, conventional chemotherapy and radiotherapy are associated with significant side effects and suppression of the immune system, leading to premature termination of treatment[2].
Traditional Chinese medicine (TCM) has high efficacy, is associated with mild and few side effects and is being explored as a safe and effective adjuvant therapy for LC. Liu-Wei-Bu-Qi capsule (LBC) is a type of TCM that consists of Panax ginseng C. A. Mey (Shengshaishen, SSS), Hedysarum Multijugum Maxim (Huangqi, HQ), Alpiniae Oxyphyliae Fructus (Yizhiren, YZR), Polygonati Odorati Rhizoma (Yuzhu, YZ), Cinnanmomi Cortex (Rougui, RG) and Citrus reticulata (Chenpi, CP). LBC can reduce radiotherapy and chemotherapy-induced side effects such as anorexia and hair loss, and improve the immune response of tumor patients[3]. Herbal compounds have been shown to have anti-tumor effects, including Ginsenoside Rh2, a ginseng extract that converts M2 macrophage phenotype to M1 phenotype in the microenvironment and prevents migration of LC cells[4]. Astragalus polysaccharide, the main active component of HQ, inhibits the proliferation of human LC cells A549 and NCI-H358 by inhibiting NF-κB signaling pathway[5].
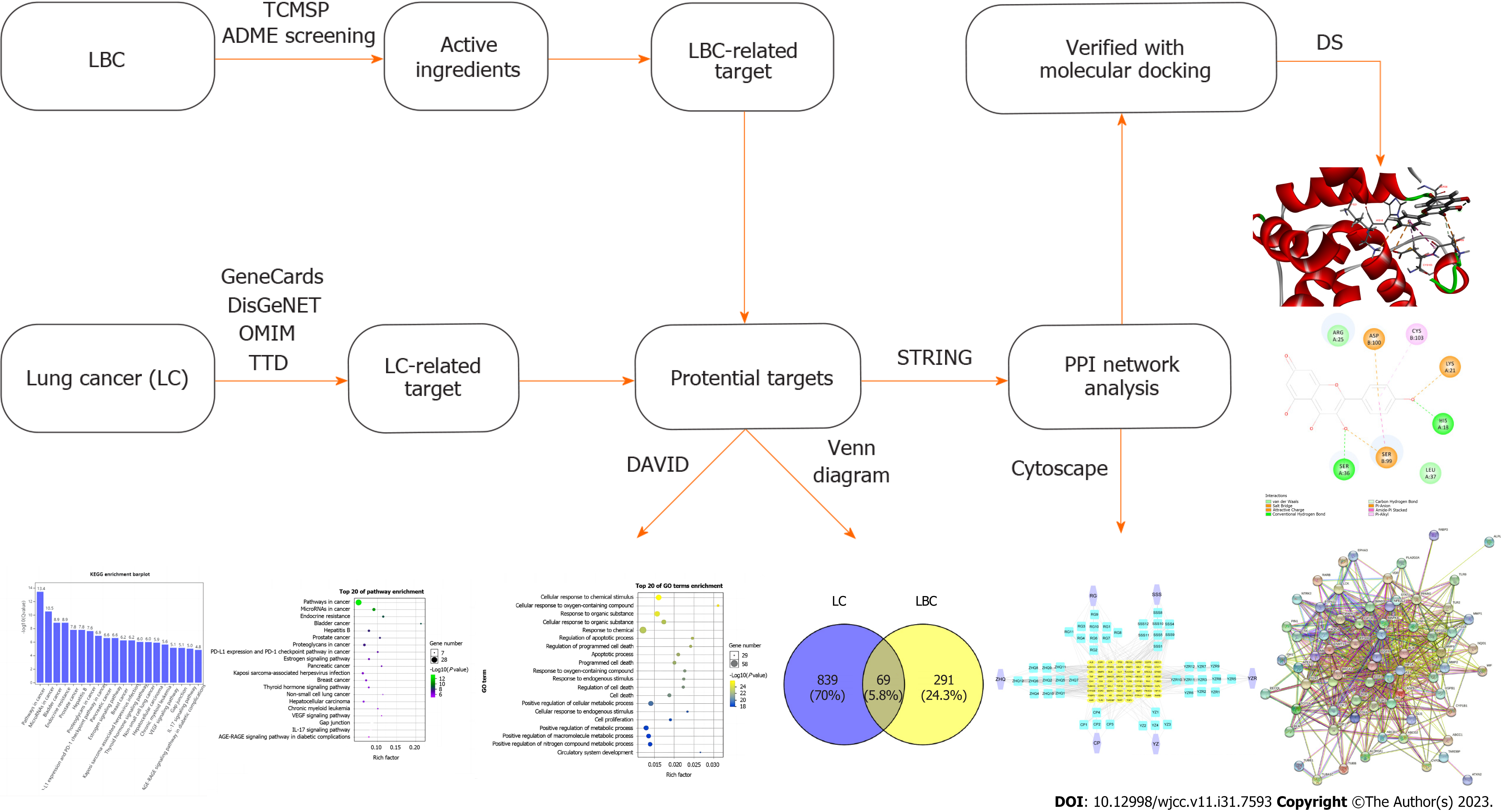
Although many herbal compounds have been found to have anti-tumor effects, most studies have focused on the anticancer effects of individual components rather than herbal complexes. The mixing of herbal compounds to achieve maximum efficacy can be aided by computer technologies such as network pharmacology and molecular docking simulation, which can be used to accurately screen the active ingredients and targets of Chinese herbs and predict their mechanisms in treatment of different diseases. Network pharmacology is the use of ‘multi-dimensional-target –pathway’ network diagrams to identify genes and proteins interactions and thus predict potential mechanism of drug action in the treatment of various diseases[6]. In this study, we used network pharmacology and molecular docking simulation to predict the potential mechanism of LBC action in the treatment of LC. Findings from this study will provide insights into further studies involving in vitro and in vivo models. The workflow diagram is shown in Figure 1.
A search of TCMSP and TCMID databases was done to identify the active ingredients of LBC. The information on potential targets of the active ingredients was imported into UniProt for searching and normalization, and the structure data files were downloaded in Pubchem after removing duplicates. The structures of the active ingredients were imported into Discovery Studio 2017R2 to predict the ADMET parameters of the LBC. Compounds with ADMET_Ab
| Database | Address |
| TCMSP | http://www.tcmspw.com/tcmsp.php |
| TCMID | http://www.megabionet.org/tcmid/ |
| UniProt | https://www.uniprot.org/ |
| PubChem | https://pubchem.ncbi.nlmnih.gov |
| SwissTargetPrediction | http://www.swisstargetprediction.ch/ |
| Genecards | https://www.genecards.org |
| TTD | http://bidd.nus.edu.sg/group/ttd/ttd.asp |
| OMIM | https://omim.org/ |
| DisGeNET | https://www.disgenet.org/home/ |
| Venn platform | https://bioinfogp.cnb.csic.es/tools/venny/index.html |
| String | https://string-db.org/ |
| DAVID 6.8 | http://david.ncifcrf.gov |
| Omicstudio | https://www.omicstudio.cn/tool |
| PDB | https://www.rcsb.org |
Lung cancer-related targets were identified using the search term “lung cancer” to search the GeneCards[7], DisGeNet[8], TTD[9] and OMIM databases[10]. Overlapping targets from the four databases were selected as LC-related targets and were used for further studies. Active ingredients-related and therapeutic LC-related targets were imported into Venn 2.1.0 platform and a Venn diagram was plotted to identify overlapping targets.
Protein-protein interaction (PPI) networks were constructed by uploading the overlapping targets to STRING database and setting the parameters as ‘human’ and ‘confidence ≥ 0.400’. The PPI network information was imported into Cytoscape 3.8.2 software based on the topological algorithm of cytoHubba plugin to identify hub genes and perform cluster analysis to find gene clusters and derive sub network[11].
We used the gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis pipeline (P < 0.05) in the DAVID 6.8 database[12] to predict the main biological processes and signaling pathways associated with LBC-based treatment of LC based on the hub genes identified. The results were visualized using Cytoscape software.
Compound information, all key targets and potential signaling pathways were imported into the Cytoscape 3.8.2 to create the ‘ingredient-target-pathway’ network and analyze the multicomponent-target-pathway mechanism of LBC-based treatment of LC.
We collected the small molecule compounds of the main active ingredients of Chinese medicine in mol2 format, downloaded the crystal structures of structurally complete, high-resolution, ligand-bearing targets from the PDB protein database, and imported the data into Discovery Studio 2017 R2 to perform molecular docking. The binding energies and hydrogen bond numbers were calculated[13].
We identified 66 active ingredients in LBC, with the main structures being phenylpropanoids, flavonoids, alkaloids and terpenoids. We then used Discovery Studio 2017R2 software to find and screen 57 active ingredients of LBC, including 14 of SSS, 12 of ZHQ, 12 of YZR, 4 of YZ, 11 of RG, and 4 of CP (Table 2). We further obtained 55 active ingredient in the target database (P > 0.9), and 360 potential targets were identified after removing duplicate values.
| No. | Compound | PubChem CID | ADMET-SOLUBLITY-level | ADMET-absorption-level | Herb |
| SSS1 | Arachidonate | 5312542 | 2 | 1 | SSS |
| SSS2 | Celabenzine | 442847 | 3 | 0 | SSS |
| SSS3 | Deoxyharringtonine | 285342 | 2 | 0 | SSS |
| SSS4 | Dianthramine | 441562 | 3 | 1 | SSS |
| SSS5 | Frutinone A | 441965 | 2 | 0 | SSS |
| SSS6 | Ginsenoside rh2 | 119307 | 2 | 2 | SSS |
| SSS7 | Girinimbine | 96943 | 1 | 0 | SSS |
| SSS8 | Gomisin B | 6438572 | 1 | 1 | SSS |
| SSS9 | Kaempferol | 5280863 | 3 | 0 | SSS |
| SSS10 | Maackiain | 91510 | 2 | 0 | SSS |
| SSS11 | Malkangunin | 90473155 | 3 | 0 | SSS |
| SSS12 | Panaxadiol | 73498 | 1 | 0 | SSS |
| SSS13 | Protopine | 4970 | 2 | 0 | SSS |
| SSS14 | Suchilactone | 132350840 | 2 | 0 | SSS |
| ZHQ1 | 3,9,10-Trimethoxypterocarpan | 15689655 | 2 | 0 | ZHQ |
| ZHQ2 | 7-O-methylisomucronulatol | 15689652 | 2 | 0 | ZHQ |
| ZHQ3 | Antibiotic FA 2097 | 102059896 | 3 | 0 | ZHQ |
| ZHQ4 | Betulinic acid | 64971 | 1 | 2 | ZHQ |
| ZHQ5 | Calycosin | 5280448 | 3 | 0 | ZHQ |
| ZHQ6 | Formononetin | 5280378 | 3 | 0 | ZHQ |
| ZHQ7 | Hederagenin | 73299 | 1 | 1 | ZHQ |
| ZHQ8 | Isoflavanone | 160767 | 2 | 0 | ZHQ |
| ZHQ9 | Isorhamnetin | 5281654 | 3 | 0 | ZHQ |
| ZHQ10 | Kaempferol | 5280863 | 3 | 0 | ZHQ |
| ZHQ11 | Kumatakenin | 5318869 | 3 | 0 | ZHQ |
| ZHQ12 | Quercetin | 5280343 | 3 | 1 | ZHQ |
| YZR1 | (+)-delta-cadinene | 441005 | 2 | 1 | YZR |
| YZR2 | Caryophyllene oxide | 1742210 | 2 | 0 | YZR |
| YZR3 | Chrysin | 5281607 | 3 | 0 | YZR |
| YZR4 | Daucosterol | 5742590 | 2 | 2 | YZR |
| YZR5 | Globulol | 12304985 | 2 | 0 | YZR |
| YZR6 | Isocyperol | 14076604 | 2 | 0 | YZR |
| YZR7 | Linolenic acid | 5280934 | 2 | 1 | YZR |
| YZR8 | Nootkatol | 182645 | 2 | 0 | YZR |
| YZR9 | O-cymene | 10703 | 3 | 0 | YZR |
| YZR10 | Oleic acid | 445639 | 2 | 2 | YZR |
| YZR11 | Protocatechuic acid | 72 | 4 | 0 | YZR |
| YZR12 | Valencene | 9855795 | 2 | 1 | YZR |
| YZ1 | 4',5,7-trihydroxy-6,8-dimethyl-homoisoflavanone | 46886731 | 2 | 0 | YZ |
| YZ2 | 4',5,7-trihydroxy-6-methyl-8-methoxy-homoisoflavanone | 46886730 | 3 | 0 | YZ |
| YZ3 | N-cis-Feruloyltyramine | 6440659 | 3 | 0 | YZ |
| YZ4 | P-Coumaroyltyramine | 5372945 | 3 | 0 | YZ |
| RG1 | (-)-alpha-cedrene | 6431015 | 2 | 0 | RG |
| RG2 | (-)-Caryophyllene oxide | 1742210 | 2 | 0 | RG |
| RG3 | ()-Sativene | 11275742 | 2 | 0 | RG |
| RG4 | (+)-Aromadendrene | 11095734 | 2 | 0 | RG |
| RG5 | Beta-Cubebene | 93081 | 2 | 0 | RG |
| RG6 | Copaene | 19725 | 2 | 0 | RG |
| RG7 | Diisobutyl phthalate | 6782 | 2 | 0 | RG |
| RG8 | Junipene | 1796220 | 2 | 0 | RG |
| RG9 | Ledene | 10910653 | 2 | 0 | RG |
| RG10 | Linoleic acid | 5280450 | 2 | 1 | RG |
| RG11 | Oleic acid | 445639 | 2 | 2 | RG |
| CP1 | 5,7-Dihydroxy-2-(3-hydroxy-4-methoxyphenyl)chroman-4-one | 676152 | 3 | 0 | CP |
| CP2 | Citromitin | 12303287 | 2 | 0 | CP |
| CP3 | Naringetol | 932 | 3 | 0 | CP |
| CP4 | Nobiletin | 72344 | 2 | 0 | CP |
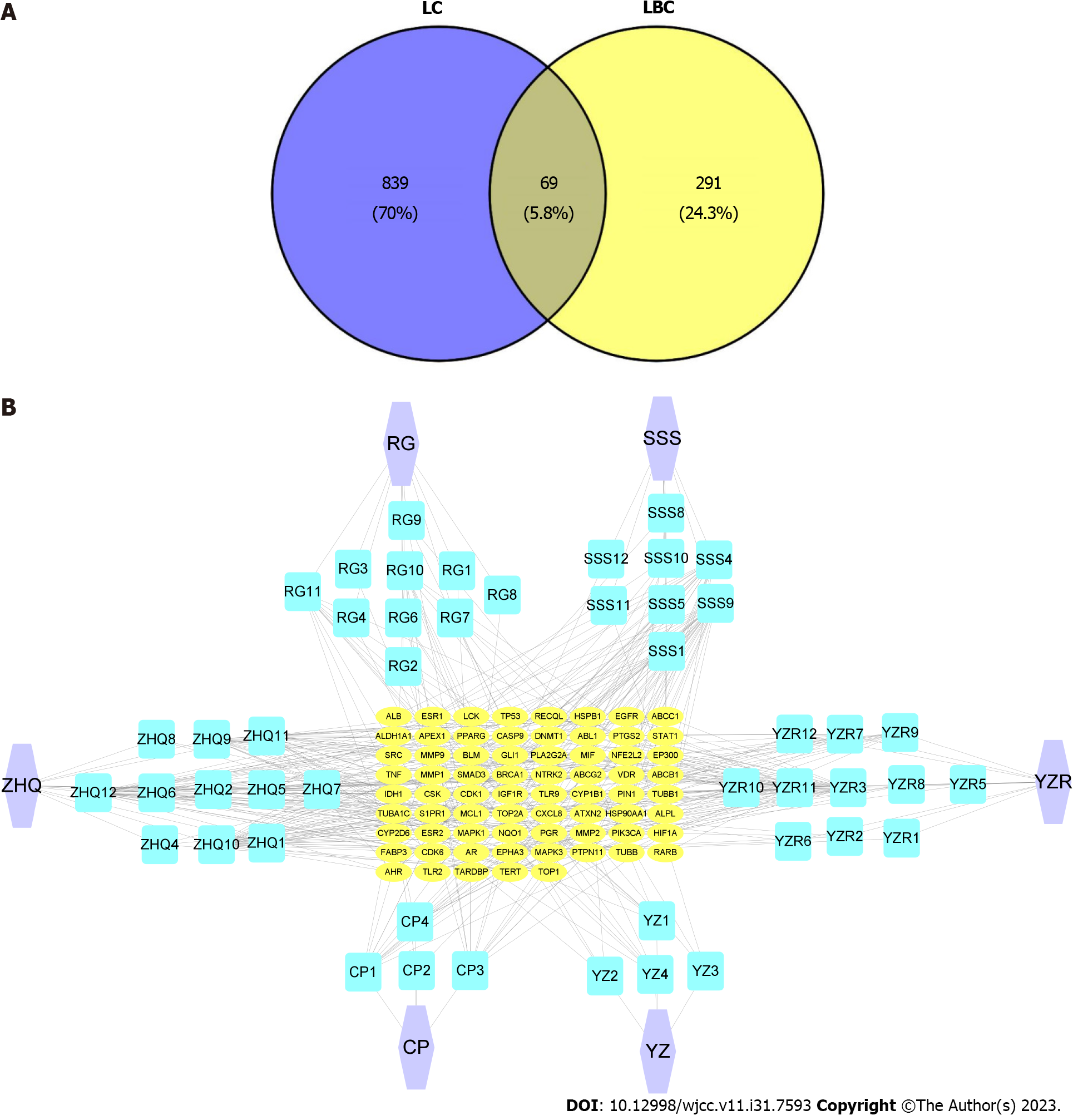
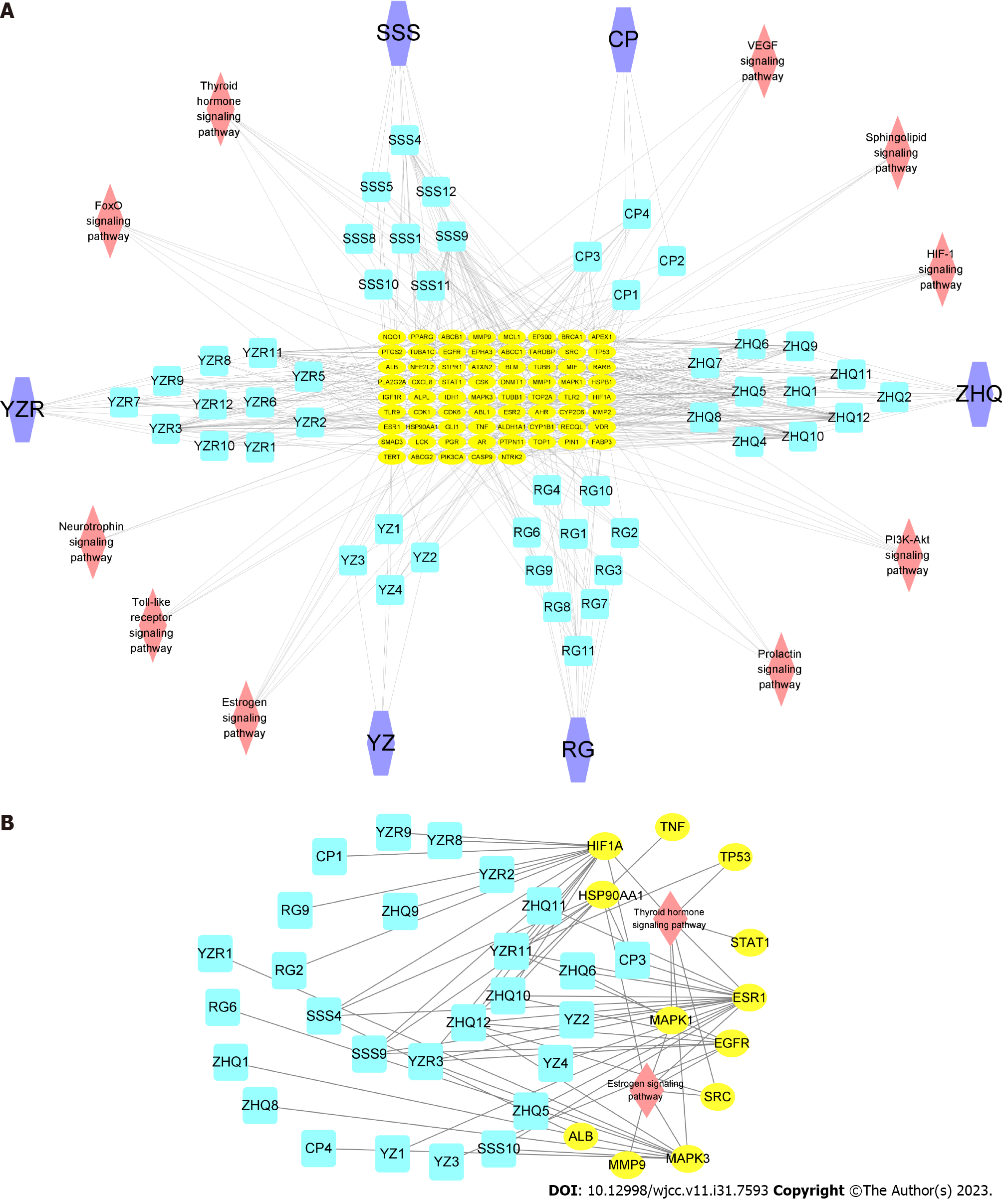
Disease-related targets were selected from the GeneCards DisGeNET, TTD, and OMIM databases using the search term ‘lung cancer’, with 908 targets being identified after deleting duplicate values. Venn diagram analysis showed that there was an overlap of 69 targets between active ingredient-related targets and LC-related targets (Figure 2A). A ‘Herbs-Ingredients-Targets’ interaction network was then constructed to illustrate the relationship among herbs, compounds and targets (Figure 2B).
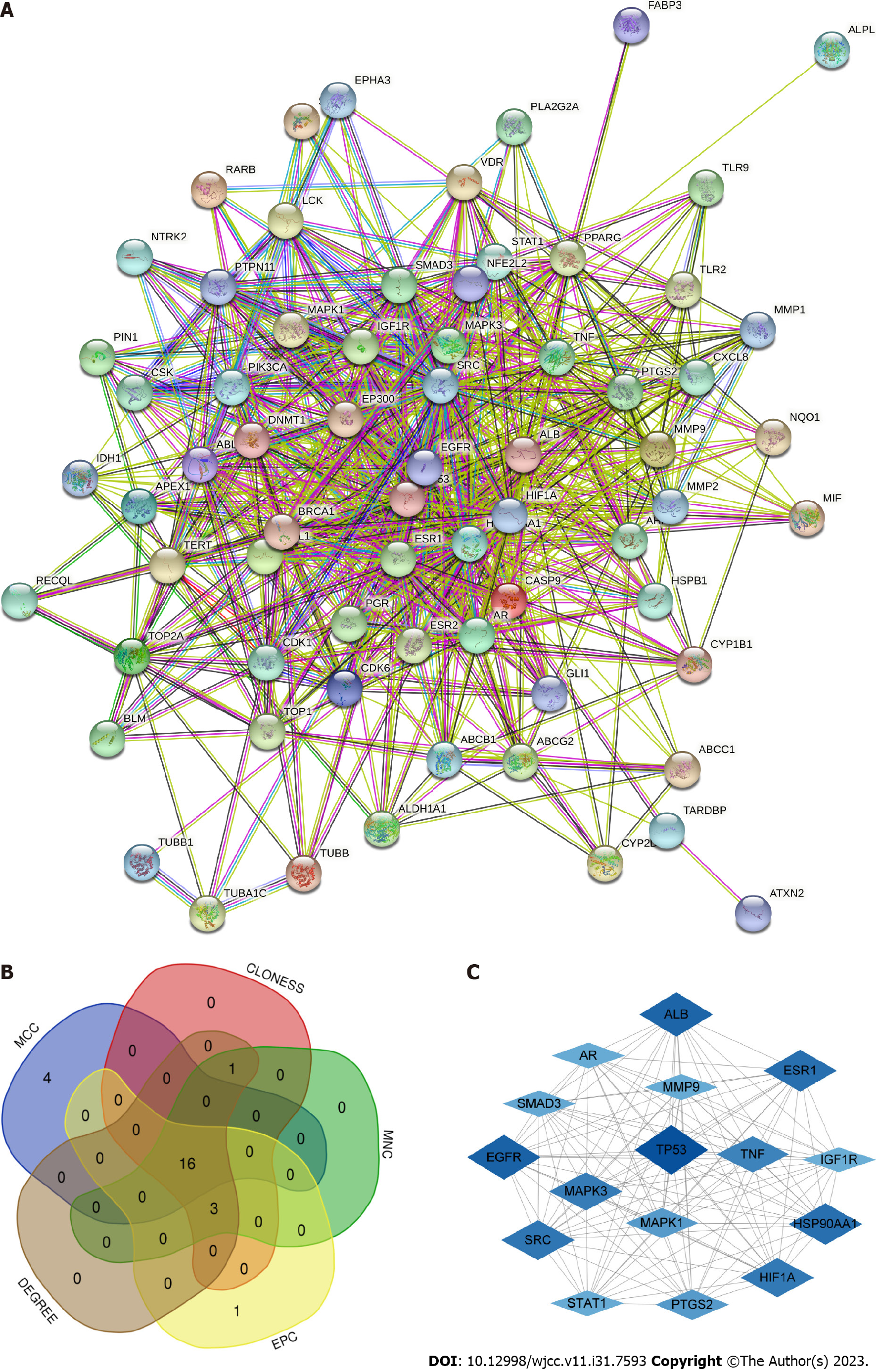
The PPI network showed a total of 69 nodes (target proteins) and 719 edges (protein interactions). The darker the red color of the target indicates the more targets that can effectively interact with the target among the predicted LC-related targets (Figure 3A). Based on the five parameters (MNC, DEGREE, MCC, CLONESS, EPC), top 20 targets were obtainedand the 16 targets that overlapped from the five parameters were considered to be hub targets (Figure 3B). The PPI network of 16 hub targets was plotted using Cytoscape, with a darker blue color indicating a larger degree value (Figure 3C, Table 3).
| Name | Closeness | Betweenness | Degree |
| TP53 | 0.839506 | 0.095155 | 56 |
| EGFR | 0.790698 | 0.051782 | 51 |
| ALB | 0.781609 | 0.077526 | 50 |
| ESR1 | 0.747253 | 0.054759 | 47 |
| HSP90AA1 | 0.764045 | 0.074274 | 47 |
| SRC | 0.73913 | 0.035499 | 45 |
| HIF1A | 0.731183 | 0.023561 | 44 |
| MAPK3 | 0.723404 | 0.033332 | 43 |
| TNF | 0.708333 | 0.027768 | 41 |
| MAPK1 | 0.660194 | 0.018393 | 34 |
| PTGS2 | 0.660194 | 0.013252 | 34 |
| SMAD3 | 0.635514 | 0.007272 | 30 |
| AR | 0.618182 | 0.005023 | 29 |
| STAT1 | 0.623853 | 0.00514 | 29 |
| MMP9 | 0.62963 | 0.006427 | 29 |
| IGF1R | 0.623853 | 0.00413 | 28 |
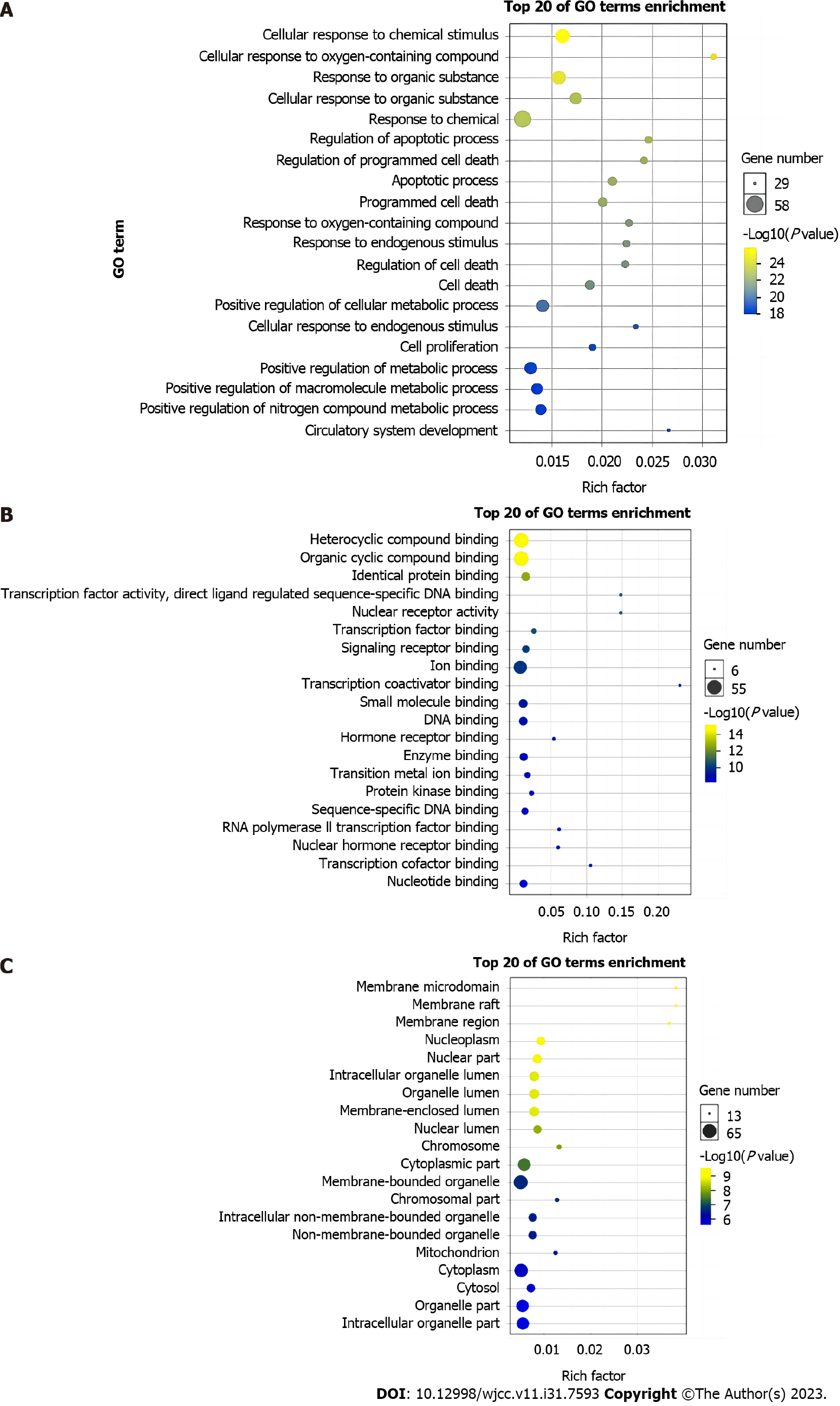
GO enrichment analysis was carried out by importing 69 targets into DAVID 6.8 database. The analysis identified 259 biological processes (BP), 78 molecular functions (MF) and 38 cellular components (CC) that were associated with the targets. The top 20 BP, CC, and MF terms were selected for visualization according to the number of genes, with significance set at P < 0.5 (Figure 4). The main BP terms associated with LBC-based treatment of LC were cellular response to chemical stimulus, cellular response to oxygen-containing compound, response to organic substance, cellular response to organic substance, and response to chemical. The main MF terms included compound binding of heterocyclic, organic cyclic and identical, transcription factor activity, and direct ligand-regulated sequence-specific DNA binding. The main CC terms were membrane raft, microdomain, and region, nucleoplasm and nuclear part.
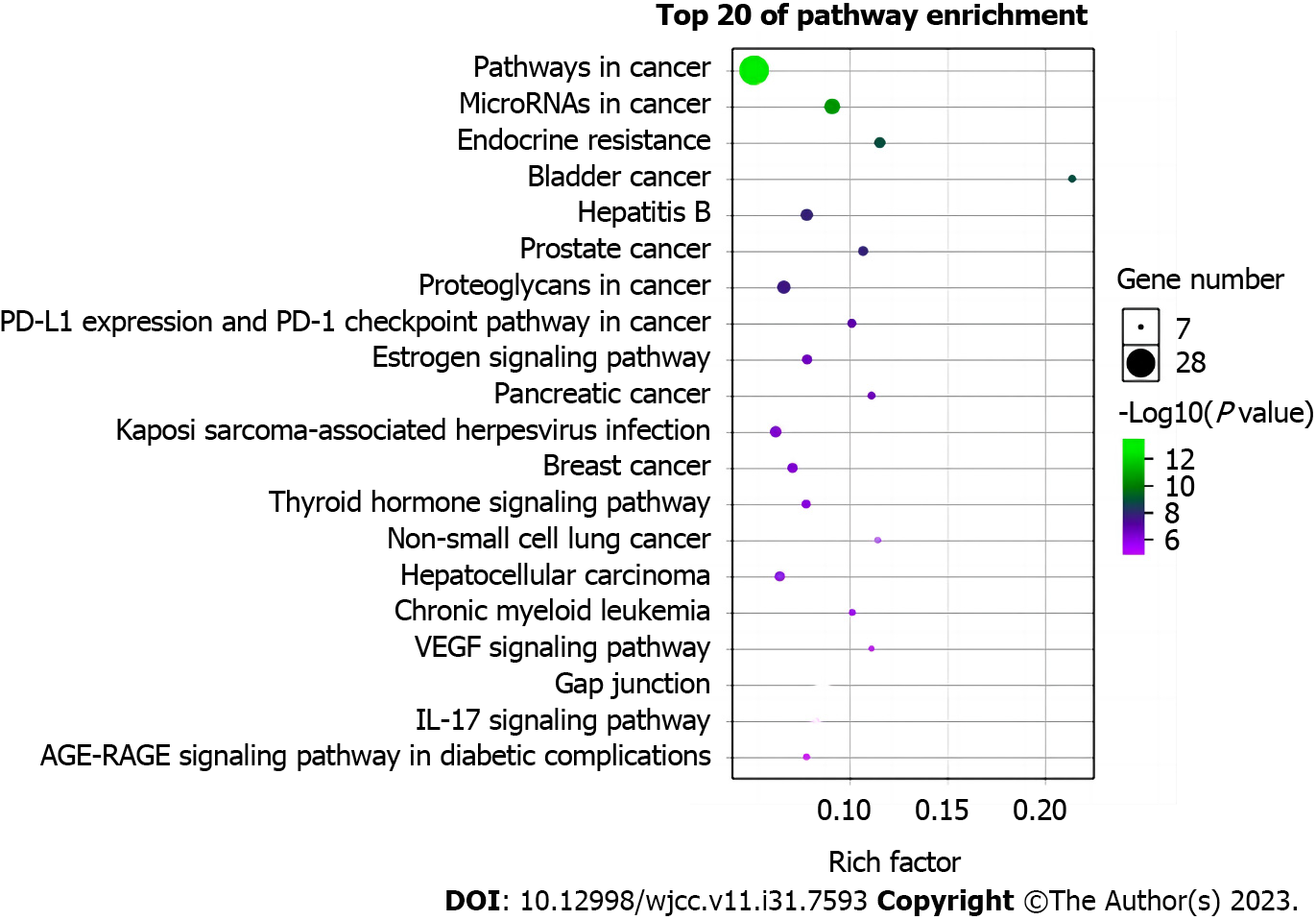
KEGG enrichment analysis was performed for potential targets through DAVID6.8 database. The top 10 pathways were estrogen signaling pathway, thyroid hormone signaling pathway, VEGF signaling pathway, toll-like receptor signaling pathway, prolactin signaling pathway, FoxO signaling pathway, PI3K-Akt signaling pathway, HIF-1 signaling pathway, sphingolipid signaling pathway, neurotrophin signaling pathway (Figure 5, Table 4). Suggesting that LBC capsule ingredients played a role in the treatment of lung cancer through the above pathways.
| Pathway | Count | P value |
| Estrogen signaling pathway | 11 | 1.09E-08 |
| Thyroid hormone signaling pathway | 10 | 5.77E-08 |
| VEGF signaling pathway | 7 | 5.6E-07 |
| Toll-like receptor signaling pathway | 8 | 1.92E-06 |
| Prolactin signaling pathway | 7 | 2.44E-06 |
| FoxO signaling pathway | 8 | 1.27E-05 |
| PI3K-Akt signaling pathway | 13 | 3.76E-05 |
| HIF-1 signaling pathway | 7 | 4.45E-05 |
| Sphingolipid signaling pathway | 7 | 5.48E-05 |
| Neurotrophin signaling pathway | 7 | 5.77E-05 |
The information on each individual herb including active compounds, key targets and pathways were imported into Cytoscape-3.8.2 to build the ‘herbs-compounds-targets-pathways’ network (Figure 6A). Analysis using cytoHubba showed that estrogen and thyroid hormone signaling pathways are closely associated with LBC-based treatment of LC. The network of these two pathways and related targets and active components was constructed (Figure 6B).
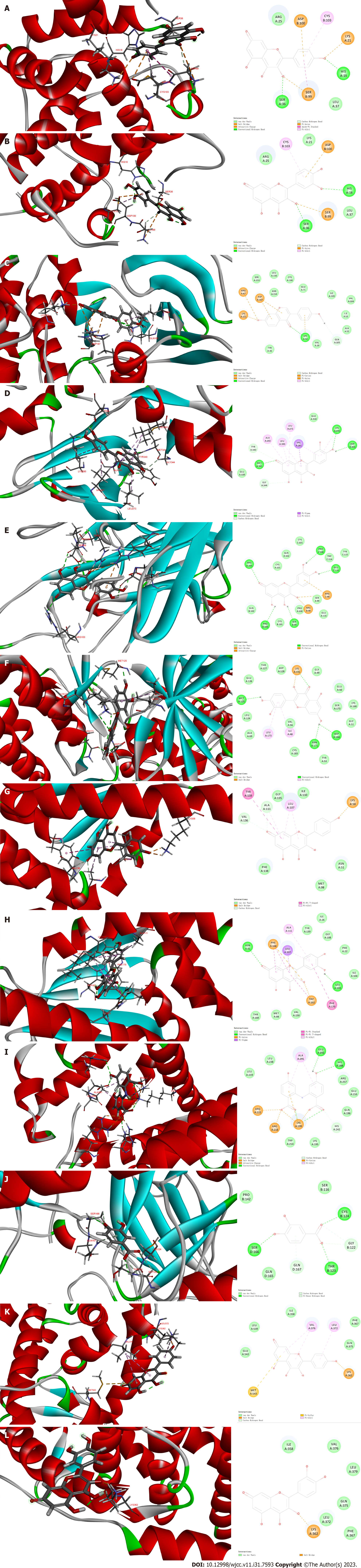
Molecular docking simulations were carried out between the ingredients, protocatechuic acid, Dianthramine, quercetin, kaempferol and quercetin, and the hub targets. The results showed that the binding energy of the targets and their related compounds were all negative. A negative binding energy indicates that the ligand can spontaneously bind to the receptor, with a smaller value indicating a more stable binding energy. Most of the potential targets docked with quercetin, with the docking energy of EGFR, ESR1, HSP90AA1, SRC, HIF1A, TNF and MAPK3 with quercetin being -21.6109, -37.218, -40.1408, -37.7868, -38.3202, -61.2015, -50.4514, respectively. Among them, MAPK3 had the lowest docking binding energy with quercetin (Figure 7, Table 5).
| Targets | PDB ID | Compounds | Binding energy (kcal/mol) |
| TP53 | 4MZR | protocatechuic acid | -42.1348 |
| ALB | 4BKE | Dianthramine | -49.0138 |
| ESR1 | 4ZNH | quercetin | -37.218 |
| kaempferol | -37.1051 | ||
| HSP90AA1 | 3WQ9 | quercetin | -40.1408 |
| kaempferol | -38.241 | ||
| SRC | 1Y57 | quercetin | -37.7868 |
| HIF1A | 1L8C | quercetin | -38.3202 |
| kaempferol | -49.377 | ||
| MAPK3 | 2ZOQ | quercetin | -61.2015 |
| TNF | 2E7A | quercetin | -50.4514 |
| MAPK1 | 1TVO | formononetin | -48.1226 |
LC is the most common malignant tumor worldwide, and its high morbidity and mortality rates pose a serious threat to public health[14]. Chinese herbal medicine has been shown to improve quality of life of patients by inducing apoptosis, inhibiting cell proliferation, and suppressing tumor metastasis. It also extends patients’ treatment cycles by reducing the side effects of radiotherapy and activating the body’s immunity[15]. Ginseng suppresses tumor migration and invasion and improves the immune function of the body through regulating the interaction between tumor-associated macrophages and LC[4].
In this study, data mining was used to identify the core active ingredients of LBC. Network pharmacology was then used to predict the multiple mechanisms of LBC-based inhibition of LC. Venn diagram analysis found that there was an overlap of 69 targets between targets of LBC active ingredients and LC, indicating that LBC may utilize the 69 genes as therapeutic targets in the treatment of LC. PPI protein network map analysis identified 16 important key targets, including tumor-related targets such as TP53, EGFR, HSP90AA1, HIF1A, and MAPK3. TP53 is a crucial tumor suppressor gene and an emerging target for tumor gene targeting therapy, which strictly regulates the initiation of the cell cycle and is able to repair damaged DNA[16]. Upregulation of the HSP90AA1 gene is associated with decrease in immunity and inhibition of the ability of DNA to repair itself[17]. EGFR is a member of ErbB β family of tyrosine kinase receptors and is considered a driver gene in tumor development[18]. VEGF is an important target in the inhibition of tumor progression, and its expression levels are negatively correlated with progression and prognosis of LC[19]. These results suggest that LBC contain a large number of antitumor compounds.
GO enrichment analysis showed that LBC components may induce apoptosis by regulating the cellular oxidative stress response through modulation of transcription factors and other factors in the treatment of LC. KEGG enrichment analysis showed that 13 and 11 potential targets were related to the PI3K-AKT and estrogen signaling pathway, respectively. Dysregulation of the PI3K/AKT pathway is associated with tumorigenesis, as well as high-grade and advanced tumors of LC. The PI3K-AKT-mTOR signaling pathway is associated with cell proliferation, differentiation, migration, apoptosis and protein synthesis, and is activated in a variety of tumors to promote tumorigenesis[20]. Hamilton DH found that targeting estrogen receptor signaling with fulvestrant enhanced immunity and reduced chemotherapy-induced cytotoxicity in LC patients. He also found that estrogen signaling pathways affect LC progression through induction of EMT[21]. Effects of estrogen signaling pathway in LC are mainly mediated through nongenetic and genetic pathways[22]. This suggests that LBC may be involved in regulating these pathways to exert anti-tumor effects in LC.
The results of molecular docking simulations demonstrated that the compounds have affinity for the potential targets. These results further demonstrate the reliability network pharmacology in predicting active compounds and their targets in relation to their interaction with LC. The targets with the highest potential to dock with quercetin were EGFR, ESR1, HSP90AA1, SRC, HIF1A, and TNF MAPK3, with binding energies of -21.6109, -37.218, -40.1408, -37.7868, -38.3202, and -61.2015, respectively. Quercetin had the best affinity for MAPK3. This illustrated that quercetin may be a crucial compound in the LBC-based treatment of LC.
In this study, we used network pharmacology to integrate information from various databases and perform preliminary validation using molecular docking to elucidate the characteristics of LBC for the treatment of LC through “multi-components-targets-pathways”. However, since the internal environment of organisms are complex, the active compounds, key targets and mechanism of action of LBC in the treatment of LC need to be verified using in vivo experiments. Results from such studies will provide more scientific basis for the use of LBC in the treatment of LC patients.
Lung cancer (LC) is the second highest disease in the world in terms of incidence rate and the main cause of cancer-related deaths.
Liu-Wei-Bu-Qi capsule (LBC) can reduce radiotherapy and chemotherapy-induced side effects such as anorexia and hair loss, and improve the immune response of tumor patients, and improve the quality of life for patients.
The purpose of this study is to investigate the potential targets and signaling pathways of LBC in the treatment of LC.
Network pharmacology and molecular docking simulations were used to analyze the relationship among the main components, targets, and signaling pathways of LBC in treatment of LC.
The analysis results indicate that the main component for treating LC in LBC may be quercetin, which may be used to treat LC by regulating the endothelial growth factor signaling pathway, Toll like receptor signaling pathway, prolactin signaling pathway, FoxO signaling pathway, PI3K-Akt signaling pathway, and HIF-1 signaling pathway. Molecular docking simulations indicate that quercetin has the best affinity for MAPK3, suggesting that quercetin in LBC may play an important role in the treatment of LC.
The results showed that the active ingredients in LBC can play a crucial role in the treatment of LC by regulating multiple signaling pathways.
Predicting potential targets and mechanisms for LBC treatment of LC based on network pharmacology and molecular docking.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Reynolds EC, Australia; Yerke LM, United States S-Editor: Liu JH L-Editor: A P-Editor: Yuan YY
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 63946] [Article Influence: 15986.5] [Reference Citation Analysis (174)] |
| 2. | Li ZH, Yu D, Huang NN, Wu JK, Du XW, Wang XJ. Immunoregulatory mechanism studies of ginseng leaves on lung cancer based on network pharmacology and molecular docking. Sci Rep. 2021;11:18201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 3. | Zuo HL, Zhang QR, Chen C, Yang FQ, Yu H, Hu YJ. Molecular evidence of herbal formula: a network-based analysis of Si-Wu decoction. Phytochem Anal. 2021;32:198-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Li H, Huang N, Zhu W, Wu J, Yang X, Teng W, Tian J, Fang Z, Luo Y, Chen M, Li Y. Modulation the crosstalk between tumor-associated macrophages and non-small cell lung cancer to inhibit tumor migration and invasion by ginsenoside Rh2. BMC Cancer. 2018;18:579. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 141] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 5. | Wu CY, Ke Y, Zeng YF, Zhang YW, Yu HJ. Anticancer activity of Astragalus polysaccharide in human non-small cell lung cancer cells. Cancer Cell Int. 2017;17:115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 6. | Li X, Wei S, Niu S, Ma X, Li H, Jing M, Zhao Y. Network pharmacology prediction and molecular docking-based strategy to explore the potential mechanism of Huanglian Jiedu Decoction against sepsis. Comput Biol Med. 2022;144:105389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 201] [Article Influence: 67.0] [Reference Citation Analysis (0)] |
| 7. | Safran M, Dalah I, Alexander J, Rosen N, Iny Stein T, Shmoish M, Nativ N, Bahir I, Doniger T, Krug H, Sirota-Madi A, Olender T, Golan Y, Stelzer G, Harel A, Lancet D. GeneCards Version 3: the human gene integrator. Database (Oxford). 2010;2010:baq020. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 718] [Cited by in RCA: 1263] [Article Influence: 84.2] [Reference Citation Analysis (0)] |
| 8. | Piñero J, Bravo À, Queralt-Rosinach N, Gutiérrez-Sacristán A, Deu-Pons J, Centeno E, García-García J, Sanz F, Furlong LI. DisGeNET: a comprehensive platform integrating information on human disease-associated genes and variants. Nucleic Acids Res. 2017;45:D833-D839. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1232] [Cited by in RCA: 1685] [Article Influence: 187.2] [Reference Citation Analysis (0)] |
| 9. | Chen X, Ji ZL, Chen YZ. TTD: Therapeutic Target Database. Nucleic Acids Res. 2002;30:412-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 486] [Cited by in RCA: 458] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 10. | Hamosh A, Scott AF, Amberger JS, Bocchini CA, McKusick VA. Online Mendelian Inheritance in Man (OMIM), a knowledgebase of human genes and genetic disorders. Nucleic Acids Res. 2005;33:D514-D517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1574] [Cited by in RCA: 1892] [Article Influence: 94.6] [Reference Citation Analysis (0)] |
| 11. | Bader GD, Hogue CW. An automated method for finding molecular complexes in large protein interaction networks. BMC Bioinformatics. 2003;4:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3477] [Cited by in RCA: 3883] [Article Influence: 176.5] [Reference Citation Analysis (0)] |
| 12. | Huang DW, Sherman BT, Tan Q, Kir J, Liu D, Bryant D, Guo Y, Stephens R, Baseler MW, Lane HC, Lempicki RA. DAVID Bioinformatics Resources: expanded annotation database and novel algorithms to better extract biology from large gene lists. Nucleic Acids Res. 2007;35:W169-W175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1781] [Cited by in RCA: 1674] [Article Influence: 93.0] [Reference Citation Analysis (0)] |
| 13. | Jin X, Awale M, Zasso M, Kostro D, Patiny L, Reymond JL. PDB-Explorer: a web-based interactive map of the protein data bank in shape space. BMC Bioinformatics. 2015;16:339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 14. | Yang D, Liu Y, Bai C, Wang X, Powell CA. Epidemiology of lung cancer and lung cancer screening programs in China and the United States. Cancer Lett. 2020;468:82-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 207] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 15. | Wang Y, Zhang Q, Chen Y, Liang CL, Liu H, Qiu F, Dai Z. Antitumor effects of immunity-enhancing traditional Chinese medicine. Biomed Pharmacother. 2020;121:109570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 191] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 16. | Donehower LA, Soussi T, Korkut A, Liu Y, Schultz A, Cardenas M, Li X, Babur O, Hsu TK, Lichtarge O, Weinstein JN, Akbani R, Wheeler DA. Integrated Analysis of TP53 Gene and Pathway Alterations in The Cancer Genome Atlas. Cell Rep. 2019;28:1370-1384.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 380] [Article Influence: 76.0] [Reference Citation Analysis (0)] |
| 17. | Zuehlke AD, Beebe K, Neckers L, Prince T. Regulation and function of the human HSP90AA1 gene. Gene. 2015;570:8-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 207] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 18. | Harrison PT, Vyse S, Huang PH. Rare epidermal growth factor receptor (EGFR) mutations in non-small cell lung cancer. Semin Cancer Biol. 2020;61:167-179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 286] [Cited by in RCA: 386] [Article Influence: 77.2] [Reference Citation Analysis (0)] |
| 19. | Ghafouri S, Burkenroad A, Pantuck M, Almomani B, Stefanoudakis D, Shen J, Drakaki A. VEGF inhibition in urothelial cancer: the past, present and future. World J Urol. 2021;39:741-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 20. | Tan AC. Targeting the PI3K/Akt/mTOR pathway in non-small cell lung cancer (NSCLC). Thorac Cancer. 2020;11:511-518. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 296] [Cited by in RCA: 333] [Article Influence: 66.6] [Reference Citation Analysis (0)] |
| 21. | Lin Z, Reierstad S, Huang CC, Bulun SE. Novel estrogen receptor-alpha binding sites and estradiol target genes identified by chromatin immunoprecipitation cloning in breast cancer. Cancer Res. 2007;67:5017-5024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 69] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 22. | Hsu LH, Chu NM, Kao SH. Estrogen, Estrogen Receptor and Lung Cancer. Int J Mol Sci. 2017;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 148] [Article Influence: 18.5] [Reference Citation Analysis (0)] |