Published online Nov 6, 2023. doi: 10.12998/wjcc.v11.i31.7570
Peer-review started: September 14, 2023
First decision: September 19, 2023
Revised: September 25, 2023
Accepted: October 23, 2023
Article in press: October 23, 2023
Published online: November 6, 2023
Processing time: 52 Days and 11.7 Hours
Transcutaneous oxygen pressure (TcpO2) is a precise method for determining oxygen perfusion in wounded tissues. The device uses either electrochemical or optical sensors.
To evaluate the usefulness of TcpO2 measurements on free flaps (FFs) in diabetic foot ulcers (DFUs).
TcpO2 was measured in 17 patients with DFUs who underwent anterolateral thigh (ALT)-FF surgery and compared with 30 patients with DFU without FF surgery.
Significant differences were observed in the ankle-brachial index; duration of diabetes; and haemoglobin, creatinine, and C-reactive protein levels between the two groups. TcpO2 values were similar between two groups except on posto
Even if the flap is clinically stable, sympathectomy due to adventitia stripping during anastomosis and arteriovenous shunt progression due to diabetic polyneuropathy could lead to low TcpO2 values in the ALT-FF owing to its thick fat tissues, which is supported by the slow recovery of the sympathetic tone following FF. Therefore, TcpO2 measurements in patients with DFU who underwent FF reconstruction may be less accurate than in those who did not.
Core Tip: Transcutaneous oxygen pressure (TcpO2) is measured low in free flap (FF) reconstruction for the following reasons. The flap itself is thick, making it difficult to accurately measure TcpO2, the adventitia stripping that occurs during vessel anastomosis semi-permanently reducing the sympathetic tone of the entire vessel, and the arteriovenous shunt caused by performing FFs and the progression of diabetes, thought to occur. For this reason, long-term periodic TcpO2 measurement is not useful when FF reconstruction is performed on diabetic foot ulcer.
- Citation: Lee DW, Hwang YS, Byeon JY, Kim JH, Choi HJ. Does the advantage of transcutaneous oximetry measurements in diabetic foot ulcer apply equally to free flap reconstruction? World J Clin Cases 2023; 11(31): 7570-7582
- URL: https://www.wjgnet.com/2307-8960/full/v11/i31/7570.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i31.7570
Free flap (FF) is a surgical procedure that involves transplanting a patient’s own tissue to the recipient site, similar to free tissue transfer or transplantation. Among the preferred options for soft tissue reconstruction, anterolateral thigh FF (ALT-FF) is a popular surgical technique because of its high reliability and versatility, ease of harvest, and low donor-site morbidity. In addition, the redundancy of the thigh skin allows harvesting of a relatively large flap area to reconstruct the defect site[1].
Several devices are used for FF monitoring; however, no equipment guarantees flap survival[2]. Conventionally, flap monitoring includes skin warmth and color, capillary refill, and hand Doppler monitoring but is subjective and does not objectively guarantee flap survival. Proper blood flow is the most important factor in ensuring flap survival, and measuring tissue oxygen levels is the basis for ensuring proper circulation[3,4]. Oxygen plays an important role in the pathophysiological process of flap survival; therefore, quantifying oxygen concentration in vivo is important. In routine clinical settings, oximetry must be noninvasive, reliable, repeatable, and capable of directly measuring partial oxygen pressure. In particular, the instrument must be able to provide repeated measurements in the region of interest to monitor changes in oxygen tension during the observation period.
The measurement of transcutaneous oxygen pressure (TcpO2) is a noninvasive and clinically approved method for measuring tissue perfusion. The necessary instrument was originally developed to measure the partial pressure of arterial oxygen and partial pressure of arterial carbon dioxide[5]. These electrodes contain heating coils that increase the temperature, thereby allowing a subsequent increase in integumentary tissue perfusion. The amount of oxygen was calculated from the amount of light absorbed[6]; TcpO2 was quantitative and free of operator bias[7].
Diabetic foot ulcer (DFU) is one of the most catastrophic complications of diabetes, and peripheral artery disease (PAD) occurs as diabetic neuropathy progresses[8]. As the morbidity period of diabetes increases, sensory nerve function decreases, and wound healing is significantly delayed[9]. In this case, TcpO2 was initially measured to determine the vascular status of DFU[10]. In patients with diabetes, TcpO2 values are significantly lower than those in patients who are not, and studies have revealed that low TcpO2 due to impaired tissue oxygenation may be the cause of DFUs[11,12]; perfusion disorders are indicated by a decrease in skin oxygenation before the appearance of clinical overt microangiopathy. The normal range of TcpO2 metric value is approximately 60 mmHg regardless of electrode placement[13-15]. Moreover, TcpO2 must be > 40 mmHg for adequate wound healing, and impaired wound healing occurs between 20 and 40 mmHg[16]. Additionally, wound healing generally fails when TcpO2 values are < 20 mmHg[17]. In June 2007, the Trans-Atlantic Inter-Society Consensus suggested a standard TcpO2 value > 30 mmHg for revascularization to prevent lower limb amputation[18,19].
Periodic measurement of TcpO2 may predict the effects of DFU amputation and diabetic neuropathy treatment based on a decrease in its value[20-22]. Even when FF reconstruction is performed in DFU cases, vessel anastomosis is difficult owing to atherosclerosis, making the operation and surgical prognosis difficult to predict owing to impaired angiogenesis[23]. Therefore, research is required to determine the abovementioned advantages of TcpO2 measurement when assessed periodically at the FF of the DFU for a longer period of time. In this study, we assumed that the reduced pattern of TcpO2 would apply equally to FF reconstruction and we decided to conduct a study to determine whether the change in TcpO2 levels would recover as wound healing occurred. Then, we intended to identify the numerical pattern of change in TcpO2 and interpret its meaning as the FF was stabilized. Furthermore, we aimed to determine whether periodic TcpO2 measurements are efficient for FF reconstruction in patients with DFU.
We retrospectively reviewed the records of patients who underwent FF reconstruction between 2021 and 2022 in Soonchunhyang Cheonan Hospital. The wounds of patients requiring FFs were all defective owing to DFU. All FFs were performed using ALT-FFs to minimize selection bias and unify results. ALT-FF was performed by three surgeons at a single institution. In addition to the FF group with DFU patients, a group of DFU patients who did not receive FFs was included to establish a control group.
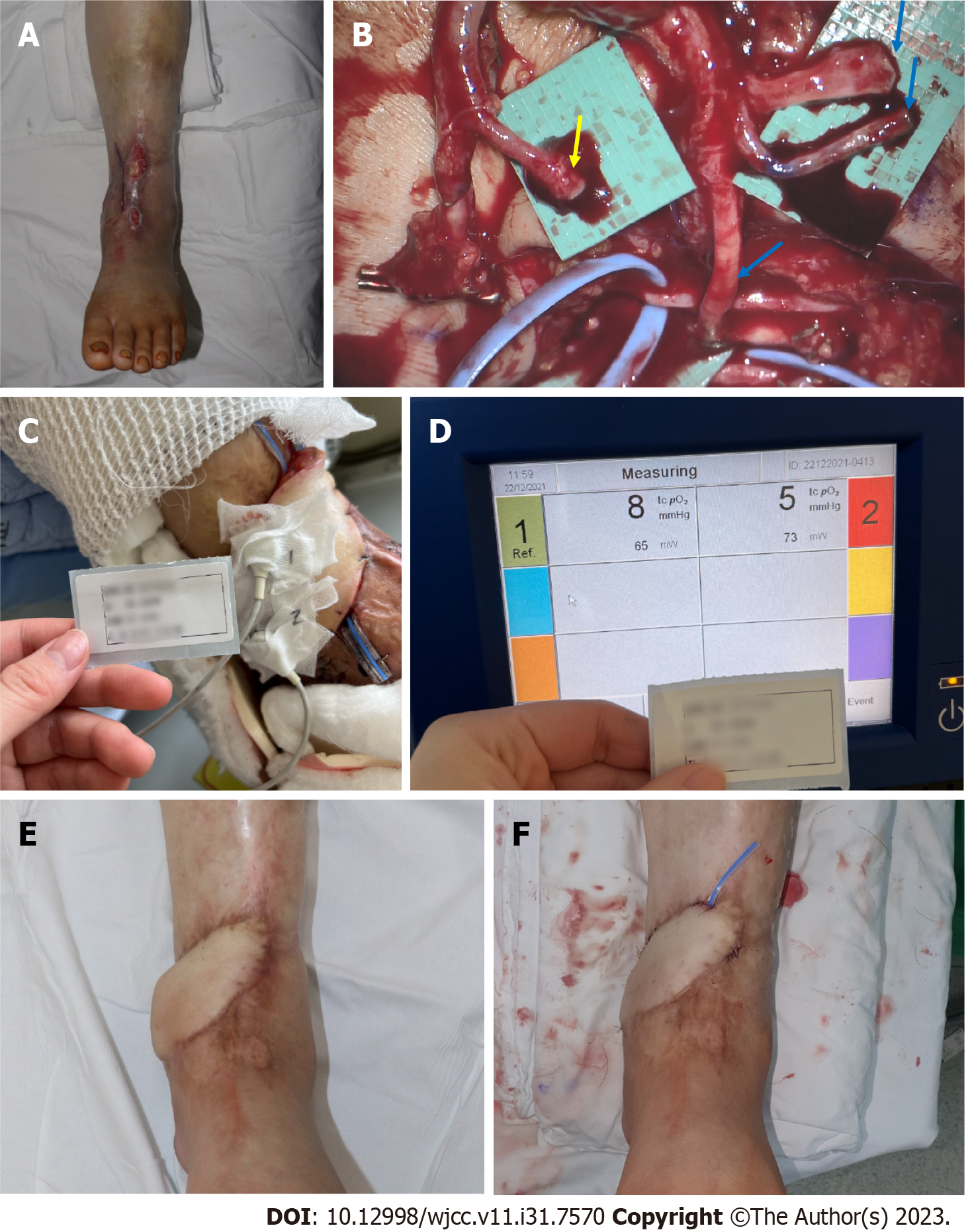
TcpO2 was measured using the TCM400® (Radiometer Medical ApS, Copenhagen, Denmark) (Figure 1). The probes were placed at two locations, and measurements were subsequently performed. One location was the skin perforator of the flap, where the flap pedicle penetrated the skin, and the other was the most distal part of the flap (Figure 2). The contact liquid, supplied by the manufacturer, was applied to the retaining ring to secure the electroprobe. The electrode was measured for 10 min to obtain a stable plateau curve. In this study, the electroprobe setting temperature was adjusted to 41 °C. The room temperature was maintained between 18 °C and 24 °C in the same place before measurement.
In total, 22 records of patients with ALT-FF were obtained. Seventeen patients were included in this study after excluding the patients who dropped out and those with incomplete clinical databases. Thirty patients with DFU who did not undergo FF were included.
After the ALT-FF, TcpO2 was measured on postoperative days (PODs) 1, 5, 10, 15, and 20 for short-term monitoring. Long-term monitoring was performed on PODs 30, 60, 90, and 180. The patients were divided into two groups, i.e., short and long terms based on the measured POD. The changes in time and TcpO2 value were then investigated. The location of the DFU was classified based on the angiosomes of the anterior tibial artery (ATA) and posterior tibial artery (PTA) in the group that did not receive FF; TcpO2 was then subsequently measured (Figure 3). Clinical photographs were taken using an ILCE-7M4 (SONY, Tokyo, Japan) camera at a standard distance (40 cm) to assess the wounds and flaps.
Age, sex, glycosylated haemoglobin A, smoking history, preoperative ankle-brachial index (ABI) after hospitalisation, haemoglobin (Hb), creatinine (Cr), nephropathy, angiosome, duration of diabetes mellitus (DM), duration of hypertension (HTN), C-reactive protein (CRP), body mass index, and TcpO2 values in the FF group and the group without FF were compared. These included variables identified as significant risk factors for DFU amputation in previous studies[24]. The TcpO2 value of the FF group was based on the perforator when the flap was sufficiently stabilized on POD 30 compared with that of the control group.
This study was performed using SPSS (version 26.0; IBM Corp., Armonk, NY, United States). Categorical variables were compared using Pearson’s χ2, Fisher’s exact, Student’s t-, and Mann-Whitney U tests. Only variables with P < 0.05 were considered statistically significant.
Based on the medical records, we reviewed 17 patients with DFU who received FFs (FF group) and 30 patients with DFU who did not receive FFs (DFU group). Table 1 presents the demographic characteristics of the FF and DFU groups. Several demographic risk factors were compared between the two groups. ABI, Hb, Cr, duration of DM, and CRP levels were significantly different between the two groups. The ABI group had a higher mean value than the FF group. The TcpO2 value was 12.01 mmHg at POD 30 in the FF group and 14.50 mmHg in the DFU group, which was significantly low in both groups; no significant difference was noted between the two groups (P = 0.812).
| Total (n = 47) | Free flap (n = 17) | DFU (n = 30) | P value | |
| Sex | ||||
| Male | 31 (65.96) | 10 (58.82) | 21 (70.00) | 0.437 |
| Female | 16 (34.04) | 7 (41.18) | 9 (30.00) | |
| Age | 61.36 ± 2.04 | 54.76 ± 3.62 | 65.10 ± 2.21 | 0.013 |
| Smoking history | ||||
| Non-smoker | 23 (48.94) | 11 (64.71) | 12 (40.00) | 0.104 |
| Smoker | 24 (51.06) | 6 (35.29) | 18 (60.00) | |
| HbA1c | 7.00 (6.00-9.10) | 6.20 (5.60-9.00) | 7.25 (6.10-9.20) | 0.084 |
| HTN | ||||
| None | 17 (36.17) | 9 (52.94) | 8 (26.67) | 0.072 |
| HTN | 30 (63.83) | 8 (47.06) | 22 (73.33) | |
| Nephropathy | ||||
| None | 34 (72.34) | 15 (88.24) | 19 (63.33) | 0.094 |
| Nephropathy | 13 (27.66) | 2 (11.76) | 11 (36.67) | |
| Angiosome | ||||
| ATA | 26 (55.32) | 7 (41.18) | 19 (63.33) | 0.138 |
| PTA | 21 (44.68) | 10 (58.82) | 11 (36.67) | |
| TcpO2 | 13.00 (6.00-22.00) | 12.01 (8.01-15.01) | 14.50 (6.00-22.00) | 0.812 |
| ABI | ||||
| Affected side | 1.02 ± 0.03 | 1.14 ± 0.03 | 0.95 ± 0.05 | 0.002 |
| Unaffected side | 1.06 ± 0.03 | 1.13 ± 0.02 | 1.03 ± 0.04 | 0.024 |
| Creatinine | 1.07 (0.72-3.12) | 0.74 (0.63-0.86) | 1.98 (0.95-6.27) | 0.001 |
| Hb | 11.10 ± 0.25 | 11.83 ± 0.31 | 10.68 ± 0.33 | 0.024 |
| Duration of DM (yr) | 10.00 (5.50-15.00) | 3.00 (1.00-10.00) | 13.50 (10.00-20.00) | < 0.001 |
| Duration of HTN (yr) | 10.50 (10.00-20.00) | 11.00 (2.00-20.00) | 10.00 (10.00-20.00) | 0.666 |
| CRP | 11.24 (4.21-35.70) | 3.50 (1.70-10.57) | 27.78 (8.20-73.71) | < 0.001 |
| BMI | 23.96 ± 0.71 | 23.84 ± 1.28 | 24.03 ± 0.87 | 0.896 |
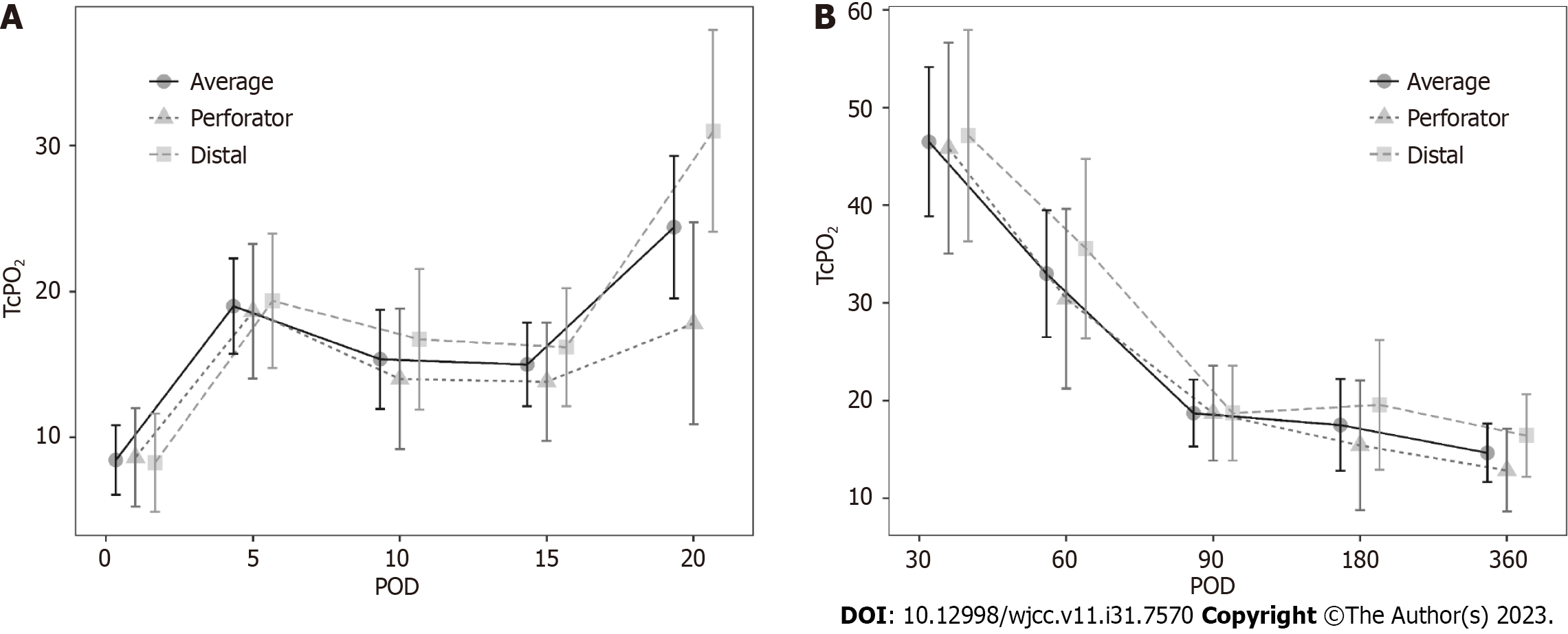
In addition, the change in TcpO2 value based on POD was investigated. A value < 10 mmHg was regarded as a ‘very low’ TcpO2 value. On POD 30, ALT FF was successfully performed without complications, despite the very low TcpO2 values in four patients (23.53%). The change in TcpO2 value over time showed a significant difference for each POD in the FF group. A comparison of the perforator (branching off from vessels and extending into the skin and fat tissue) and distal portion of the flap based on POD showed no difference according to position (Table 2). However, a significant difference was observed when comparing the short- (until POD 20) and long-term (after POD 30) results through division. The short-term P value was 0.006, and the long-term P value was 0.002, showing significant changes as POD progressed. Upon short-term follow-up, a gradual increase was observed (Figure 4A), while upon long-term follow-up (POD 30 onwards), a gradual decrease was observed. A similar pattern was observed (Figure 4B). This pattern showed a similar trend in the perforator and distal portions of the flap. However, the TcpO2 value was low (< 30 mmHg), except for PODs 30 and 60.
| Value | Perforator | Distal | P value |
| POD 1 | 8.64 ± 10.72 | 8.27 ± 11.62 | > 0.099 |
| POD 5 | 18.64 ± 14.43 | 19.36 ± 16.11 | > 0.99 |
| POD 10 | 14.00 ± 16.35 | 16.73 ± 15.51 | > 0.99 |
| POD 15 | 13.82 ± 13.41 | 16.18 ± 13.40 | > 0.99 |
| POD 20 | 17.82 ± 18.21 | 31.00 ± 26.81 | 0.972 |
| POD 30 | 45.86 ± 33.20 | 47.14 ± 23.13 | > 0.99 |
| POD 60 | 30.43 ± 21.95 | 35.57 ± 26.48 | > 0.99 |
| POD 90 | 18.71 ± 5.35 | 18.71 ± 17.37 | > 0.99 |
| POD 180 | 15.43 ± 11.10 | 19.57 ± 22.23 | > 0.99 |
| POD 360 | 12.86 ± 7.78 | 16.43 ± 13.78 | > 0.99 |
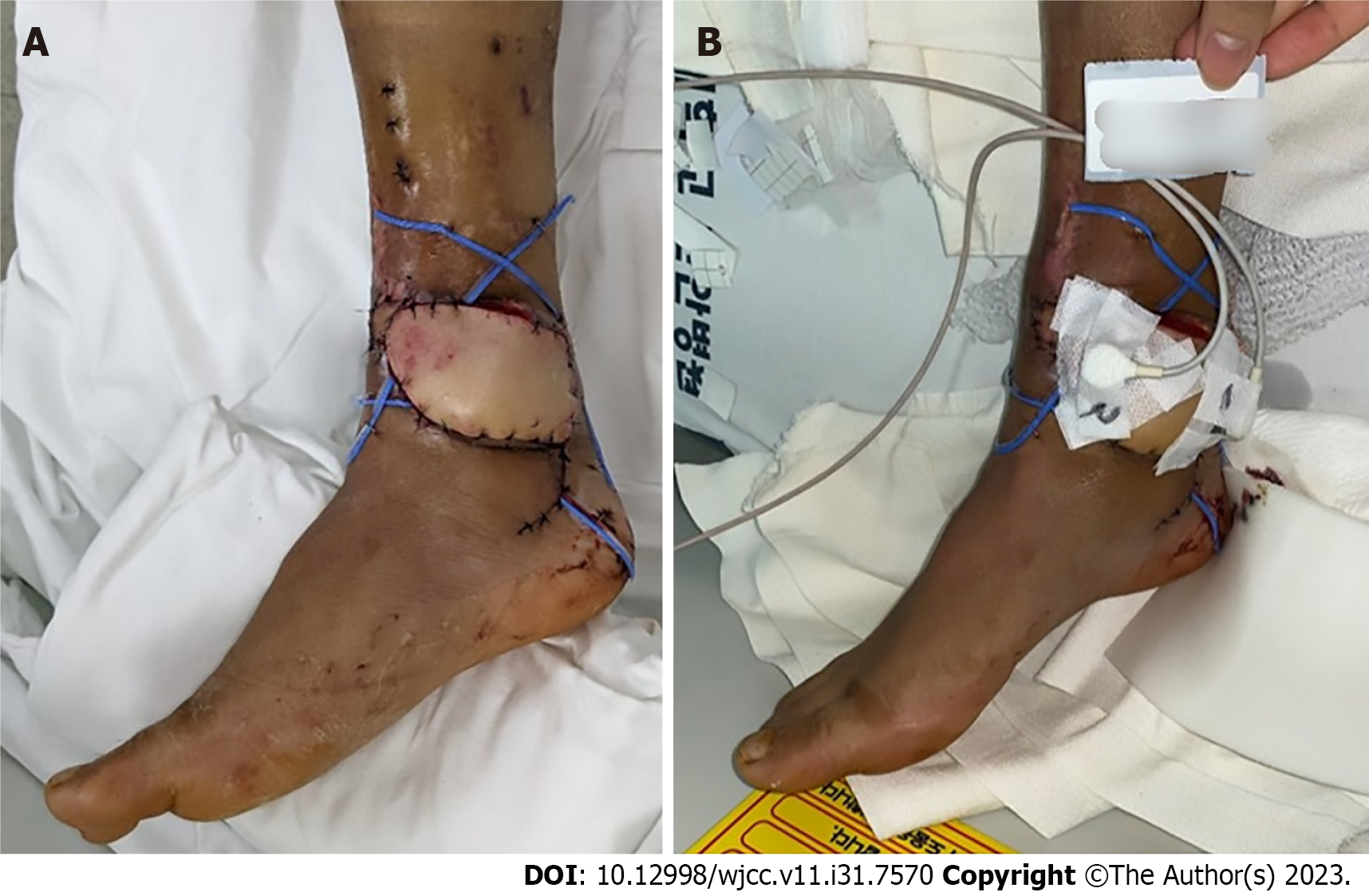
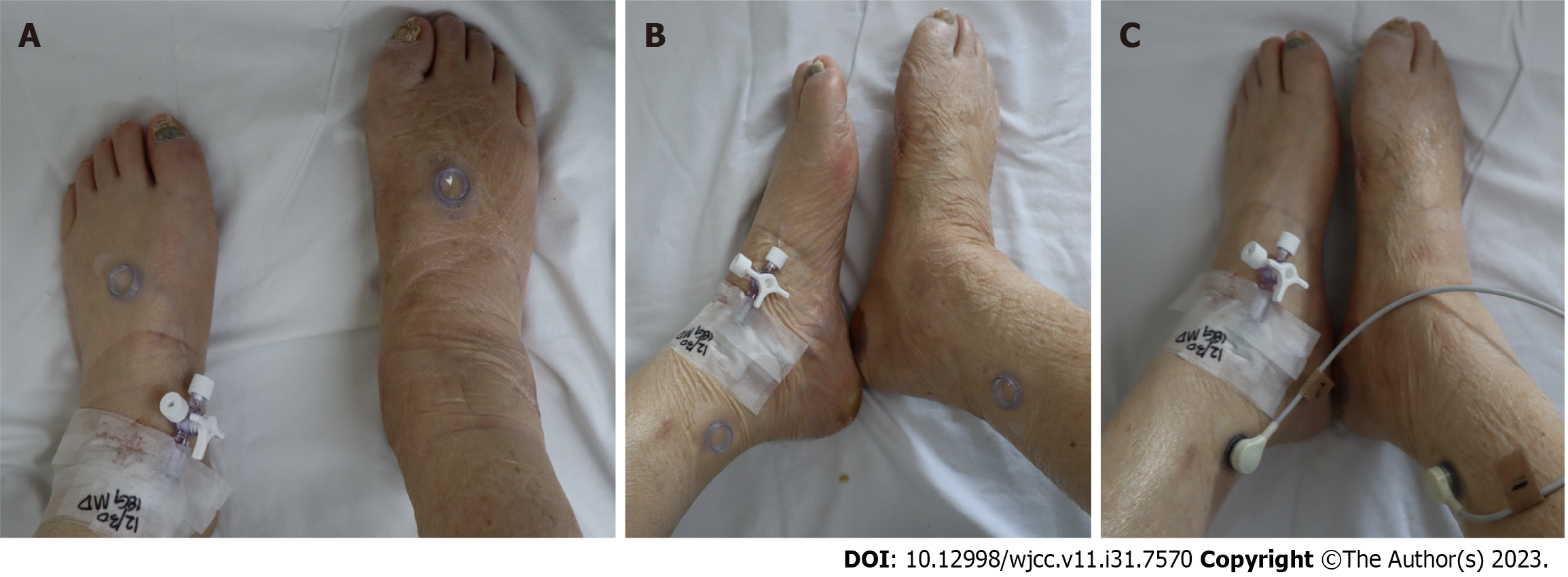
Case 1: A 61-year-old man presented with a necrotic DFU, 5 cm × 8 cm in size, on the dorsum of the left foot. The patient had been diagnosed with DM and HTN 20 years prior. The patient underwent reconstruction with an ALT-FF after removing the necrotic tissue and performing several debridements. End-to-end anastomosis was also performed on the dorsalis pedis artery and two vena comitans. Flap failure was not observed, and TcpO2 was measured according to POD. Limb salvage was successfully performed, and no wound recurrence was noted until POD 360 (Figure 5).
Case 2: A 62-year-old man presented with multiple skin defects on the left ankle. The patient had been diagnosed with DM and HTN 3 years prior. The DFU was reconstructed with an ALT-FF after removing the necrotic tissue and performing several debridements. The end-to-side anastomosis was performed on the PTA; end-to-end anastomosis was performed on two vena comitans. Additionally, end-to-end anastomosis was performed in a flow-through manner (conserving vessels through performing end-to-end anastomosis on both sides of the recipient vessel) after identifying one dorsal recipient vein. Flap failure was not observed, and TcpO2 was measured according to POD. Flap debulking was performed on POD 192 owing to discomfort during ankle joint movement. No wound recurrence was observed until POD 360 (Figure 6).
In our study, the proportion of TcpO2 levels in FFs that did not increase by more than 30 mmHg even after POD 30 was 35.3% (6/17). Before discussing, let us firstly mention the relationship between DFU and TcpO2. Wound formation in DFU is mostly due to PAD, and there are several ways to examine the peripheral blood flow state[25]. TcpO2, skin perfusion pressure, and fluorescence angiography were performed to identify regional tissue perfusion. The advantages and disadvantages of each device are listed in Table 3. The ABI is routinely used to screen for PAD. However, these results are less reliable in patients with diabetes with severe neuropathy and arterial calcification[34]. In such case, TcpO2 may be helpful in predicting the risk of amputation and disease progression[35-37].
| Advantages | Limitations | |
| Assessment of disease severity | ||
| ABI (normal range 1.1-1.3) | Widely used, easy to measure | Unreliable in patients with severe PAD, DFU[26] |
| TBI (normal range 1.0-1.1) | Compensating for ABI limitations, easy to repeat measurement | Decreased accuracy at severe vessel calcification, limitation of diagnostic threshold[27] |
| Continuous wave Doppler | High accuracy in PAD diagnosis[28] | Decreased accuracy in DFU[28] |
| Pulse volume recording | Used in PAD diagnosis | Unable to determine exact location, many other variables[29] |
| Assessment of morphological distribution | ||
| Duplex ultrasound | Noninvasive | Complemented by more detailed image required |
| Angiography | Detailed images can be provided, fast | Vulnerable to artifacts, risk for contrast nephropathy |
| Assessment of regional tissue perfusion | ||
| TcpO2 (normal range 40-70 mmHg) | More sensitive than ABI | DFU, autonomic neuropathy, low accuracy for severe vascular calcification[12] |
| Skin perfusion pressure | Useful when ABI, TBI are not possible | Requires special equipment and further validation[30] |
| Fluorescence angiography | Low toxicity compared with angiography, good discriminatory ability[31] | Expensive, requires special equipment |
| Laser Doppler | Evaluate blood flux rather than blood flow | Vulnerable to motion artifacts and temperature changes, inter-operator variation |
| Hyperspectral imaging | Useful for determining the effect of DFU treatment | Lack of research on interpretation of results[32] |
| Molecular imaging | ||
| PET and SPECT | High resolution | Expensive, requires special equipment |
| Contrast-enhanced ultrasound | Excellent for PAD discrimination[33] | Low accuracy for DFU[33] |
| Multi-modal MRI | Semi-quantitative evaluations based on relative patient conditions are possible | Take long time, no evidence of effectiveness for DFU |
Various factors affect the measurement of TcpO2. First, measurement of TcpO2 is affected by the partial pressure of oxygen in the artery. TcpO2 decreases in the presence of vascular injury and atherosclerosis[38]. Skin thickness also affects TcpO2 measurements[39]. Therefore, thick fatty tissues and bones should be avoided. In addition, determining the area of good capillary phenomena remains achievable[40]. Finally, the temperature of the capillary under the measuring electroprobe also has an effect. The electroprobe for TcpO2 uses heated metal electrodes placed directly on the skin. During the measurement, the skin temperature under the electrode increased by up to 44 °C, thereby dilating the blood vessels of the subdermal plexus and subsequently increasing the oxygen permeability.
Several limitations to TcpO2 measurement have been noted during wound assessment. First, it requires a considerable amount of time for measurement, which lasts 10-15 min. Second, its sensitivity is low, although it does not cause pain to patients unlike the nerve conduction velocity method. Third, the presence of cellulitis or significant oedema in the tissue interferes with measurement[41]. Fourth, it reflects the state of blood flow only in parts of the skin and not in major blood vessels. Therefore, obtaining accurate values for thick tissues is difficult. Finally, TcpO2 measurement is heavily influenced by skin temperature. This measurement is possible only when skin perfusion is measured using a heated metal electrode.
According to our study, the percentage of TcpO2 levels in FFs that did not increase > 30 mmHg, even after sufficient POD (POD 30), was 35.3% (6/17), which accounted for a fairly large percentage. No increase in the value was noted in these patients even though the flap thickness was significantly decreased. Almost all the TcpO2 average values were < 30 mmHg except for PODs 30 and 60. In addition, the TcpO2 value did not increase even though a significant temperature increase was observed when monitored with a thermometer. In addition, low TcpO2 was measured regardless of which recipient artery (ATA or PTA) was used or whether the anastomosis method was end-to-end or end-to-side. Therefore, the following are reasons for the low TcpO2 during measurement:
First, mid-thigh tissue was used as the donor site in the case of ALT-FF performed in this study. The ALT-FF is usually at least 1-cm thick during flap elevation. The subcutaneous fat tissue of the thigh is thicker than the other tissues, making it difficult to accurately measure TcpO2[42].
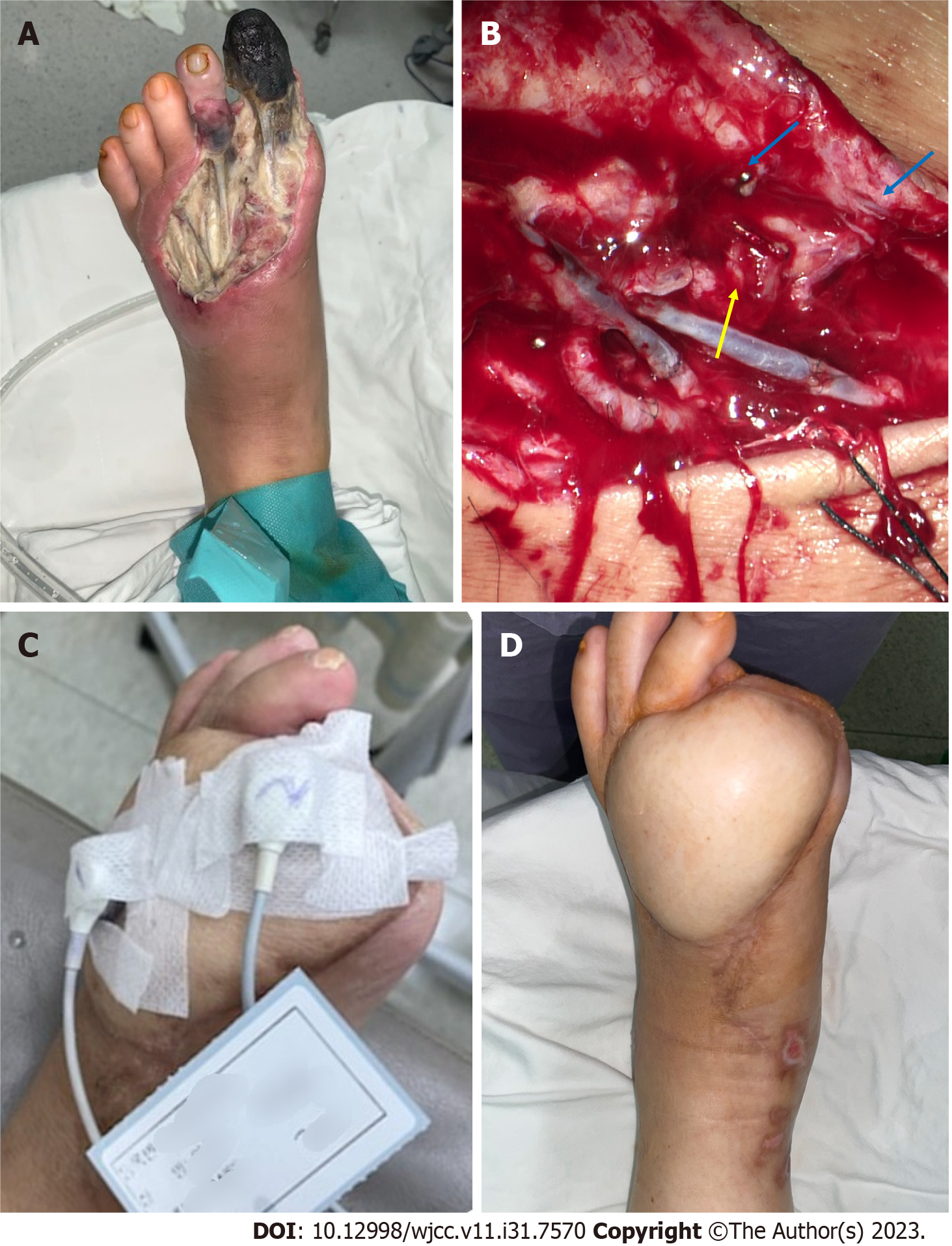
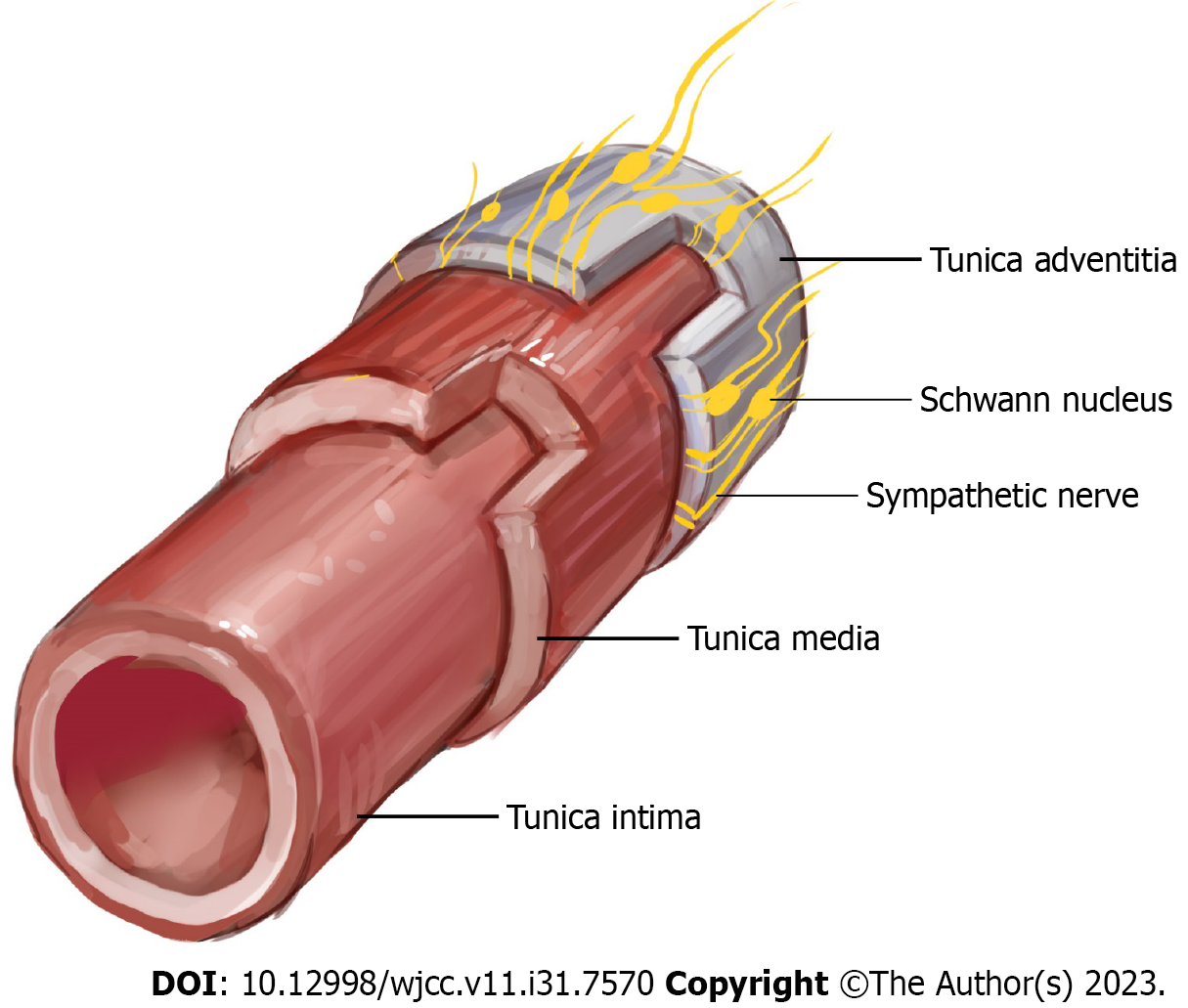
Second, since the electroprobe of TcpO2 consumes oxygen during measurement, it is difficult to measure if the oxygen level that may present on the skin temperature is low. Moreover, the method is prone to calibration errors. In essence, the TcpO2 value can be measured at 0 mmHg even if the skin flap has significant perfusion[6]. As the temperature of the electroprobe increased, the skin temperature also increased, thereby correcting the value of TcpO2. However, in the FF of the DFU, the normal response of the flap skin temperature did not increase even when the electroprobe temperature increased. This may be due to insufficient sympathetic tone[43]. Moreover, this may also be due to the surgical removal of the arterial adventitia by many surgeons during artery anastomosis to prevent vasospasm[44]. Sympathetic innervation of the major vessels of the foot is most evident in the adventitia, which is the outermost layer of the artery (Figure 7)[45]. Nerve regeneration did not occur when a periarterial sympathectomy was performed. Based on these findings, the removal of the adventitia from the FF resulted in semi-permanent sympathetic nerve denervation. A significant TcpO2 value could not be obtained if the dominance of the sympathetic nerve decreased and the temperature of the flap skin did not increase in response to the temperature of the sensor. Chao and Cheing[46] reported that when the sympathetic nerve present in the adventitia is removed, the functions of the smooth muscle and endothelium, which are the vascular structures inside the adventitia, also decrease. Adventitial damage may result in extensive vascular insufficiency. In this study, the ABIs of the FF group were > 0.9, and the average value was higher than that of the DFU group, regardless of whether a wound was present on the left or right side. This indicates that patients in the FF group did not have severe PAD or neuropathy compared with those in the DFU group. Nevertheless, the TcpO2 value remains low even after a sufficient period of time. Thus, sympathectomy performed during anastomosis may not have been recoverable, and the recovery was insignificant. Furthermore, flap skin reperfusion was not restored. Additionally, if a low TcpO2 value was observed even with a normal ABI, this finding suggested that diabetic neuropathy was caused by a decrease in sympathetic tone[47].
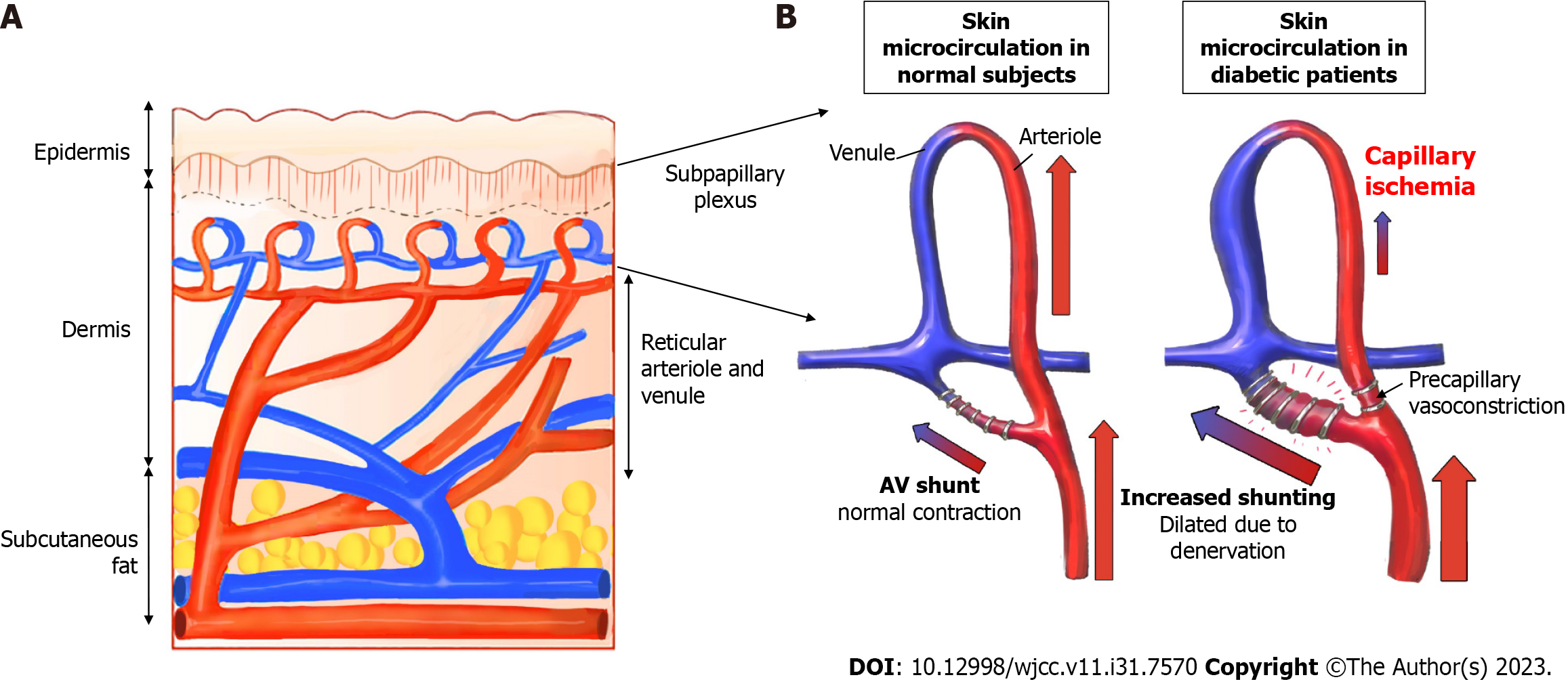
Third, as diabetic angiopathy progresses, an arteriovenous (AV) shunt develops in the peripheral blood vessels. The skin microcirculation consists of two horizontal plexus structures: The deeper subpapillary plexus and the superficial cutaneous plexus. Among these, the subpapillary plexus is responsible for more than 80% of blood flow[48]. Their actions are regulated by a complex interplay between neurogenic and neurovascular regulation[46]. Arteriovenous vessels have thicker walls and lower resistance than capillaries, allowing blood to flow at a relatively higher rate directly from the arterioles to the venules. In advanced DFU lesions, peripheral capillary resistance increases, and blood flow decreases owing to microvasculopathy caused by advanced diabetic polyneuropathy and vasodilation impairment. Therefore, AV shunting to the subpapillary system, which has a lower resistance than the capillaries, occurs more frequently than in the normal foot (Figure 8). Early venous enhancement occurs during the early inflammatory reaction of diabetic angiopathy, which supports the maldistribution of blood flow between the nutritional capillaries and subpapillary vessels, and the AV shunt caused by diabetic angiopathy occurs more peripherally[49]. An increased subpapillary AV shunt reduces the exchange of oxygen and metabolites between the capillaries and peripheral tissues, thus leading to ischemia and delayed wound healing. In this study, the FF group had an inadequate oxygen supply to the AV shunt and peripheral tissues. In addition, removal of the adventitia resulted in sympathectomy, whereas regeneration of the removed sympathetic nerve did not occur, resulting in failure to recover from the AV shunt. Even with flap operation, if blood flow was greater to the artery than before or after anastomosis, the theory that the AV shunt increases without the choke vessel opening would have been proven through cadaver and animal studies[50]. Thus, AV shunt can become more severe as diabetic angiopathy progresses. In summary, measuring TcpO2 appropriately in FFs is difficult because of decreased sympathetic tone, malfunctioning vasculature, and AV shunting.
This study has a statistical limitation; it compared the data of only 17 patients with complete TcpO2 values, which was insufficient to highlight the complete interpretation of the TcpO2 numerical pattern. Measuring TcpO2 at more frequent intervals is also necessary. Thus, a larger multi-institutional study with a larger number of patients is required. In addition, a prospective study may also be required to validate the effectiveness of the risk factors identified in our study for TcpO2 pattern analysis. In particular, sympathectomy and AV shunt formation, which were suggested as the prime causes of low TcpO2, require identification through additional experiments rather than proven only with TcpO2 or ABI measurements.
TcpO2 helps assess the therapeutic effect and blood flow in patients with DFU and predicts vascular occlusion in FFs. However, when FF reconstruction was performed in patients with DFU, a successful flap was achieved even with low TcpO2; no significant increase in TcpO2 was noted even after a long-term follow-up of > 1 year, which is thought to be owing to the thickness of the ALT-FF, sympathectomy owing to adventitia removal for intraoperative anastomosis, and diabetic AV shunt formation. TcpO2 becomes low when sympathectomy was performed without regard to the presence of diabetic neuropathy. Therefore, TcpO2 measurements in FFs after FF reconstruction may not be as useful as those with DFU who did not undergo FF reconstruction owing to the characteristics of FF operation and DFU.
Patients with diabetes have lower transcutaneous oxygen pressure (TcpO2) levels than those without diabetes, indicating that poor tissue oxygenation may lead to diabetic foot ulcers (DFUs). TcpO2 measurements can help assess the risk of DFU amputation and the effectiveness of diabetic neuropathy treatment by showing changes in TcpO2 levels.
Vessel anastomosis is challenging in free flap (FF) reconstruction for DFU cases because of atherosclerosis and narrowed vessel. Therefore, it is important to study the benefits of periodic TcpO2 measurement at the FF site of the DFU for a longer period of time.
Our goal was to find out how TcpO2 values changed over time and what it meant for the FF stability. We also wanted to see if TcpO2 measurements were useful for FF reconstruction in DFU patients.
We retrospectively reviewed the records of patients requiring FFs were all defective owing to DFU. In addition to the FF group with DFU patients, a group of DFU patients who did not receive FFs was included as a control group. We then examined how TcpO2 value changed over time.
The FF group had higher levels of ankle-brachial index (ABI), haemoglobin, creatinine, diabetes mellitus duration, and C-reactive protein than the control group. Both groups had low TcpO2 levels. TcpO2 levels increased gradually in the short term, but decreased gradually in the long term (after postoperative day 30).
There is no significant increase in TcpO2 was noted even after a long-term follow-up of > 1 year. This may be due to the flap thickness, the sympathectomy from adventitia removal, and the diabetic arteriovenous (AV) shunts. Therefore, TcpO2 measurements may not be very helpful for FF reconstruction in DFU patients, unlike in DFU patients who did not have FF reconstruction, because of the features of FF surgery and DFU.
Future studies should measure TcpO2 more frequently. A larger and multi-center study with more patients is needed. A prospective study may also confirm the risk factors we found for TcpO2 pattern analysis. Especially, sympathectomy and AV shunt formation, which may cause low TcpO2, need more experiments than just TcpO2 or ABI measurements.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Glumac S, Croatia; Primadhi RA, Indonesia S-Editor: Wang JJ L-Editor: A P-Editor: Zhao S
| 1. | Lee JH, Choi HJ, Kwak SH, Lee DW, Tak MS, Kang JS. Anterolateral thigh free flaps with T-shaped pedicles and multiple venous anastomosis for extremity reconstruction. Medicine (Baltimore). 2021;100:e26575. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 2. | Furnas H, Rosen JM. Monitoring in microvascular surgery. Ann Plast Surg. 1991;26:265-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 68] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Howell ST, Seaber AV, Urbaniak JR. Microcirculatory responses to vascular washout following ischemia. Microsurgery. 1989;10:264-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Carroll WR, Esclamado RM. Ischemia/reperfusion injury in microvascular surgery. Head Neck. 2000;22:700-713. [PubMed] [DOI] [Full Text] |
| 5. | Hashimoto I, Nakanishi H, Takiwaki H, Takase MT, Yamano M, Sedo H. Flap monitoring by transcutaneous PO2 and PCO2: importance of transcutaneous PCO2 in determining follow-up treatment for compromised free flaps. J Reconstr Microsurg. 2007;23:269-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Ballard JL, Bianchi C. Transcutaneous Oxygen Tension: Principles and Applications. In: AbuRahma AF, Bergan JJ. Noninvasive Vascular Diagnosis. London: Springer London, 2000: 403-409. |
| 7. | Abe Y, Hashimoto I, Goishi K, Kashiwagi K, Yamano M, Nakanishi H. Transcutaneous PCO2 Measurement at Low Temperature for Reliable and Continuous Free Flap Monitoring: Experimental and Clinical Study. Plast Reconstr Surg Glob Open. 2013;1:1-8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Yazdanpanah L, Nasiri M, Adarvishi S. Literature review on the management of diabetic foot ulcer. World J Diabetes. 2015;6:37-53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 291] [Cited by in RCA: 322] [Article Influence: 32.2] [Reference Citation Analysis (11)] |
| 9. | Ashcroft GS, Horan MA, Ferguson MW. The effects of ageing on cutaneous wound healing in mammals. J Anat. 1995;187:1-26. [PubMed] |
| 10. | Ladurner R, Küper M, Königsrainer I, Löb S, Wichmann D, Königsrainer A, Coerper S, Beckert S. Predictive value of routine transcutaneous tissue oxygen tension (tcpO2) measurement for the risk of non-healing and amputation in diabetic foot ulcer patients with non-palpable pedal pulses. Med Sci Monit. 2010;16:CR273-CR277. [PubMed] |
| 11. | Boyko EJ, Ahroni JH, Stensel V, Forsberg RC, Davignon DR, Smith DG. A prospective study of risk factors for diabetic foot ulcer. The Seattle Diabetic Foot Study. Diabetes Care. 1999;22:1036-1042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 430] [Cited by in RCA: 409] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 12. | Williams DT, Price P, Harding KG. The influence of diabetes and lower limb arterial disease on cutaneous foot perfusion. J Vasc Surg. 2006;44:770-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Cina C, Katsamouris A, Megerman J, Brewster DC, Strayhorn EC, Robison JG, Abbott WM. Utility of transcutaneous oxygen tension measurements in peripheral arterial occlusive disease. J Vasc Surg. 1984;1:362-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 14. | Clyne CA, Ryan J, Webster JH, Chant AD. Oxygen tension of the skin of ischemic legs. Am J Surg. 1982;143:315-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 45] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Hauser CJ, Shoemaker WC. Use of a transcutaneous PO2 regional perfusion index to quantify tissue perfusion in peripheral vascular disease. Ann Surg. 1983;197:337-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 94] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 16. | Ruangsetakit C, Chinsakchai K, Mahawongkajit P, Wongwanit C, Mutirangura P. Transcutaneous oxygen tension: a useful predictor of ulcer healing in critical limb ischaemia. J Wound Care. 2010;19:202-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 17. | Quigley FG, Faris IB. Transcutaneous oxygen tension measurements in the assessment of limb ischaemia. Clin Physiol. 1991;11:315-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 36] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG, Rutherford RB; TASC II Working Group. Inter-society consensus for the management of peripheral arterial disease. Int Angiol. 2007;26:81-157. [PubMed] |
| 19. | Faglia E, Clerici G, Caminiti M, Quarantiello A, Curci V, Morabito A. Predictive values of transcutaneous oxygen tension for above-the-ankle amputation in diabetic patients with critical limb ischemia. Eur J Vasc Endovasc Surg. 2007;33:731-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 70] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 20. | Iino K, Yoshinari M, Doi Y, Shinohara N, Iwase M, Fujishima M. Reduced tissue oxygenation and its reversibility by glycemic control in diabetic patients. Diabetes Res Clin Pract. 1997;34:163-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | McNeely MJ, Boyko EJ, Ahroni JH, Stensel VL, Reiber GE, Smith DG, Pecoraro RF. The independent contributions of diabetic neuropathy and vasculopathy in foot ulceration. How great are the risks? Diabetes Care. 1995;18:216-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 218] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 22. | Berli MC, Wanivenhaus F, Kabelitz M, Götschi T, Böni T, Rancic Z, Waibel FWA. Predictors for reoperation after lower limb amputation in patients with peripheral arterial disease. Vasa. 2019;48:419-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 23. | Valentini V, Cassoni A, Marianetti TM, Mitro V, Gennaro P, Ialongo C, Iannetti G. Diabetes as main risk factor in head and neck reconstructive surgery with free flaps. J Craniofac Surg. 2008;19:1080-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 83] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 24. | Lee DW, Kwak SH, Kim JH, Choi HJ. Prediction of diabetic foot amputation using newly revised DIRECT coding system: Comparison of accuracy with that of five existing classification systems. Int Wound J. 2023;20:359-371. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 25. | Forsythe RO, Hinchliffe RJ. Assessment of foot perfusion in patients with a diabetic foot ulcer. Diabetes Metab Res Rev. 2016;32 Suppl 1:232-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 26. | Boyko EJ, Ahroni JH, Davignon D, Stensel V, Prigeon RL, Smith DG. Diagnostic utility of the history and physical examination for peripheral vascular disease among patients with diabetes mellitus. J Clin Epidemiol. 1997;50:659-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 59] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 27. | Høyer C, Sandermann J, Petersen LJ. The toe-brachial index in the diagnosis of peripheral arterial disease. J Vasc Surg. 2013;58:231-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 153] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 28. | Williams DT, Harding KG, Price P. An evaluation of the efficacy of methods used in screening for lower-limb arterial disease in diabetes. Diabetes Care. 2005;28:2206-2210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 175] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 29. | Lewis JE, Owens DR. The pulse volume recorder as a measure of peripheral vascular status in people with diabetes mellitus. Diabetes Technol Ther. 2010;12:75-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 30. | Yamada T, Ohta T, Ishibashi H, Sugimoto I, Iwata H, Takahashi M, Kawanishi J. Clinical reliability and utility of skin perfusion pressure measurement in ischemic limbs--comparison with other noninvasive diagnostic methods. J Vasc Surg. 2008;47:318-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 135] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 31. | Perry D, Bharara M, Armstrong DG, Mills J. Intraoperative fluorescence vascular angiography: during tibial bypass. J Diabetes Sci Technol. 2012;6:204-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 32. | Jeffcoate WJ, Clark DJ, Savic N, Rodmell PI, Hinchliffe RJ, Musgrove A, Game FL. Use of HSI to measure oxygen saturation in the lower limb and its correlation with healing of foot ulcers in diabetes. Diabet Med. 2015;32:798-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 33. | Thomas KN, Cotter JD, Lucas SJ, Hill BG, van Rij AM. Reliability of contrast-enhanced ultrasound for the assessment of muscle perfusion in health and peripheral arterial disease. Ultrasound Med Biol. 2015;41:26-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 34. | Beutner F, Teren A, Gielen S, Schuler G, Wirkner K, Tiller D, Loeffler M, Scholz M. Automated photoplethysmography-based determination of ankle-brachial index: a validation study against Doppler sonography. Clin Res Cardiol. 2012;101:875-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 35. | Gazzaruso C, Coppola A, Falcone C, Luppi C, Montalcini T, Baffero E, Gallotti P, Pujia A, Solerte SB, Pelissero G, Giustina A. Transcutaneous oxygen tension as a potential predictor of cardiovascular events in type 2 diabetes: comparison with ankle-brachial index. Diabetes Care. 2013;36:1720-1725. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 36. | Gernigon M, Marchand J, Ouedraogo N, Leftheriotis G, Piquet JM, Abraham P. Proximal ischemia is a frequent cause of exercise-induced pain in patients with a normal ankle to brachial index at rest. Pain Physician. 2013;16:57-64. [PubMed] |
| 37. | Pardo M, Alcaraz M, Bernal FL, Felices JM, Achel GD, Canteras M. Transcutaneous oxygen tension measurements following peripheral transluminal angioplasty procedure has more specificity and sensitivity than ankle brachial index. Br J Radiol. 2015;88:20140571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 38. | Matsen FA 3rd, Wyss CR, Robertson CL, Oberg PA, Holloway GA. The relationship of transcutaneous PO2 and laser Doppler measurements in a human model of local arterial insufficiency. Surg Gynecol Obstet. 1984;159:418-422. [PubMed] |
| 39. | de Meijer VE, Van't Sant HP, Spronk S, Kusters FJ, den Hoed PT. Reference value of transcutaneous oxygen measurement in diabetic patients compared with nondiabetic patients. J Vasc Surg. 2008;48:382-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 40. | Lamah M, Mortimer PS, Dormandy JA. Quantitative study of capillary density in the skin of the foot in peripheral vascular disease. Br J Surg. 1999;86:342-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 41. | Ballard JL, Eke CC, Bunt TJ, Killeen JD. A prospective evaluation of transcutaneous oxygen measurements in the management of diabetic foot problems. J Vasc Surg. 1995;22:485-90; discussion 490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 114] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 42. | Luo S, Raffoul W, Luo J, Luo L, Gao J, Chen L, Egloff DV. Anterolateral thigh flap: A review of 168 cases. Microsurgery. 1999;19:232-238. [PubMed] [DOI] [Full Text] |
| 43. | Vetrugno R, Liguori R, Cortelli P, Montagna P. Sympathetic skin response: basic mechanisms and clinical applications. Clin Auton Res. 2003;13:256-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 271] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 44. | Turin SY, Walton RL, Dumanian GA, Hijjawi JB, LoGiudice JA, Alghoul M. Current Practices in the Management of Postoperative Arterial Vasospasm in Microsurgery. J Reconstr Microsurg. 2018;34:242-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 45. | Dellon AL, Höke A, Williams EH, Williams CG, Zhang Z, Rosson GD. The sympathetic innervation of the human foot. Plast Reconstr Surg. 2012;129:905-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 46. | Chao CY, Cheing GL. Microvascular dysfunction in diabetic foot disease and ulceration. Diabetes Metab Res Rev. 2009;25:604-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 148] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 47. | Deng W, Dong X, Zhang Y, Jiang Y, Lu D, Wu Q, Liang Z, Yang G, Chen B. Transcutaneous oxygen pressure (TcPO₂): a novel diagnostic tool for peripheral neuropathy in type 2 diabetes patients. Diabetes Res Clin Pract. 2014;105:336-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 48. | Flynn MD, Edmonds ME, Tooke JE, Watkins PJ. Direct measurement of capillary blood flow in the diabetic neuropathic foot. Diabetologia. 1988;31:652-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 77] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 49. | Byeon JY, Kwak SH, Choi HJ, Kim JH, Lee DW. Clinical significance of early venous enhancement on CT angiography of the ischemic lower limbs. Medicine (Baltimore). 2022;101:e30560. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (1)] |
| 50. | Gascoigne AC, Taylor GI, Corlett RJ, Briggs C, Ashton MW. Increasing Perfusion Pressure Does Not Distend Perforators or Anastomoses but Reveals Arteriovenous Shuntings. Plast Reconstr Surg Glob Open. 2020;8:e2857. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |