Published online Oct 26, 2023. doi: 10.12998/wjcc.v11.i30.7475
Peer-review started: August 21, 2023
First decision: September 13, 2023
Revised: September 25, 2023
Accepted: October 8, 2023
Article in press: October 8, 2023
Published online: October 26, 2023
Processing time: 65 Days and 6.6 Hours
Although neonatal Staphylococcus aureus pneumonia is common and usually curable, it can also be refractory and life-threatening. Herein, we report a case of severe neonatal community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA) necrotizing pneumonia with bilateral recurrent pyopneumothorax, respiratory failure, heart failure, and cardiac arrest. We hope our report will add to the understanding of this disease.
An 18-d-old boy presented with cough for five days, fever for three days, and dyspnea for two days. Preadmission chest radiograph revealed high-density shadows in both lungs. On admission, his oxygen saturation fluctuated around 90% under synchronized intermittent mandatory ventilation. He was uncon
Neonatal MRSA pneumonia can be refractory and lethal, especially in cases where necrotizing pneumonia leads to extensive lung necrosis and recurrent pneumothorax. Despite treatment with linezolid and other medical measures, it may still be ineffective. Currently, ECMO has been a remedial therapy, but if the lung tissue is too severely eroded to be repaired, it may be useless unless the infection can be controlled and lung transplantation can be performed. Regardless of whether ECMO is initiated, the key to successful treatment is to achieve control over the pneumonia caused by MRSA as soon as possible and to reverse lung injury as much as possible.
Core Tip: Neonatal pneumonia can usually be prevented, controlled and cured, regardless of whether it is caused by hospital-associated methicillin-resistant Staphylococcus aureus or community-acquired methicillin-resistant Staphylococcus aureus, but sometimes it is refractory or incurable. We report a case of severe neonatal community-acquired methicillin-resistant Staphylococcus aureus necrotizing pneumonia with bilateral recurrent pyopneumothorax, respiratory failure, heart failure, and cardiac arrest. We hope our report furthers our understanding of this disease.
- Citation: Li XC, Sun L, Li T. Neonatal methicillin-resistant Staphylococcus aureus pneumonia–related recurrent fatal pyopneumothorax: A case report and review of literature. World J Clin Cases 2023; 11(30): 7475-7484
- URL: https://www.wjgnet.com/2307-8960/full/v11/i30/7475.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i30.7475
With the development and widespread application of antibiotics, methicillin-resistant Staphylococcus aureus (MRSA) has spread globally[1-3], exhibiting multidrug resistance and extensive colonization[1-2,4-5]. MRSA causes both hospital-associated infections and community-acquired infections[2,6-10]. In addition to skin and soft tissue infections (SSTIs)[5,9], MRSA can lead to pneumonia, sepsis, intracranial infections, etc.[6,9], which are common in the elderly population, children, patients with neutrophil dysfunction, and inpatients[6]. Pneumonia caused by MRSA is severe and refractory, with acute onset, rapid progression and high mortality, manifested by repeated episodes of fever, symptoms of severe infection and dyspnea, and it can lead to shock and multiple-organ failure[11-12]. The characteristics of MRSA pneumonia on chest imaging vary, including such features as pneumothorax[11], empyema[11,13], and pneumatoceles[11,14-15]. Neonatal MRSA infections usually occur in critically ill newborns with long-term hospitalization[16-17], such as extremely premature infants[18-19], low-birth-weight infants[17,20], term infants undergoing surgery[18], and neonates with congenital heart disease[17]. MRSA infections in newborns can also involve multiple systems (e.g., SSTIs[17], pneumonia[13-15,17,19,21], bacteremia[13,15,17], central nervous system infections[17], bone and joint infections[17,22], peritonitis[17], and liver abscess[23]) and cause related injuries that are severe and refractory[17,23].
Hospital-associated MRSA (HA-MRSA) infection is common[19,24-26], while community-acquired MRSA (CA-MRSA) infection[6,21,27], especially severe neonatal CA-MRSA refractory necrotizing pneumonia[13,15], is relatively rare. Although some neonates with MRSA pneumonia will recover[13-15,21], some cases cannot be cured[17]. Caregivers must pay attention to the importance of hand hygiene and other protective measures in the daily care of newborns and try to avoid MRSA infections in multiple ways[16,20,26,28-29].
Herein, we report a case of severe neonatal CA-MRSA necrotizing pneumonia with bilateral recurrent pyopneumothorax, respiratory failure, heart failure, and cardiac arrest.
An 18-d-old boy presented with cough for five days, fever for three days, and dyspnea for two days.
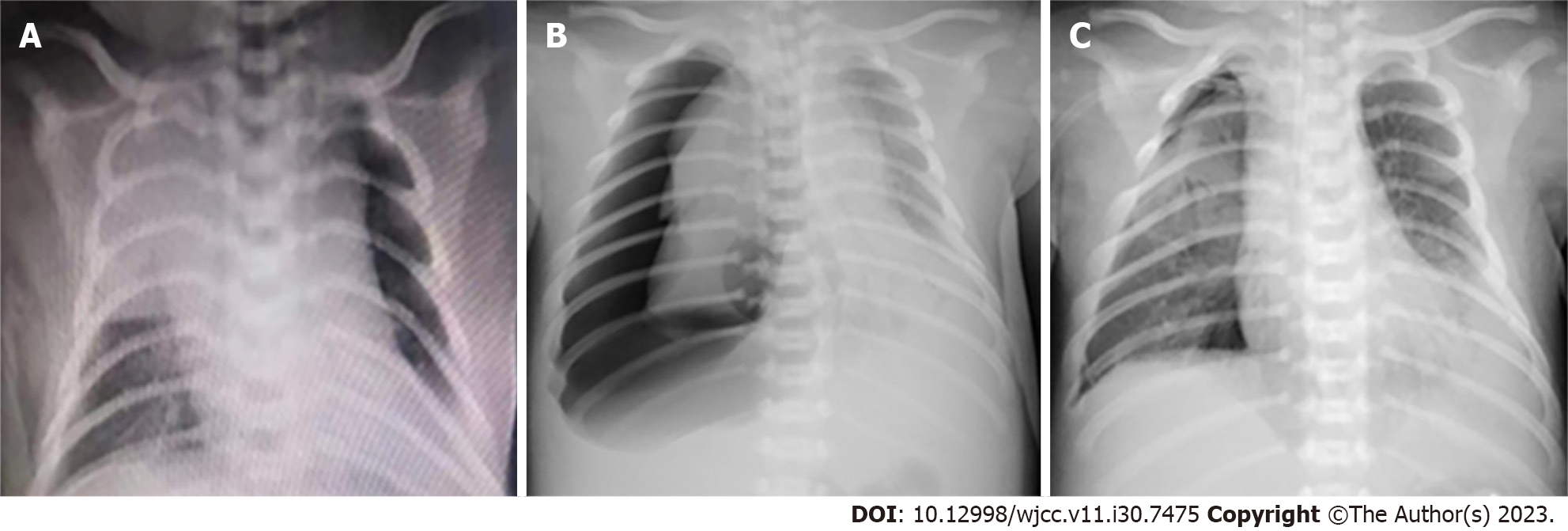
His body temperature peaked at 37.5 °C. Chest radiograph revealed high-density shadows in both lungs (Figure 1A). Antibiotics (meropenem and vancomycin) and mechanical ventilation had been performed at the local hospital. For further treatment, the newborn was transferred to our neonatal intensive care unit through a vehicle-mounted ventilator by ambulance.
The boy, G3P2, was born to a 34-year-old woman at 38 wk 3 d of gestational age with a birth weight of 4.5 kg and an Apgar score of 9 points at 1 min after birth. His parents were not close relatives and were in good health. They lived in a rural area.
He had a fever of 38.2 °C, a heart rate of 210 beats/min, a respiratory rate of 100 breaths/min, a blood pressure of 95/65 mmHg, and a body weight of 4.75 kg. His oxygen saturation fluctuated around 90% under synchronized intermittent mandatory ventilation (FiO2 100%). He was unconscious, with dyspnea, weak heart sounds and hepatomegaly. Moist crackles were present throughout his left lung, while the breath sounds in the right lung were decreased.
Changes in leukocyte, erythrocyte, hemoglobin, platelet, neutrophil (%), C-reactive protein, creatine kinase-MB, lactate dehydrogenase, and hydroxybutyrate dehydrogenase parameters (Table 1) indicated that the above items had increased to varying degrees, except for the decreases in erythrocytes, hemoglobin, and platelets. MRSA infection was confirmed by multiple cultures (sputum and pleural fluid) (Table 2), while other pathogen tests, such as Mycoplasma pneumoniae, Chlamydia pneumoniae, cytomegalovirus, herpes simplex virus, rubella virus, Toxoplasma gondii, Mycobacterium tuberculosis, adenovirus, parainfluenza virus 2, parainfluenza virus 3, human coronavirus 229E/NL63, influenza virus A, rhinovirus, and respiratory syncytial virus, along with cerebrospinal fluid culture and blood cultures, were all negative.
| DOH 1 | DOH 2 | DOH 3 | DOH 4 | DOH 5 | DOH 6 | DOH 7 | DOH 8 | DOH 9 | DOH 10 | DOH 11 | DOH 12 | |
| Leukocyte (109/L) | 6.69 | 5.18 | 12.58 | 22.31 | 75.77 | 35.57 | 43.45 | 35.73 | 27.9 | 25.73 | 22.69 | 19.95 |
| Erythrocyte (1012/L) | 3.78 | 3.36 | 3.14 | 3.18 | 3.44 | 3.44 | 3.44 | 2.93 | 3.13 | 3.92 | 3.12 | 3.09 |
| Hemoglobin (g/L) | 134 | 119 | 113 | 111 | 125 | 122 | 122 | 102 | 109 | 133 | 106 | 103 |
| Platelet (109/L) | 290 | 197 | 199 | 187 | 269 | 105 | 149 | 166 | 88 | 169 | 115 | 95 |
| Neutrophil (%) | 63.2 | 75.6 | 75.6 | 74.9 | 80.6 | 80.0 | NA | 79.9 | 76.6 | 75.5 | 80.4 | 80.4 |
| CRP (mg/L) | 290.41 | 260.3 | 152.96 | 114.7 | 132.6 | 128.0 | 227.3 | 187.8 | 151.2 | 129.0 | 126.6 | 229.7 |
| CK-MB (U/L) | - | - | - | 171 | 150 | - | 47 | - | - | - | - | - |
| LDH (U/L) | - | - | - | 865 | 1885 | - | 1128 | - | - | - | - | - |
| HBDH (U/L) | - | - | - | 548 | 1006 | - | 684 | - | - | - | - | - |
| DOH 6 | DOH 6 | DOH 6 | DOH 6 | DOH 10 | DOH 12 | |
| Specimen | Sputum | Sputum | Sputum | Pleural fluid | Pleural fluid | Pleural fluid |
| Pathogen | MRSA | MRSA | MRSA | MRSA | MRSA | MRSA |
| Chloromycetin | S | S | S | S | S | S |
| Erythromycin | R | R | R | R | R | R |
| Cefoxitin | R | R | R | R | R | R |
| Gentamicin | S | S | S | S | S | S |
| Oxacillin | S | R | R | R | S | S |
| Penicillin | R | R | R | R | R | R |
| Rifampin | S | S | S | S | S | S |
| Teicoplanin | S | S | S | S | S | S |
| Vancomycin | S | S | S | S | S | S |
| Clarithromycin | R | R | R | R | R | R |
| Clindamycin | R | R | R | R | R | R |
| Linezolid | S | S | S | S | S | S |
| Tigecycline | S | S | S | S | S | S |
| Amikacin | S | S | S | S | S | S |
| Azithromycin | R | R | R | R | R | R |
| Levofloxacin | S | S | S | S | S | S |
| Moxifloxacin | S | S | S | S | S | S |
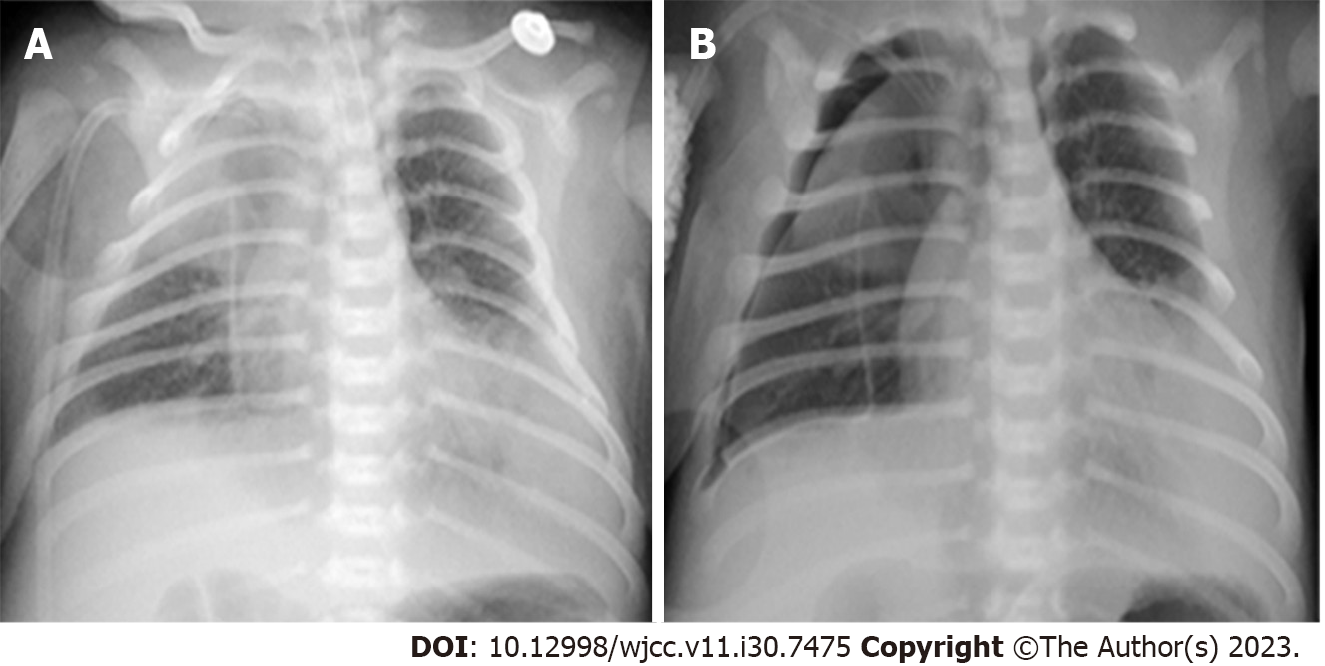
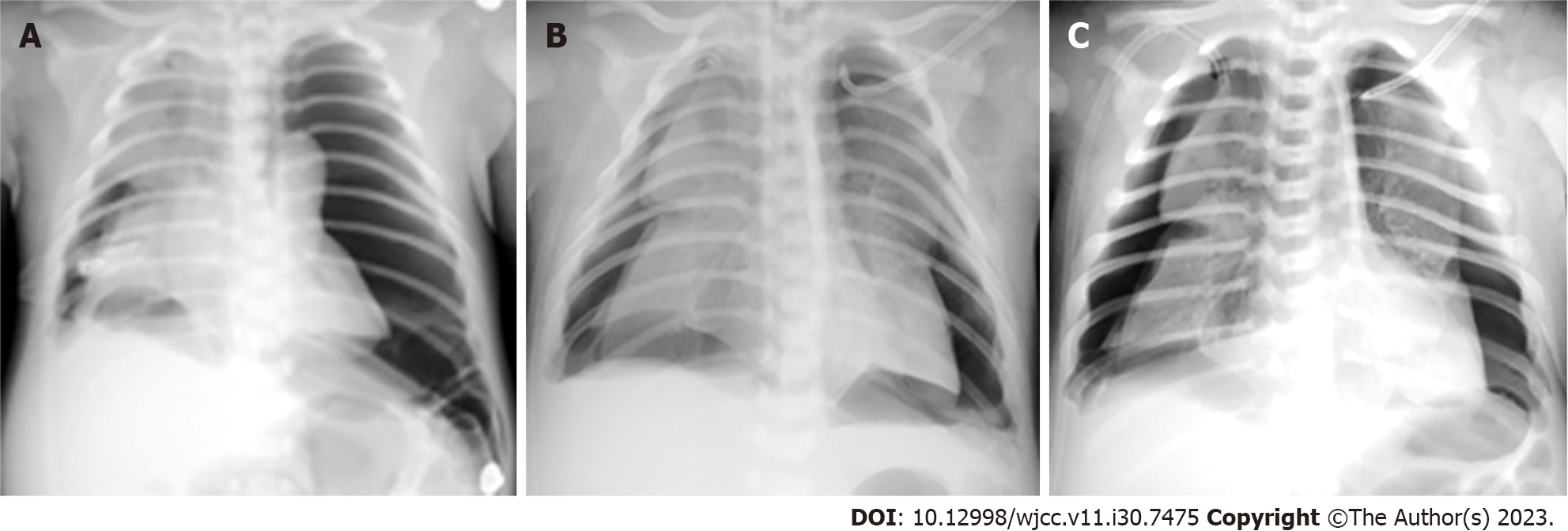
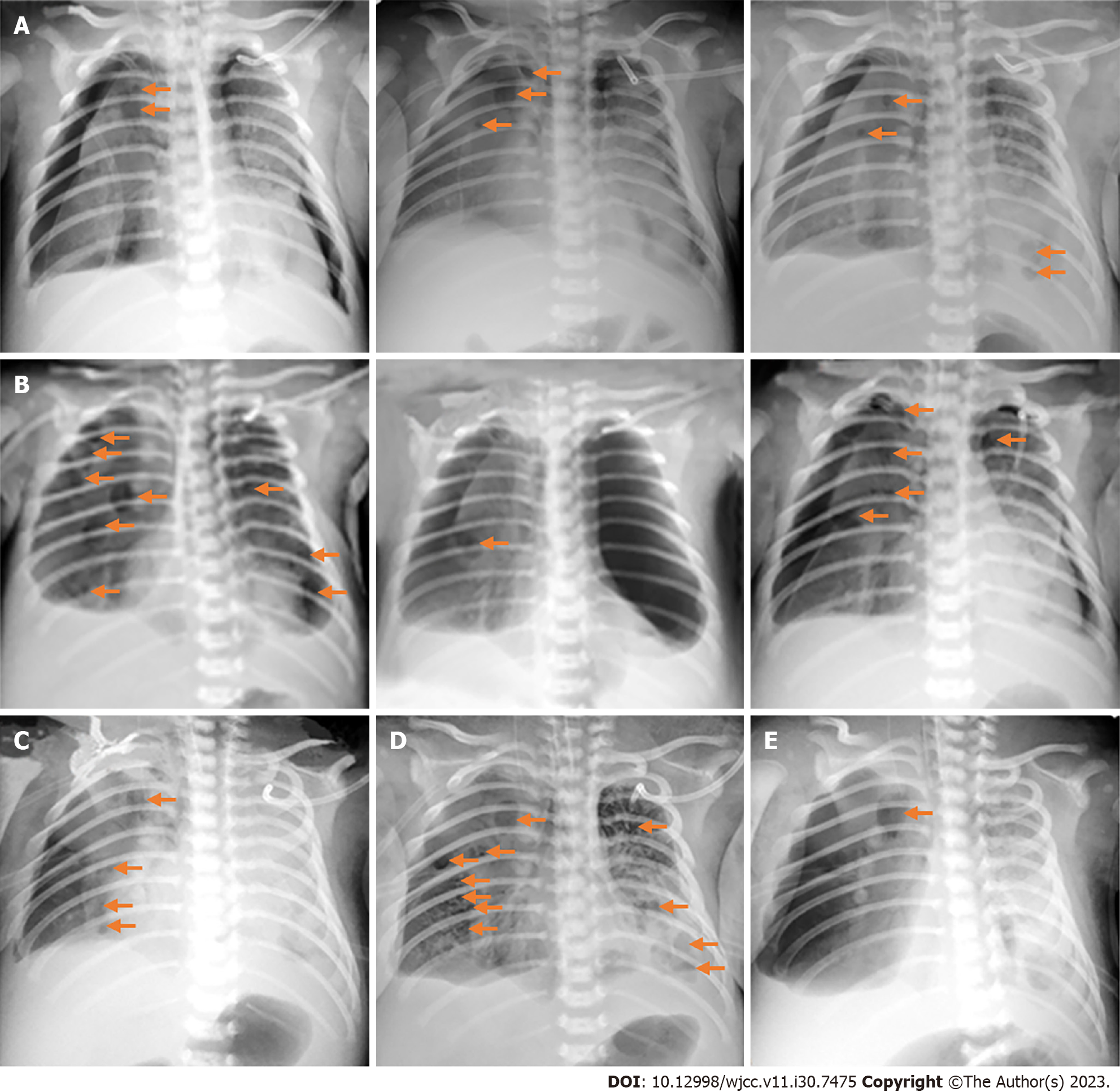
Chest radiographs at the local hospital (Figure 1A) and in Taihe Hospital (Figure 1B and C and Figures 2-4) are shown in detail in the History of present illness and Treatment sections, respectively. The latter revealed recurrent bilateral pyopneumothorax, multiple high-density shadows and pneumatoceles (Figure 4, orange arrows) in both lungs.
According to the clinical definition of CA-MRSA infection used by the United States Centers for Disease Control and Prevention[30], the diagnosis was severe neonatal CA-MRSA necrotizing pneumonia.
Upon admission, after timely high-frequency oscillatory ventilation (HFOV), empiric antimicrobials (meropenem 40 mg/kg IV q8h plus vancomycin 15 mg/kg IV q8h), improved circulation, and right pleural cavity drainage for right pneumothorax (approximately 90% compression) (Figure 1B), his oxygen saturation level stayed above 95%, and recruitment of the right lung was observed (Figure 1C). Dynamic monitoring was performed by chest radiography (Figure 2), and his condition did not deteriorate until the 5th day of hospitalization (DOH 5).
On the morning of DOH 5, his oxygen saturation decreased. Subsequent chest radiograph showed bilateral pneumothorax with nearly 100% compression of the left lung (Figure 3A). Desaturation was not relieved after urgent left pleural cavity drainage, and cardiac arrest with nonshockable rhythm occurred soon thereafter. Emergency neonatal cardiopulmonary resuscitation (CPR) (including intravenous 1:10000 epinephrine 0.2 mL/kg every 3 to 5 min) was performed for 42 min, but he still had no spontaneous heartbeat. Then, his spontaneous heartbeat returned within 18 minutes by adjusting the resuscitation [infusion of 5% sodium bicarbonate (3 mL/kg), 1:10000 epinephrine (0.2 mL/kg), atropine (0.02 mg/kg), and 10% calcium gluconate (2 mL/kg) via a preset peripherally inserted central catheter (PICC) sequentially], and the left lung was partially recruited (Figure 3B and C).
Afterward, MRSA infection was confirmed by multiple cultures (Table 2). Although salvage antibacterial therapy (linezolid 10 mg/kg IV q8h plus levofloxacin 10 mg/kg IV q12h) was administered on DOH 6 according to the culture and antimicrobial susceptibility test results and the child did not experience cardiac arrest again, his condition showed no improvement. He had recurrent pyopneumothorax (Figure 4) and continued drainage of purulent fluid and necrotic lung tissue fragments from the pleural cavity.
Eventually, owing to the high probability of poor prognosis, the parents refused extracorporeal membrane oxygenation (ECMO) and gave up all the treatments, and the newborn passed away soon after withdrawal on DOH 13.
As this case shows, neonatal MRSA pneumonia can be extremely challenging. The following aspects of this case are discussed in a clinical problem-oriented manner.
On admission, given the severity and rapid progression of the child’s condition and the presence of pneumothorax, the primary diagnosis was severe neonatal community-acquired pneumonia. Initial antibacterial therapy with meropenem and vancomycin was administered for coverage of gram-negative bacteria and gram-positive bacteria.
Afterward, the diagnosis of severe neonatal CA-MRSA necrotizing pneumonia with bilateral pyopneumothorax was made. Based on the culture and antimicrobial susceptibility test results (Table 2) and a literature review, the antibiotics were switched to linezolid[9,31-35] and levofloxacin[36] for further salvage antibacterial therapy.
First, pathogens such as MRSA invade the body, proliferate, and cause damage in a gradual process. Second, neonatal diseases (including MRSA pneumonia) tend to be atypical and relatively less serious in the early stage. Third, MRSA strains should proliferate to a certain critical level and cause extensive damage before the patient’s outward condition rapidly deteriorates. Furthermore, the various treatments administered (e.g., vancomycin, HFOV, and pleural cavity drainage in this case) may have slowed the progression of the disease to a certain extent. However, the initial vancomycin probably could not eliminate the accumulated MRSA from the lungs, thereby increasing the chance of rapid replication of MRSA[33]. All of the above factors may have contributed to the transient stability of the child’s condition after initial treatment and the subsequent rapid deterioration. Most importantly, this strain of MRSA caused severe necrotizing pneumonia with extensive necrosis of the lung tissue and recurrent pyopneumothorax, which may be the primary reason for the rapid deterioration of this child’s condition.
Compared with vancomycin[35,37], linezolid has a stronger ability to penetrate tissues and is more likely to concentrate in the lungs[35,38-39], which may be more helpful for controlling pneumonia caused by MRSA[7,33,35]. Although MRSA detection methods have improved[1,29], it is still difficult to make an early diagnosis of MRSA infection and to determine which antibiotics will be effective in a timely manner, let alone to make an accurate prognostic prediction early on. Thus, it is difficult to choose linezolid as the initial therapy.
Acute respiratory and circulatory dysfunction secondary to myocardial damage (e.g., weak heart sounds, elevated creatine kinase-MB), heart failure and pneumothorax resulting from severe necrotizing pneumonia caused by MRSA led to cardiac arrest in this patient. Among these factors, pneumothorax was the most important, and the failure of conventional neonatal CPR may also be related to the negative effects of pneumothorax on his breathing and circulation.
In our case, apart from continuing to address the pneumothorax, the resuscitation strategy should be adjusted in a timely manner. While undergoing infusion of sodium bicarbonate, the child still had no heartbeat. Upon reinjection of 1:10000 epinephrine, although he had not yet regained his own heartbeat, short bursts of ventricular escape rhythm occurred. After administration of atropine, his spontaneous sinus rhythm was elicited, and the weak heart sounds became stronger subsequent to infusion of 10% calcium gluconate. Soon afterward, the oxygen saturation rose to 97%. All first-aid drugs were injected via a preset PICC. If the above treatments do not work, extracorporeal cardiopulmonary resuscitation (ECPR) could be attempted[40].
Prolonged asphyxia can lead to acidosis[41-42], which not only inhibits cardiac contractility[42-43] but also reduces the sensitivity of cardiomyocytes to catecholamine[43] and can even result in death[42]. Therefore, when the effect of conventional neonatal CPR is not ideal, to antagonize the inhibition of cardiomyocytes by acidosis, sodium bicarbonate should be promptly injected to increase the blood pH[42]. In this patient, after a subsequent administration of epinephrine, short bursts of ventricular escape rhythm were observed, indicating that the myocardial inhibition had been partially relieved and that the sensitivity of cardiomyocytes to epinephrine had been partially restored after infusion of sodium bicarbonate, although sinus node function remained poor. Given the repeated administration of epinephrine, atropine was needed immediately to improve sinus node automaticity to restore sinus rhythm[43-44], and it was successful in this patient. At this time, although the sinus rhythm had been restored, the myocardial contraction was too weak to support adequate circulation. Prolonged asphyxia can not only lead to myocardial injury and depression[42] but also lead to hypocalcemia[45], which may further reduce the contractility of the already hypoxic and damaged myocardium, thereby decreasing cardiac output and resulting in persistent poor circulation. Neonatal hypocalcemia is common in neonatal asphyxia[45] and during plasma exchange[46]. Generally, intravenous calcium supplementation can quickly enhance myocardial contractility[43], thereby increasing cardiac output and stabilizing circulation, which occurred in this patient. The success of resuscitation in this case also benefited from the witnessed in-hospital cardiac arrest, the preexisting PICC, and the close monitoring of his heart rhythm. However, the abovementioned drugs also have potential side effects that cannot be ignored. For instance, calcium may lead to myocardial damage, while sodium bicarbonate can cause hypercapnia, metabolic alkalosis, hypernatremia and hyperosmolality[43]. Therefore, during neonatal CPR, these drugs should not be used initially (unless there are indications), let alone routinely[43].
This is possible, but the following factors should be considered. First, the chief indications for neonatal ECMO are that the primary disease is reversible and that the patient meets certain criteria, such as the absence of lethal congenital malformations, irreversible organ damage (unless transplantation is considered), severe intracranial hemorrhage, and uncontrollable bleeding[40]. Second, ECMO can only temporarily replace cardiopulmonary function, gaining some time to allow the comprehensive treatment of primary disease[47]. In this patient, it is unclear whether the MRSA infection could ultimately be controlled and whether the recurrent pyopneumothorax could be eliminated, especially given the extensive erosion of the lung tissue. If those conditions could not be met and lung transplantation could not be performed, ECMO would certainly fail. Moreover, ECMO itself can also lead to infection and organ dysfunction[47]. In short, ECMO has its own pros and cons[47]. If ECMO is used relatively early, it may be beneficial for reversing the course of a disease, but its premature application, especially its long-term application, may also lead to many ECMO-related complications, while its delayed initiation will inevitably decrease the success rate.
Neonatal ECMO is commonly used in patients with persistent pulmonary hypertension of the newborn (ECMO survival: 73%), meconium aspiration syndrome (ECMO survival: 92%), neonatal sepsis (ECMO survival: 45%), neonatal pneumonia (ECMO survival: 60%), congenital diaphragmatic hernia (ECMO survival: < 50%), ECPR (ECMO survival: 40%-50%), etc.[40] This child’s lungs were so severely damaged by MRSA that he experienced recurrent lethal pyopneumothorax. If the MRSA infection cannot be controlled and/or the damaged lungs cannot be repaired, the prognosis will be poor, which is not even ameliorated by ECMO. However, during the coronavirus disease 2019 (COVID-19) pandemic, lung transplantation has been performed for COVID-19 patients supported with ECMO, improving the prognosis of COVID-19 patients with irreversible infection-related lung injury[48-52]. Moreover, a 7-year-old girl recovered from severe inhalational burn injury and secondary Pseudomonas necrotizing pneumonia after 605 d of ECMO support, providing us with new insight into “irreversible” lung injury and the exciting possibility of lung tissue regeneration or recovery[53]. Regrettably, long-term ECMO support and lung transplantation in newborns are difficult, expensive and challenging.
Neonatal MRSA pneumonia can be refractory and lethal, especially in cases where necrotizing pneumonia leads to extensive lung necrosis and recurrent pneumothorax. Despite treatment with linezolid and other medical measures, it may still be ineffective. ECMO has been a remedial therapy, but if the lung tissue is too severely eroded to be repaired, it may be useless unless the infection can be controlled and lung transplantation can be performed. Regardless of whether ECMO is initiated, the key to successful treatment is to achieve control over the pneumonia caused by MRSA as soon as possible and to reverse lung injury as much as possible.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Schwan WR, United States; Sunder T, India S-Editor: Li L L-Editor: A P-Editor: Yu HG
| 1. | Miao J, Chen L, Wang J, Wang W, Chen D, Li L, Li B, Deng Y, Xu Z. Current methodologies on genotyping for nosocomial pathogen methicillin-resistant Staphylococcus aureus (MRSA). Microb Pathog. 2017;107:17-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 2. | Guo J, Xie Z, Ruan W, Tang Q, Qiao D, Zhu W. Thiazole-based analogues as potential antibacterial agents against methicillin-resistant Staphylococcus aureus (MRSA) and their SAR elucidation. Eur J Med Chem. 2023;259:115689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 3. | Wei J, Wang Y, Chen C, Lin J. Risk Factors Associated with Methicillin Resistance in Hospitalized Newborn Infants with Staphylococcus aureus Infection. Infect Drug Resist. 2022;15:2921-2928. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 4. | Zaghen F, Sora VM, Meroni G, Laterza G, Martino PA, Soggiu A, Bonizzi L, Zecconi A. Epidemiology of Antimicrobial Resistance Genes in Staphyloccocus aureus Isolates from a Public Database in a One Health Perspective-Sample Characteristics and Isolates' Sources. Antibiotics (Basel). 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 5. | Su W, Liu Y, Wang Q, Yuan L, Gao W, Yao KH, Yang YH, Ma L. Antibiotic susceptibility and clonal distribution of Staphylococcus aureus from pediatric skin and soft tissue infections: 10-year trends in multicenter investigation in China. Front Cell Infect Microbiol. 2023;13:1179509. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 6. | Kumar P. A review on quinoline derivatives as anti-methicillin resistant Staphylococcus aureus (MRSA) agents. BMC Chem. 2020;14:17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 7. | Algammal AM, Hetta HF, Elkelish A, Alkhalifah DHH, Hozzein WN, Batiha GE, El Nahhas N, Mabrok MA. Methicillin-Resistant Staphylococcus aureus (MRSA): One Health Perspective Approach to the Bacterium Epidemiology, Virulence Factors, Antibiotic-Resistance, and Zoonotic Impact. Infect Drug Resist. 2020;13:3255-3265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 213] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 8. | Hsieh RC, Liu R, Burgin DJ, Otto M. Understanding mechanisms of virulence in MRSA: implications for antivirulence treatment strategies. Expert Rev Anti Infect Ther. 2023;21:911-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 9. | Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, Kaplan SL, Karchmer AW, Levine DP, Murray BE, J Rybak M, Talan DA, Chambers HF. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis. 2011;52:285-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1171] [Cited by in RCA: 1247] [Article Influence: 89.1] [Reference Citation Analysis (0)] |
| 10. | David MZ, Daum RS. Community-associated methicillin-resistant Staphylococcus aureus: epidemiology and clinical consequences of an emerging epidemic. Clin Microbiol Rev. 2010;23:616-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1363] [Cited by in RCA: 1423] [Article Influence: 94.9] [Reference Citation Analysis (0)] |
| 11. | Vardakas KZ, Matthaiou DK, Falagas ME. Incidence, characteristics and outcomes of patients with severe community acquired-MRSA pneumonia. Eur Respir J. 2009;34:1148-1158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 74] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 12. | Arumairaj A, Safavi A, Amin H, Poor A, Trenard N. Methicillin-Resistant Staphylococcus aureus (MRSA) Empyema Post-COVID Infection Causing Severe Septic Shock and Multiorgan Failure. Cureus. 2023;15:e41054. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 13. | Zapata H, Wahba A. Severe necrotizing pneumonia complicated by empyema in a neonate. Respir Med Case Rep. 2020;31:101248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 14. | Perdue S, Newberry D. Methicillin-Resistant Staphylococcus aureus Pneumatoceles in a Neonate With Sotos Syndrome : A Case Report. Adv Neonatal Care. 2023;23:409-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 15. | Yee-Guardino S, Kumar D, Abughali N, Tuohy M, Hall GS, Kumar ML. Recognition and treatment of neonatal community-associated MRSA pneumonia and bacteremia. Pediatr Pulmonol. 2008;43:203-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Heigl K, Zamfir M, Adler AC, Dammeyer A, Schomacher L, Karlin B, Franitza M, Hörmansdorfer S, Tuschak C, Valenza G, Ochmann U, Herr C, Heinze S. Prevalence of methicillin-sensitive, methicillin-resistant Staphylococcus aureus, and extended-spectrum beta-lactamase-producing Escherichia coli in newborns: a cross-sectional study. J Matern Fetal Neonatal Med. 2022;35:4243-4249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Wu X, Wang C, He L, Xu H, Jing C, Chen Y, Deng J, Lin A, Deng H, Cai H, Yang J, Zhang T, Cao Q, Hao J, Huang Y, Yu H. Clinical characteristics and antibiotic resistance profile of invasive MRSA infections in newborn inpatients: a retrospective multicenter study from China. BMC Pediatr. 2023;23:264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 18. | Carey AJ, Duchon J, Della-Latta P, Saiman L. The epidemiology of methicillin-susceptible and methicillin-resistant Staphylococcus aureus in a neonatal intensive care unit, 2000-2007. J Perinatol. 2010;30:135-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 19. | Salerno SN, Bernhardt J, Laughon M, Jhaveri R, Massaro M, Gonzalez D. Pharmacokinetics of Ceftaroline in a Preterm Infant With Methicillin-Resistant Staphylococcus Aureus Pneumonia. J Pediatric Infect Dis Soc. 2018;7:342-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 20. | Sakaki H, Nishioka M, Kanda K, Takahashi Y. An investigation of the risk factors for infection with methicillin-resistant Staphylococcus aureus among patients in a neonatal intensive care unit. Am J Infect Control. 2009;37:580-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 21. | Nicolaou EV, Bartlett AH. Necrotizing Pneumonia. Pediatr Ann. 2017;46:e65-e68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Sankaran G, Zacharia B, Roy A, Purayil SP. Current clinical and bacteriological profile of septic arthritis in young infants: a prospective study from a tertiary referral centre. Eur J Orthop Surg Traumatol. 2018;28:573-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Heger ML, Al-Sayyad B. Ceftaroline and Daptomycin Combination Antibiotic Therapy for a Methicillin-Resistant Staphylococcus Aureus Liver Abscess in a Premature Infant. J Pediatr Pharmacol Ther. 2022;27:754-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 24. | Chew CH, Yeo CC, Che Hamzah AM, Al-Trad EI, Jones SU, Chua KH, Puah SM. Multidrug-Resistant Methicillin-Resistant Staphylococcus aureus Associated with Hospitalized Newborn Infants. Diagnostics (Basel). 2023;13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 25. | Shi ZY, Hou SL, Li XW. Silver dressing in the management of an infant's urachal anomaly infected with methicillin-resistant Staphylococcus aureus: A case report. World J Clin Cases. 2022;10:2629-2636. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Barrett RE, Fleiss N, Hansen C, Campbell MM, Rychalsky M, Murdzek C, Krechevsky K, Abbott M, Allegra T, Blazevich B, Dunphy L, Fox A, Gambardella T, Garcia L, Grimm N, Scoffone A, Bizzarro MJ, Murray TS. Reducing MRSA Infection in a New NICU During the COVID-19 Pandemic. Pediatrics. 2023;151. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 27. | Budimir A. MRSA in Croatia: prevalence and management. Expert Rev Anti Infect Ther. 2016;14:167-176. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 28. | Tidwell J, Kirk L, Luttrell T, Pike CA. CA-MRSA Decolonization Strategies: Do They Reduce Recurrence Rate? J Wound Ostomy Continence Nurs. 2016;43:577-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 29. | Hassoun A, Linden PK, Friedman B. Incidence, prevalence, and management of MRSA bacteremia across patient populations-a review of recent developments in MRSA management and treatment. Crit Care. 2017;21:211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 351] [Cited by in RCA: 393] [Article Influence: 49.1] [Reference Citation Analysis (0)] |
| 30. | Pichereau S, Rose WE. Invasive community-associated MRSA infections: epidemiology and antimicrobial management. Expert Opin Pharmacother. 2010;11:3009-3025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 31. | Kullar R, Sakoulas G, Deresinski S, van Hal SJ. When sepsis persists: a review of MRSA bacteraemia salvage therapy. J Antimicrob Chemother. 2016;71:576-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 32. | Rae N, Jarchow-MacDonald A, Nathwani D, Marwick CA. MRSA: treating people with infection. BMJ Clin Evid. 2016;2016. [PubMed] |
| 33. | Wunderink RG, Rello J, Cammarata SK, Croos-Dabrera RV, Kollef MH. Linezolid vs vancomycin: analysis of two double-blind studies of patients with methicillin-resistant Staphylococcus aureus nosocomial pneumonia. Chest. 2003;124:1789-1797. [PubMed] |
| 34. | Chavanet P. The ZEPHyR study: a randomized comparison of linezolid and vancomycin for MRSA pneumonia. Med Mal Infect. 2013;43:451-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 35. | Pletz MW, Burkhardt O, Welte T. Nosocomial methicillin-resistant Staphylococcus aureus (MRSA) pneumonia: linezolid or vancomycin? - Comparison of pharmacology and clinical efficacy. Eur J Med Res. 2010;15:507-513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 36. | Bhagwat SS, Nandanwar M, Kansagara A, Patel A, Takalkar S, Chavan R, Periasamy H, Yeole R, Deshpande PK, Bhavsar S, Bhatia A, Ahdal J, Jain R, Patel M. Levonadifloxacin, a Novel Broad-Spectrum Anti-MRSA Benzoquinolizine Quinolone Agent: Review of Current Evidence. Drug Des Devel Ther. 2019;13:4351-4365. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 37. | Cruciani M, Gatti G, Lazzarini L, Furlan G, Broccali G, Malena M, Franchini C, Concia E. Penetration of vancomycin into human lung tissue. J Antimicrob Chemother. 1996;38:865-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 204] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 38. | Honeybourne D, Tobin C, Jevons G, Andrews J, Wise R. Intrapulmonary penetration of linezolid. J Antimicrob Chemother. 2003;51:1431-1434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 103] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 39. | Conte JE Jr, Golden JA, Kipps J, Zurlinden E. Intrapulmonary pharmacokinetics of linezolid. Antimicrob Agents Chemother. 2002;46:1475-1480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 222] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 40. | Wild KT, Rintoul N, Kattan J, Gray B. Extracorporeal Life Support Organization (ELSO): Guidelines for Neonatal Respiratory Failure. ASAIO J. 2020;66:463-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 65] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 41. | Hoeger H, Labudova O, Mosgoeller W, Herrera-Marschitz M, Fuerst G, Kitzmüller E, Lubec B. Deficient transcription of subunit RPA 40 of RNA polymerase I and III in heart of rats with neonatal asphyxia. Life Sci. 1998;62:275-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 42. | Dawes GS, Jacobson HN, Mott JC, Shelley HJ, Stafford A. The Treatment of Asphyxiated, Mature Foetal Lambs and Rhesus Monkeys with Intravenous Glucose and Sodium Carbonate. J Physiol. 1963;169:167-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 107] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 43. | Papastylianou A, Mentzelopoulos S. Current pharmacological advances in the treatment of cardiac arrest. Emerg Med Int. 2012;2012:815857. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 44. | Yano T, Kawana R, Yamauchi K, Endo G, Nagamine Y. The Additive Effect of Atropine Sulfate during Cardiopulmonary Resuscitation in Out-of-hospital Non-traumatic Cardiac Arrest Patients with Non-shockable Rhythm. Intern Med. 2019;58:1713-1721. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 45. | Aldana Valenzuela C, Romaro Maldonado S, Vargas Origel A, Hernández Arriaga J. [Acute complications in full term neonates with severe neonatal asphyxia]. Ginecol Obstet Mex. 1995;63:123-127. [PubMed] |
| 46. | Sawyer T, Billimoria Z, Handley S, Smith K, Yalon L, Brogan TV, DiGeronimo R. Therapeutic Plasma Exchange in Neonatal Septic Shock: A Retrospective Cohort Study. Am J Perinatol. 2020;37:962-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 47. | Lin JC. Extracorporeal Membrane Oxygenation for Severe Pediatric Respiratory Failure. Respir Care. 2017;62:732-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 48. | Chen JY, Qiao K, Liu F, Wu B, Xu X, Jiao GQ, Lu RG, Li HX, Zhao J, Huang J, Yang Y, Lu XJ, Li JS, Jiang SY, Wang DP, Hu CX, Wang GL, Huang DX, Jiao GH, Wei D, Ye SG, Huang JA, Zhou L, Zhang XQ, He JX. Lung transplantation as therapeutic option in acute respiratory distress syndrome for coronavirus disease 2019-related pulmonary fibrosis. Chin Med J (Engl). 2020;133:1390-1396. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 125] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 49. | Han W, Zhu M, Chen J, Zhang J, Zhu S, Li T, Cai H, Fang Q, Wei G, Liang T. Lung Transplantation for Elderly Patients With End-Stage COVID-19 Pneumonia. Ann Surg. 2020;272:e33-e34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 65] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 50. | Lang C, Jaksch P, Hoda MA, Lang G, Staudinger T, Tschernko E, Zapletal B, Geleff S, Prosch H, Gawish R, Knapp S, Robak O, Thalhammer F, Indra A, Koestenberger M, Strassl R, Klikovits T, Ali K, Fischer G, Klepetko W, Hoetzenecker K, Schellongowski P. Lung transplantation for COVID-19-associated acute respiratory distress syndrome in a PCR-positive patient. Lancet Respir Med. 2020;8:1057-1060. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 100] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 51. | Bharat A, Querrey M, Markov NS, Kim S, Kurihara C, Garza-Castillon R, Manerikar A, Shilatifard A, Tomic R, Politanska Y, Abdala-Valencia H, Yeldandi AV, Lomasney JW, Misharin AV, Budinger GRS. Lung transplantation for pulmonary fibrosis secondary to severe COVID-19. medRxiv. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 52. | Cozzi E, Faccioli E, Marinello S, Loy M, Congedi S, Calabrese F, Romagnoli M, Cattelan AM, Rea F. COVID-19 pneumonia in lung transplant recipients: Report of 2 cases. Am J Transplant. 2020;20:2933-2937. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 53. | Nelson-McMillan K, Vricella LA, Stewart FD, Young J, Shah AS, Hibino N, Coulson JD. Recovery from Total Acute Lung Failure After 20 Months of Extracorporeal Life Support. ASAIO J. 2020;66:e11-e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |