Published online Oct 26, 2023. doi: 10.12998/wjcc.v11.i30.7432
Peer-review started: July 21, 2023
First decision: September 13, 2023
Revised: September 26, 2023
Accepted: October 8, 2023
Article in press: October 8, 2023
Published online: October 26, 2023
Processing time: 95 Days and 19.9 Hours
The prognosis of patients with advanced diffuse large B-cell lymphoma (DLBCL) is poor, with a 5-year survival rate of approximately 50%. The mainstay of treatment is multidrug combination chemotherapy, which has been associated with serious side effects. Amplified natural killer (ANK) cell therapy amplifies and activates natural killer (NK) cells to attack only malignant tumors. As ANK cells attack programmed death ligand 1 (PD-L1)-positive tumor cells, ANK therapy is considered effective against adult T-cell lymphoma and malignant lymphoma.
Herein, we report a case of an older patient with advanced DLBCL who was successfully treated with ANK immunotherapy. A 91-year-old female visited our hospital with sudden swelling of the right axillary lymph node in April 2022. The patient was diagnosed with stage II disease, given the absence of splenic involvement or contralateral lymphadenopathy. ANK therapy was administered. Six rounds of lymphocyte sampling were performed on July 28, 2022. To reduce the occurrence of side effects, the six samples were diluted by half to obtain 12 samples. Cultured NK cells were administered twice weekly. The treatment efficacy was evaluated by performing computed tomography and serological tests every 1 or 2 mo. The treatment suppressed lesion growth, and the antitumor effect persisted for several months. The patient experienced mild side effects. PD-L1 immunostaining was positive, indicating that the treatment was highly effective.
ANK therapy can be used as a first-line treatment for malignant lymphoma; the PD-L1 positivity rate can predict treatment efficacy.
Core Tip: The current patient was programmed death ligand 1 (PD-L1) positive, and the treatment was highly effective. Accordingly, amplified natural killer (ANK) therapy could be employed as a first-line treatment for malignant lymphoma based on the therapeutic efficacy and minimal side effects. Moreover, the PD-L1 positivity rate may serve as a biomarker for predicting the efficacy of ANK therapy.
- Citation: Nagai K, Nagai S, Okubo Y, Teshigawara K. Diffuse large B-cell lymphoma successfully treated with amplified natural killer therapy alone: A case report. World J Clin Cases 2023; 11(30): 7432-7439
- URL: https://www.wjgnet.com/2307-8960/full/v11/i30/7432.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i30.7432
Malignant lymphomas are hematopoietic tumors that originate from mature lymphocytes. Historically, Hodgkin's first described Hodgkin's lymphoma in 1834. Malignant lymphomas are broadly classified into those derived from B lymphocytes (B-cell) and those derived from T lymphocytes or natural killer (NK) cells (T/NK cells). Currently, the World Health Organization classification is widely used for categorizing this disease[1]. Malignant lymphomas are further classified into three types based on the general growth rate before treatment: Slowly progressive (indolent), rapidly progressive, and very rapidly progressive. In Japan, malignant lymphoma impacts approximately 35000 individuals annually, with an incidence rate of 28 cases per 100000 individuals and 13000 deaths estimated annually[2].
The present case report discusses diffuse large B-cell lymphoma (DLBCL), the largest category of non-Hodgkin's lymphoma. Morphologically, DLBCL is characterized by diffuse proliferation of medium- to large-sized cells, and a heterogeneous case population may develop DLBCL due to histological transformation during the course of follicular or mucosa-assisted lymphoid tissue lymphoma[1]. Malignant lymphoma is diagnosed by histopathological examination of biopsy samples of the lesion. If malignant lymphoma is suspected, surgical incisional biopsy is preferred to obtain a pathological specimen of sufficient size. In addition, ancillary tests, such as flow cytometry, chromosome testing (including the fluorescence in situ hybridization method), and various genetic tests are performed when feasible.
DLBCL is classified into germinal center B-cell-like and activated B-cell-like subtypes based on differences in gene expression derived from cancer cells[3,4]. Furthermore, disease classification can be achieved using the Ann Arbor classification[5]. The International Prognostic Index of the National Comprehensive Cancer Network has been proposed as a prognostic model[6]. Treatment type is determined based on a combination of these factors. Drug therapy forms the treatment basis, which depends on the type of disease. The main pharmacotherapeutic approach involves combination chemotherapy comprising cytotoxic antineoplastic agents: rituximab, cyclophosphamide, vincristine, and prednisolone (R-CHOP) or bendamustine and rituximab therapy for B-cell lymphoma, and doxorubicin, bleomycin, vinblastine, and dacarbazine therapy for Hodgkin’s lymphoma. Radiotherapy may also be administered depending on the location of the lesion.
The recommended treatment for early and advanced-stage DLBCL is 6–8 courses of R-CHOP therapy[7,8]. Regarding prognosis, the 5-year survival rate for early and advanced DLBCL is 58%. However, the prognosis remains markedly poor in the case of resistance to initial treatment or early recurrence. CD19-targeted chimeric antigen receptor (CAR)-T-cell therapy has recently been developed. However, the 1-year survival rate is limited to approximately 50%, and the therapeutic effect is insufficient, accompanied by serious side effects such as cytokine release syndrome, encephalopathy, neurotoxicity, and cytopenia. Hence, a preferred treatment strategy is currently lacking[9].
In 1985, Rosenberg et al[10] introduced immunotherapy at the United States National Cancer Institute, developing a treatment called lymphokine-activated killer (LAK) cell immunotherapy[10,11]. In LAK cell immunotherapy, a large volume of blood, approximately 50 L, is drawn from the patient over five days in a single week, and the extracted lymphocytes are cultured with recombinant interleukin (IL)-2 for 3–4 d to induce LAK cells, which are subsequently injected into the patient over a short period. Although this treatment showed a certain level of efficacy, it is not commonly available owing to its high cost and intense side effects. The amplified NK (ANK) immunotherapy used in the current case focuses on the fact that NK cells exert the strongest anti-cancer activity among various lymphocytes. The amount of blood collected is ~5 L; however, by increasing the number and activity of NK cells and returning them to the patient, a safe and highly effective treatment can be provided.
Theoretically, ANK immunotherapy is effective against all cancers. This unique treatment method, researched and developed by Lymphocyte Bank Co., Ltd., is distinct from conventional immunotherapy, including LAK and CAR-T-cell therapy[12-14].
A 91-year-old female visited our hospital with sudden swelling of the right axillary lymph node.
The symptom was observed in April 2022.
The patient developed reflux esophagitis and a hiatus hernia at 85 and 86 years of age, respectively.
No relevant family history was reported.
No special notes.
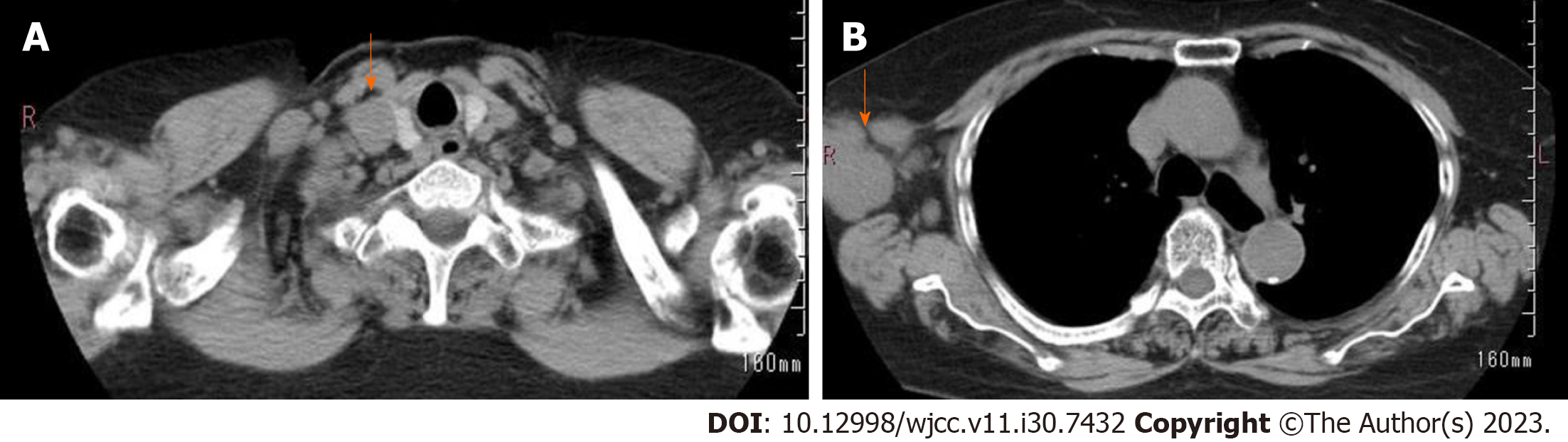
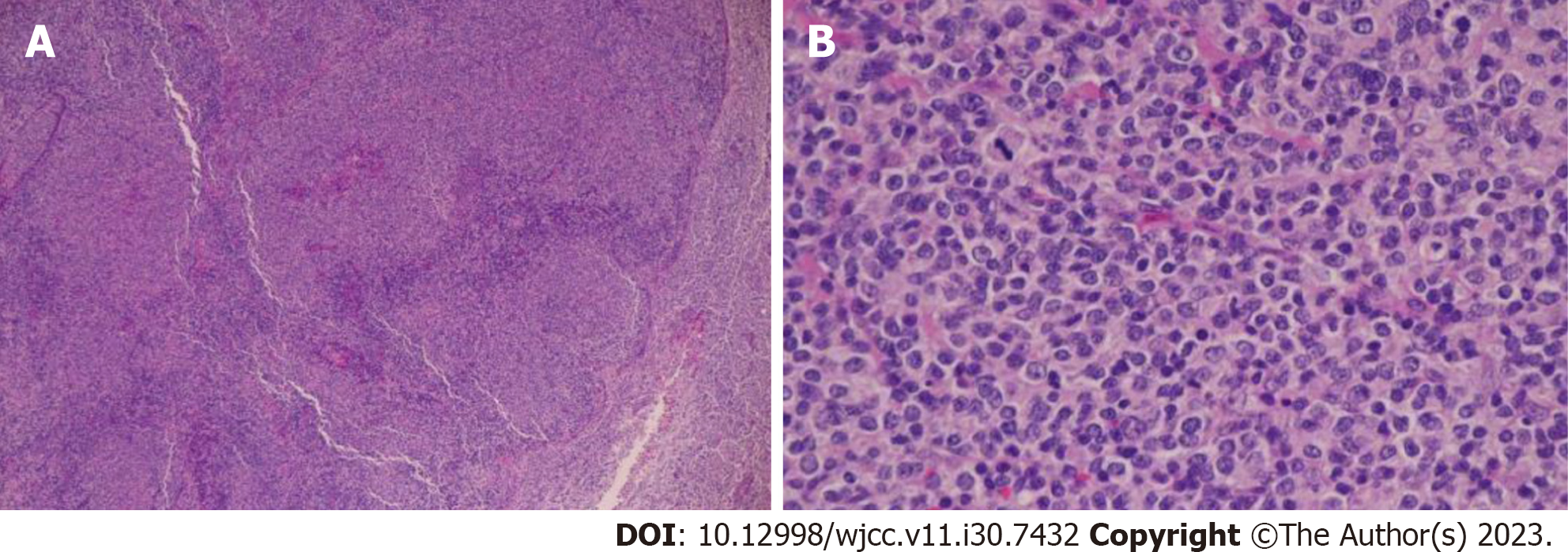
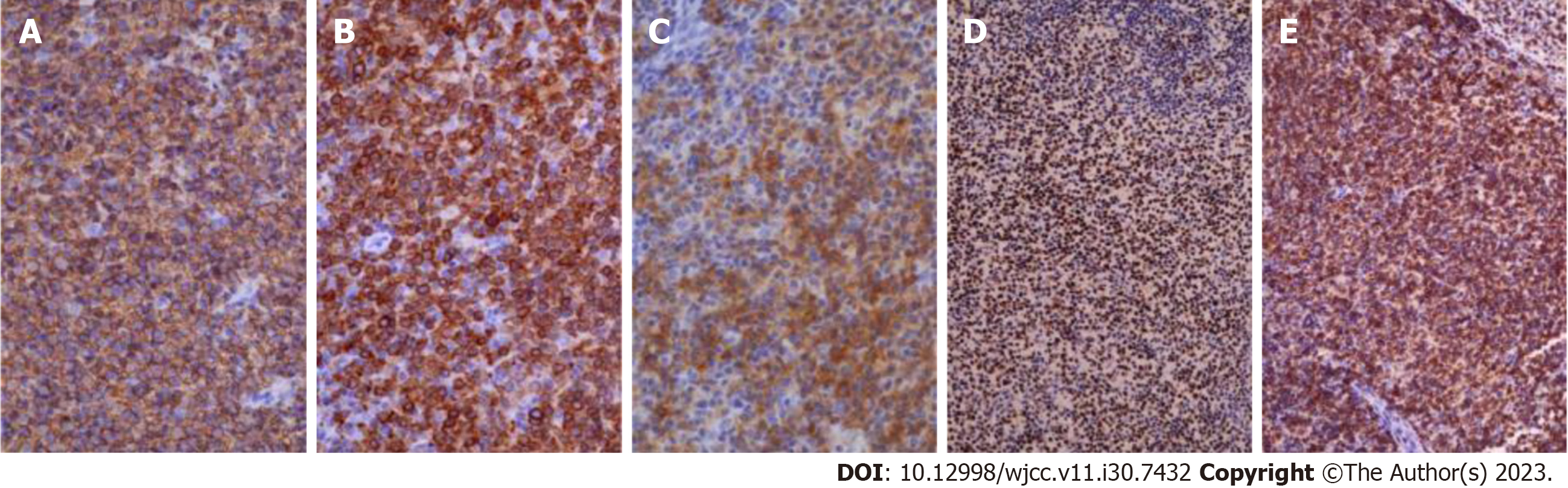
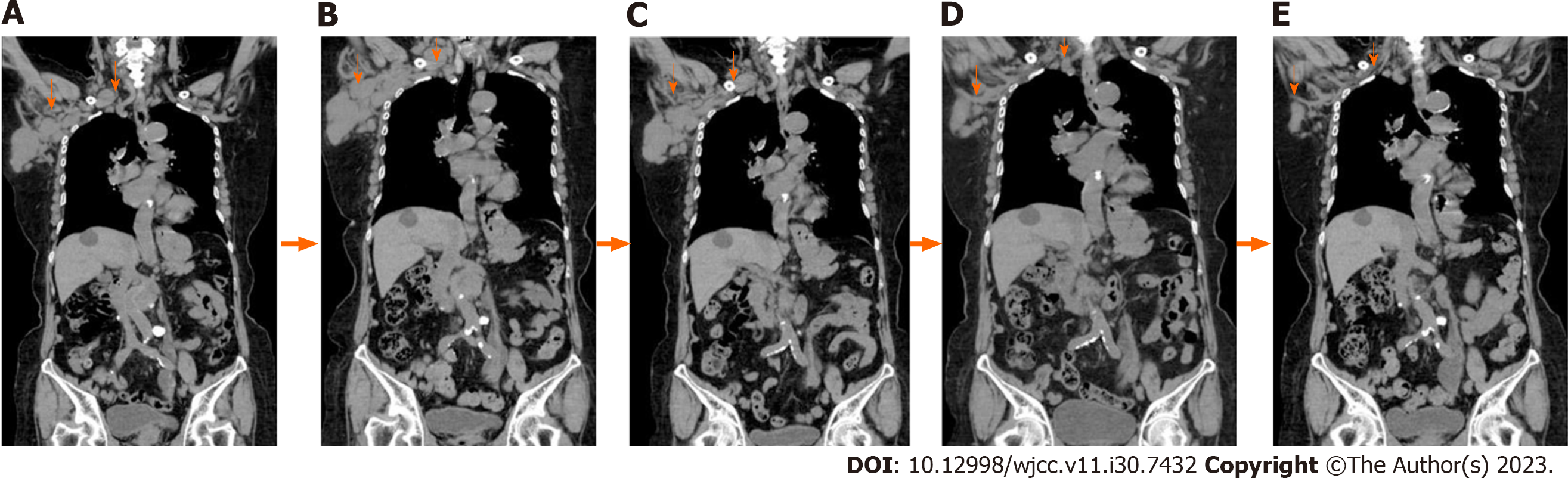
Detailed laboratory data is presented in Table 1. Puncture cytology and culture of the right axillary lymph node showed no malignant findings nor any findings suspicious of mycobacterium infection. Given that the hematologic examination revealed the presence of toxoplasma IgG antibodies and the patient had a cat, the condition was initially judged to be lymphadenopathy due to acute toxoplasmosis, and antimicrobial treatment was administered. The tumor temporarily shrank, but the right axillary lymph node appeared swollen again after approximately a month, accompanied by enlargement of the subclavian lymph node (Figure 1). Hence, a right subclavian lymph node biopsy was performed. Histological examination of the sample showed pathological results of follicular lymphoma or DLBCL (Figure 2). For differentiation, immunostaining was performed, and CD20(+), CD79a(+), CD10(+), bcl2, and bcl6(+) were detected (Figure 3).
| Hematology | Value | Unit | Biochemistry | Value | Unit |
| White blood cells | 4310 | /μL | CEA | 2.1 | ng/mL |
| Neutrophils | 69.3 | % | CA 125 | 12.6 | U/mL |
| Lymphocytes | 21.5 | % | CA 15-3 | 13.4 | U/mL |
| Mononuclear cells | 7.5 | % | Toxoplasma IgG | 11.1 | IU/mL |
| Eosinophils | 1.5 | % | Toxoplasma IgM | 0.50 | IU/mL |
| S-IL2 | 1772 | U/mL | |||
| T-SPOT | (-) | ||||
| Red blood cells | 3.80 × 106 | /μL | |||
| Hemoglobin | 12.0 | g/dL | |||
| Platelets | 205 × 103 | /μL | |||
| Biochemistry | |||||
| Total protein | 6.3 | g/dL | |||
| Albumin | 3.7 | g/dL | |||
| Blood urea nitrogen | 16.0 | mg/dL | |||
| Creatinine | 1.00 | g/dL | |||
| Ca | 9.5 | g/dL | |||
| Total bilirubin | 0.4 | mg/dL | |||
| Aspartate aminotransferase | 22 | U/L | |||
| Alanine transaminase | 14 | U/L | |||
| Alkaline phosphatase | 117 | U/L | |||
| γ-glutamyl transferase | 18 | U/L | |||
| Na | 143 | mmol/L | |||
| K | 4.5 | mmol/L | |||
| Cl | 109 | mmol/L | |||
| Creatine kinase | 51 | U/L | |||
| C-reactive protein | 0.3 | mg/dL | |||
| Lactate dehydrogenase | 257 | U/L | |||
CT image: Subclavian lymph node enlargement; right axillary lymph node enlargement.
The patient was diagnosed with stage II disease owing to a lack of splenic involvement or contralateral lymphadenopathy. Disease severity was low-to-moderate according to the International Prognostic Index (IPI), considering age and serum lactate dehydrogenase (LDH) levels.
Given that the patient was over 90 years of age, adapting the standard of care for DLBCL (drug combination che
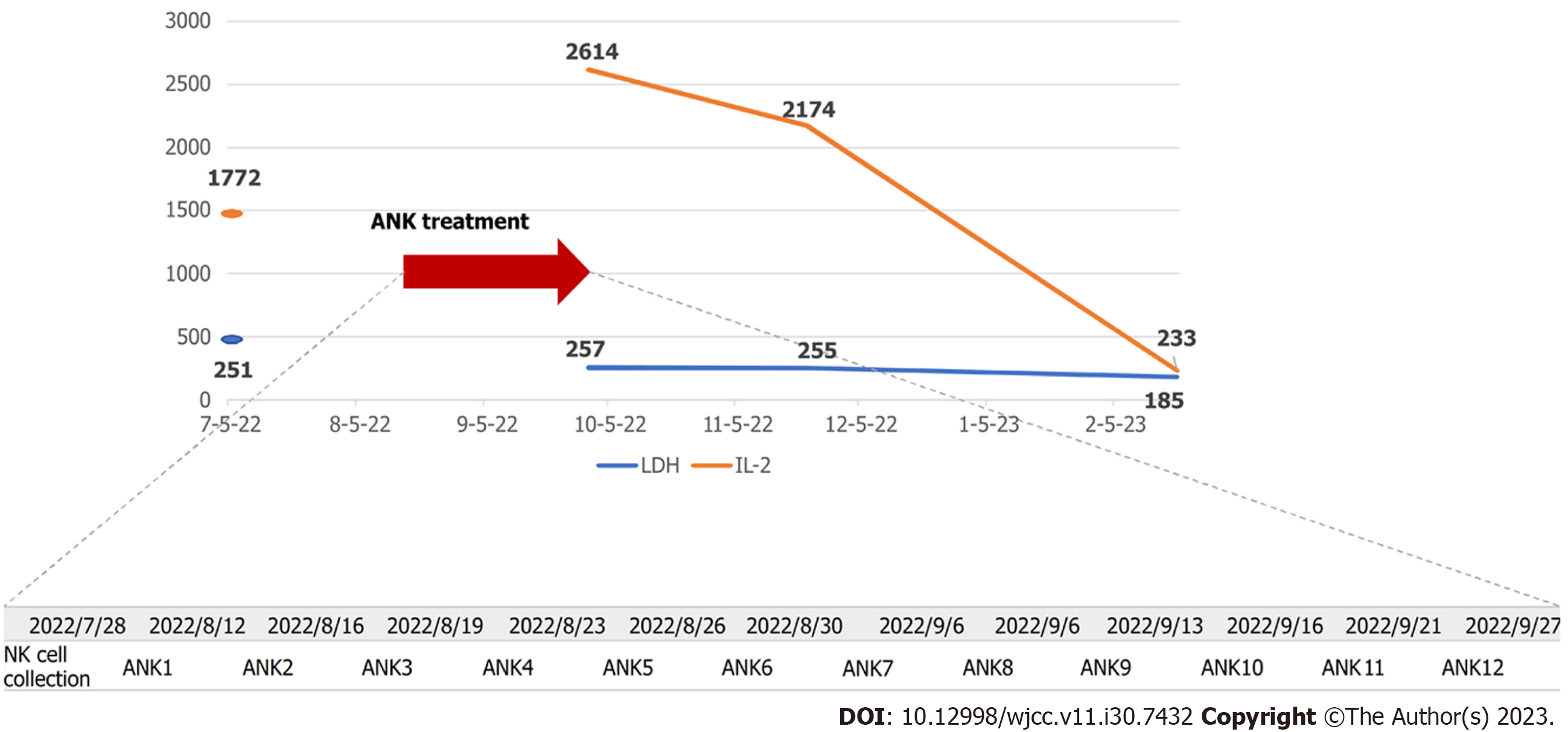
Six rounds of lymphocyte sampling were performed on July 28, 2022. Considering the patient's advanced age, the six samples were diluted to half the initial concentration to achieve 12 samples to reduce the occurrence of side effects. The cultured NK cells were administered twice weekly (Figure 4) via outpatient care. The treatment efficacy was evaluated by performing computed tomography (CT) and serological tests every 1 or 2 mo.
At one month, the right axillary lymph node was mildly enlarged, and the subclavian lymph node size was unchanged (Figure 5B). IL-2 and LDH levels were 2614 U/mL and 261 U/L, respectively (Figure 4). At two months, the size of the right axillary lymph node showed a prominent reduction, and the subclavian lymph node showed a mild reduction (Figure 5C). IL-2 and LDH levels were 2174 U/mL and 255 U/L, respectively (Figure 4). At five months, the right axillary lymph node, which initially was the size of a fist, had shrunk to the size of the head of a thumb. The subclavian lymph node also shrank remarkably; the swelling was substantially reduced, such that the site of swelling was imperceptible (Figure 5D). At six months, the size of the axillary and subclavian lymph nodes showed further reduction (Figure 5E), and the IL-2 and LDH levels were 233 U/mL and 185 U/L, respectively (Figure 4). Although it is necessary to carefully monitor the treatment progress to establish the duration of efficacy, we found that the treatment suppressed lesion growth from administration onward, and the antitumor effect persisted for several months. After administration, the patient experienced general malaise and fever, although the temperature did not exceed 37 °C. The side effects were mild.
To the best of our knowledge, this is the first report of a markedly old patient diagnosed with DLBCL who was successfully treated with ANK therapy alone. Typically, the main drug therapy for DLBCL is combination chemotherapy comprising cytotoxic antineoplastic agents. It is well-established that chemotherapy is not indicated for markedly older patients owing to difficulties such as the occurrence of side effects, with most older patients receiving only palliative care. The established guidelines recommend repeat R-CHOP therapy in patients diagnosed with DLBCL and advanced-stage II Ann Arbor disease with a positive bulky mass at initial treatment[8,15]. Typical side effects of R-CHOP therapy include anorexia, nausea, constipation, numbness in the limbs, fever, hair loss, bone marrow suppression, and decreased renal function, known to pose a substantial burden on patients. Although the patient was in her 90s and markedly old, her cognition was sufficiently robust to allow decision-making regarding the treatment risks. According to the guidelines, combination chemotherapy has benefits and risks. A meta-analysis published in 2021 has reported the efficacy of R-CHOP. However, R-CHOP therapy involves multidrug chemotherapy with the potential to induce severe side effects; hence, it is considered a high-risk treatment, especially among older patients. Our team is well-versed in the efficacy and risks of ANK therapy. Patients and their families have previously requested ANK therapy, which is associated with fewer side effects than chemotherapy, can be received on an outpatient basis, and is expected to gain momentum as an effective treatment strategy[16-18].
It has been reported that NK cells extracted from blood and then cultured and activated can attack tumors better than T cells, regardless of tumor suppressor molecule expression. This finding implies that ANK therapy is tumor cell-specific and carries a low risk of serious damage to the normal immune system. ANK cells have been reported to kill PD-L1-positive tumor cells[19]. Kataoka et al[20] have reported that structures with missing or elevated PD-L13'-UTR are frequently observed in adult T-cell lymphoma (ATL), inducing substantially high PD-L1 expression. Therefore, the therapeutic efficacy of ANK therapy is reported to be high. Considering side effects, serious side effects observed with the commonly used anti-CCR4 antibody have not been reported with ANK therapy[21-23]. Therefore, it differs from existing immunotherapies in terms of efficacy and safety.
In addition to ATL, other cancers have shown similar genomic abnormalities, with such anomalies frequently observed in diffuse large-cell lymphoma, gastric cancer, esophageal cancer, and cervical cancer[22]. These findings suggest that repeated administration of NK cells, including ANK cells, can alleviate immunosuppression via the PD-1-PD-L1 pathway[23,24]. Regarding DLBCL, immunostaining studies of intravascular large B-cell lymphoma have reported a high rate of PD-L1 expression[24].
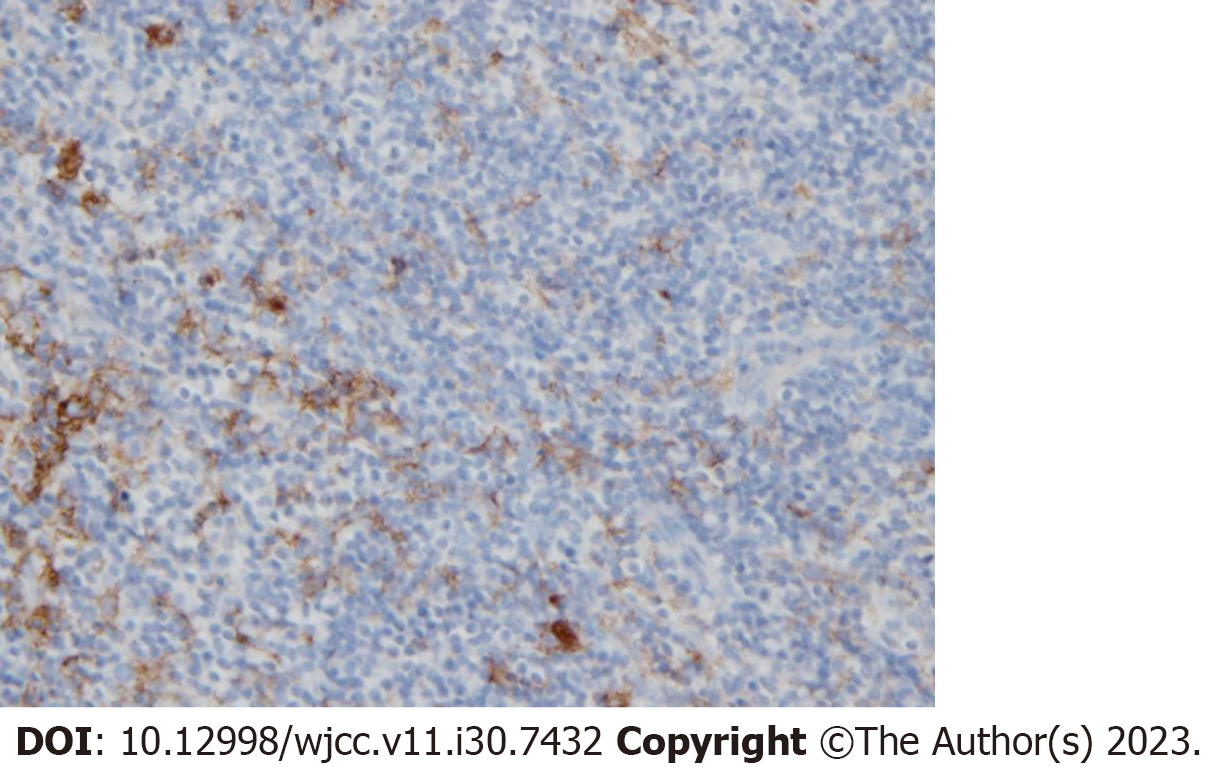
Based on the abovementioned prior reports, we considered that ANK therapy would be more effective in tumor cells with high PD-L1 positivity. Pathological tissues derived from the patient were subjected to PD-L1 immunostaining (Figure 6), revealing positive immunostaining.
Accordingly, in the current patient, DLBCL was associated with a high number of PD-L1-positive tumor cells, and ANK therapy was likely to be very effective. Notably, the results were markedly good, and ANK therapy was adm
ANK therapy could be effective in ATL and several other solid tumors. Considering the potential mechanism, it can be postulated that the greater the number of PD-L1-positive tumor cells, the more effective the therapy. These points have been successfully demonstrated in the current case report[8,22,23]. Although additional cases need to be accumulated to determine and confirm the efficacy and side effects of ANK therapy, it could be used as a first-line therapy to replace R-CHOP therapy in treating malignant lymphoma. Moreover, PD-L1 could be an effective biomarker to establish the efficacy of ANK therapy in other types of cancers.
The authors would like to thank the lymphocyte bank for providing the materials for ANK therapy. We also thank Professor Manabu Fukumoto for teaching us about pathology.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Chemistry, organic
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Baysal M, Turkey; Shahriari M, Iran S-Editor: Liu JH L-Editor: A P-Editor: Zhang YL
| 1. | Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, 4th ed. Lyon: IARC Press, 2008.. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1268] [Cited by in RCA: 1446] [Article Influence: 103.3] [Reference Citation Analysis (0)] |
| 2. | National Cancer Center Cancer Information Service. Statistical information by cancer type, malignant lymphoma 2021. 2021. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 3. | Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, Advani R, Ghielmini M, Salles GA, Zelenetz AD, Jaffe ES. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127:2375-2390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4245] [Cited by in RCA: 5427] [Article Influence: 603.0] [Reference Citation Analysis (0)] |
| 4. | Brandsma D, Bromberg JEC. Primary CNS lymphoma in HIV infection. Handb Clin Neurol. 2018;152:177-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 5. | Carbone PP, Kaplan HS, Musshoff K, Smithers DW, Tubiana M. Report of the Committee on Hodgkin's Disease Staging Classification. Cancer Res. 1971;31:1860-1861. [PubMed] |
| 6. | Zhou Z, Sehn LH, Rademaker AW, Gordon LI, Lacasce AS, Crosby-Thompson A, Vanderplas A, Zelenetz AD, Abel GA, Rodriguez MA, Nademanee A, Kaminski MS, Czuczman MS, Millenson M, Niland J, Gascoyne RD, Connors JM, Friedberg JW, Winter JN. An enhanced International Prognostic Index (NCCN-IPI) for patients with diffuse large B-cell lymphoma treated in the rituximab era. Blood. 2014;123:837-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 503] [Cited by in RCA: 659] [Article Influence: 54.9] [Reference Citation Analysis (0)] |
| 7. | Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, Morel P, Van Den Neste E, Salles G, Gaulard P, Reyes F, Lederlin P, Gisselbrecht C. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:235-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3975] [Cited by in RCA: 4044] [Article Influence: 175.8] [Reference Citation Analysis (0)] |
| 8. | Pfreundschuh M, Trümper L, Osterborg A, Pettengell R, Trneny M, Imrie K, Ma D, Gill D, Walewski J, Zinzani PL, Stahel R, Kvaloy S, Shpilberg O, Jaeger U, Hansen M, Lehtinen T, López-Guillermo A, Corrado C, Scheliga A, Milpied N, Mendila M, Rashford M, Kuhnt E, Loeffler M; MabThera International Trial Group. CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: a randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol. 2006;7:379-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1509] [Cited by in RCA: 1535] [Article Influence: 80.8] [Reference Citation Analysis (0)] |
| 9. | Bishop MR, Dickinson M, Purtill D, Barba P, Santoro A, Hamad N, Kato K, Sureda A, Greil R, Thieblemont C, Morschhauser F, Janz M, Flinn I, Rabitsch W, Kwong YL, Kersten MJ, Minnema MC, Holte H, Chan EHL, Martinez-Lopez J, Müller AMS, Maziarz RT, McGuirk JP, Bachy E, Le Gouill S, Dreyling M, Harigae H, Bond D, Andreadis C, McSweeney P, Kharfan-Dabaja M, Newsome S, Degtyarev E, Awasthi R, Del Corral C, Andreola G, Masood A, Schuster SJ, Jäger U, Borchmann P, Westin JR. Second-Line Tisagenlecleucel or Standard Care in Aggressive B-Cell Lymphoma. N Engl J Med. 2022;386:629-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 338] [Article Influence: 112.7] [Reference Citation Analysis (0)] |
| 10. | Rosenberg SA, Lotze MT, Muul LM, Leitman S, Chang AE, Ettinghausen SE, Matory YL, Skibber JM, Shiloni E, Vetto JT. Observations on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 to patients with metastatic cancer. N Engl J Med. 1985;313:1485-1492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1802] [Cited by in RCA: 1669] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 11. | International Non-Hodgkin's Lymphoma Prognostic Factors Project. A predictive model for aggressive non-Hodgkin's lymphoma. N Engl J Med. 1993;329:987-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3971] [Cited by in RCA: 4201] [Article Influence: 131.3] [Reference Citation Analysis (0)] |
| 12. | Nagai K, Nagai S, Okubo Y, Teshigawara K. ANK Therapeutic Prospects and Usefulness of PD-L1 and NK Activity as Biomarkers for Predicting Treatment Efficacy Revealed from the Treatment Course of Patients with HTLV-1-Associated Bronchioloalveolar Disease. Cancer Med J. 2023;6:30-36. [PubMed] |
| 13. | Nagai K, Nagai S. Effectiveness of Amplified Natural Killer (ANK) Therapy for Adult T-cell Leukemia/Lymphoma (ATL) and Future Prospects of ANK Therapy. Cancer Med J. 2022;5:27-33. [PubMed] |
| 14. | Nagai K, Nagai S, Okubo Y, Teshigawara K. Efficacy and future prospects of ANK therapy for atl, malignant lymphoma and solid tumors. J Blood Lymph. 2021;11:290. [DOI] [Full Text] |
| 15. | Persky DO, Unger JM, Spier CM, Stea B, LeBlanc M, McCarty MJ, Rimsza LM, Fisher RI, Miller TP; Southwest Oncology Group. Phase II study of rituximab plus three cycles of CHOP and involved-field radiotherapy for patients with limited-stage aggressive B-cell lymphoma: Southwest Oncology Group study 0014. J Clin Oncol. 2008;26:2258-2263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 212] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 16. | Azoulay T, Slouzky I, Karmona M, Filatov M, Hayun M, Ofran Y, Sarig G, Ringelstein-Harlev S. Compromised activity of natural killer cells in diffuse large b-cell lymphoma is related to lymphoma-induced modification of their surface receptor expression. Cancer Immunol Immunother. 2023;72:707-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Wang Y, Ren X, Huang K, Liang X, Pu L, Hu L, Zhai Z. Comparison of first-line treatments for elderly patients with diffuse large B-cell lymphoma: A systematic review and network meta-analysis. Front Immunol. 2022;13:1082293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 18. | Tavares A, Moreira I. Diffuse large B-cell lymphoma in very elderly patients: Towards best tailored treatment - A systematic review. Crit Rev Oncol Hematol. 2021;160:103294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 19. | Ishikawa T, Imawari M, Moriyama T, Ohnishi S, Matsuhashi N, Suzuki G, Takaku F. Immunotherapy of hepatocellular carcinoma with autologous lymphokine-activated killer cells and/or recombinant interleukin-2. J Cancer Res Clin Oncol. 1988;114:283-290. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Kataoka K, Ogawa S. PD-L1 genomic abnormalities and its potential as a biomarker. Cytometry Res. 2016;26:15-20. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Ishida T, Ito A, Sato F, Kusumoto S, Iida S, Inagaki H, Morita A, Akinaga S, Ueda R. Stevens-Johnson Syndrome associated with mogamulizumab treatment of adult T-cell leukemia / lymphoma. Cancer Sci. 2013;104:647-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 111] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 22. | Mohammed TO, Chagan-Yasutan H, Ashino Y, Nakayama W, Takahashi Y, Shimomura T, Fujimoto T, Watanabe Y, Niki T, Suzushima H, Hattori T. Galectin-9 as a Predictive Marker for the Onset of Immune-Related Adverse Effects Associated with Anti-CCR4 MoAb Therapy in Patients with Adult T Cell Leukemia. Tohoku J Exp Med. 2017;241:201-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 23. | Ratner L, Waldmann TA, Janakiram M, Brammer JE. Rapid Progression of Adult T-Cell Leukemia-Lymphoma after PD-1 Inhibitor Therapy. N Engl J Med. 2018;378:1947-1948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 175] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 24. | Lanuza PM, Vigueras A, Olivan S, Prats AC, Costas S, Llamazares G, Sanchez-Martinez D, Ayuso JM, Fernandez L, Ochoa I, Pardo J. Activated human primary NK cells efficiently kill colorectal cancer cells in 3D spheroid cultures irrespectively of the level of PD-L1 expression. Oncoimmunology. 2018;7:e1395123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |