Published online Oct 26, 2023. doi: 10.12998/wjcc.v11.i30.7380
Peer-review started: July 1, 2023
First decision: August 16, 2023
Revised: September 2, 2023
Accepted: September 28, 2023
Article in press: September 28, 2023
Published online: October 26, 2023
Processing time: 115 Days and 21.1 Hours
Intractable postherpetic neuralgia (PHN) can be difficult to manage even with aggressive multimodal therapies. Patients who experience uncontrolled refractory cranial PHN despite conservative treatment may benefit from an intrathecal drug delivery system (IDDS). For craniofacial neuropathic pain, the traditional appro
We describe a 69-year-old man with a 1-year history of PHN after developing a vesicular rash in the ophthalmic division of cranial nerve V (trigeminal nerve) distribution. The pain was rated 7-8 at rest and 9-10 at breakthrough pain (BTP) on a numeric rating scale. Despite receiving aggressive multimodal therapies including large doses of oral analgesics (gabapentin 150 mg q12 h, oxycodone 5 mg/acetaminophen 325 mg q6 h, and lidocaine 5% patch 700 mg q12 h) and sphenopalatine ganglion block, there was no relief of pain. Subsequently, the patient elected to have an implantable IDDS with the catheter tip placed at the interpeduncular cistern. The frequency of BTP episodes decreased. The patient’s continuous daily dose was adjusted to 0.032 mg/d after 3 mo of follow-up and stopped 5 mo later. He did not report pain or other discomfort at outpatient follow-up 6 mo and 1 year after stopping intracisternal hydromorphone.
The use of interpeduncular cistern intrathecal infusion with low-dose hydro
Core Tip: We describe a case of refractory postherpetic neuralgia in the trigeminal nerve area that was successfully treated by implanting of an intrathecal drug delivery system with catheter tip placement at the interpeduncular cistern. We discuss the key points and difficulties in the surgical process and the future expansion of this technique.
- Citation: Fu F, Jiang XF, Wang JJ, Gong L, Yun C, Sun HT, Tang FW. Interpeduncular cistern intrathecal targeted drug delivery for intractable postherpetic neuralgia: A case report. World J Clin Cases 2023; 11(30): 7380-7385
- URL: https://www.wjgnet.com/2307-8960/full/v11/i30/7380.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i30.7380
Postherpetic neuralgia (PHN) is defined as persistent pain with dermatomal distribution in patients who have recovered from herpes zoster (HZ). In addition, PHN is accompanied by physical and mental pain and sleep disturbances, resulting in diminished quality of life. In general, PHN occurs in about 20% of patients with HZ and > 50% of PHN occurs in patients who are aged ≥ 60 years[1,2]. Similarly, a cross-sectional study involving 24 hospitals in China reported that an estimated 29.8% of patients with zoster infection developed PHN, of which 66.3% were > 60 years of age[3].
Currently, first-stage treatment of PHN is medication, followed by interventional therapies such as botulinum toxin injection, ganglion block, pulsed radiofrequency, nerve or spinal cord stimulation as second-stage therapies[4]. However, patients who still have refractory pain after conservative treatment may benefit from intrathecal drug delivery systems (IDDS), which is referred to as third-stage therapy by the polyanalgesic consensus conference[5].
The traditional teaching of many best practices and guidelines has recommended that the optimal placement of the catheter tip is nearest the site of pain[5]. However, the traditional IDDS therapy for craniofacial neuropathic pain is to place the tip of catheter far away from the cranial nerve root entry area, thus potentially leading to an unsatisfactory analgesic effect[6].
Limited data exist as to the appropriate and best catheter tip placement. To provide a reference for clinical treatment, we describe a case of IDDS with catheter tip placement at the interpeduncular cistern for treatment of PHN of the ophthalmic branch.
This is a 69-year-old man with PHN of the ophthalmic branch, he had experienced variable intensity facial pain about the region of the top of forehead and upper eyelid for over one year.
The patient was originally treated with antiviral medication, and the rash subsequently resolved. However, he developed PHN with sharp, burning, and electrical type of pain in the distribution of his prior rash. On an 10-point numeric rating scale (NRS), his resting pain intensity was 7-8 and breakthrough pain (BTP) was 9-10. The pain initially responded to gabapentin (150 mg q12 h), oxycodone 5mg/acetaminophen 325 mg q6 h, and lidocaine 5% patch (700 mg q12 h), but then became refractory to these treatments. The patient was also treated twice with sphenopalatine ganglion block (SGB), with no relief of his pain.
The patient had documented hypertension and type 2 diabetes, although blood pressure and glucose were well managed.
The patient had a clear personal and family history.
The patient had cutaneous scarring on an area of HZ ophthalmicus, and hypersensitivity in the ophthalmic division at cranial nerve V (trigeminal nerve) distribution, where a light touch produced pain. Other physical examination results were normal. His resting pain intensity was 7-8 and BTP was 9-10 on an NRS. The patient’s blood pressure and blood glucose were well controlled by medication before the operation.
No abnormalities were found in the blood and urine tests.
There were no significant abnormalities in the head magnetic resonance imaging and computed tomography.
We arrived at a final diagnosis of PHN.
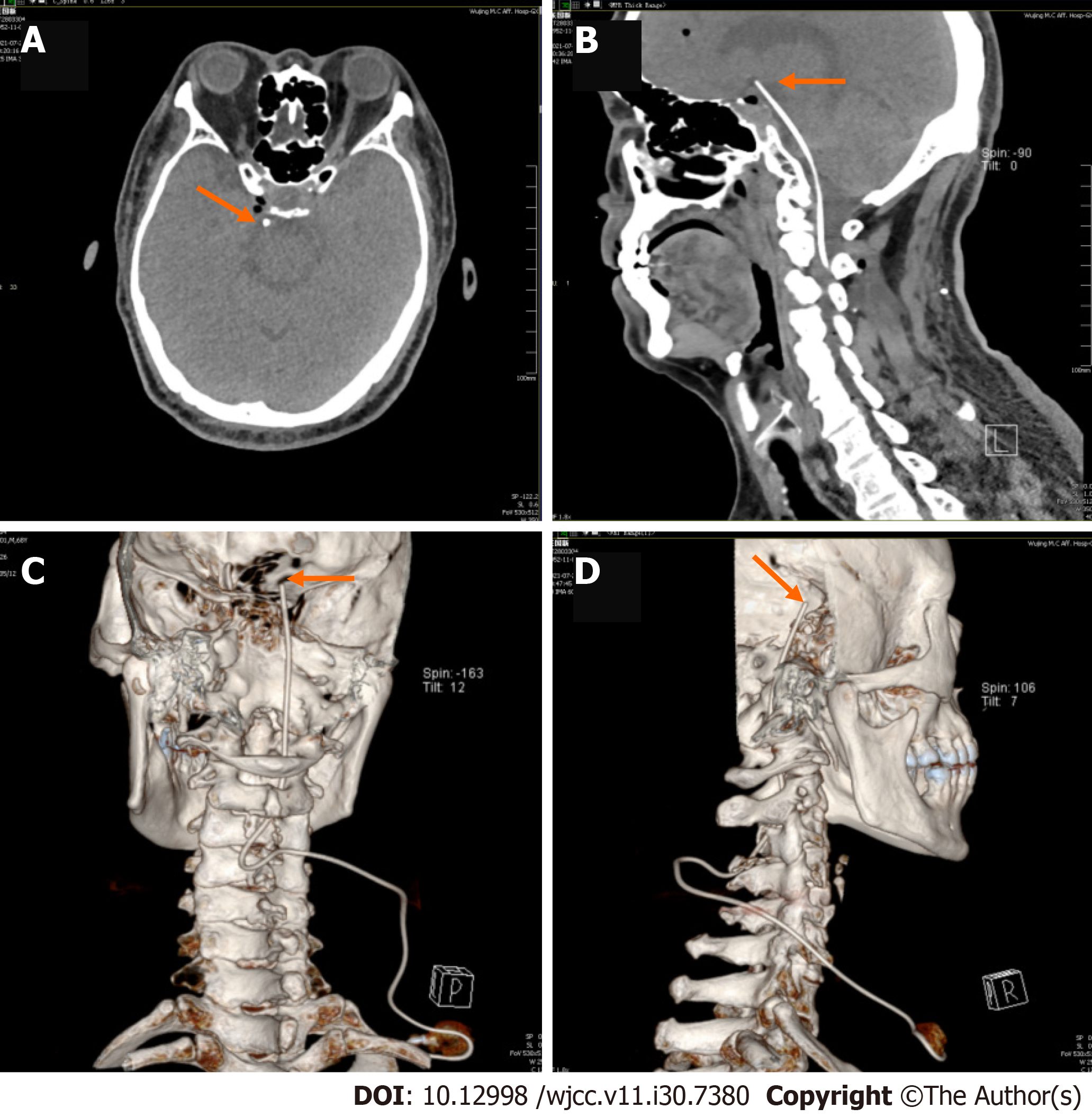
The patient’s anxiety and insomnia became worse due to the severity of pain, and he agreed to proceed with the IDDS implantation procedure. The patient gave informed consent, and the procedure was conducted in accordance with the declaration of Helsinki and approved by the Ethics Committee of The Characteristic Medical Center of People’s Armed Police. The patient was put in left decubitus position on the operating table after induction of general anesthesia. Intraoperative fluoroscopy was mandatory to confirm access to the C3-4 intrathecal space and the catheter tip (SP-10210, Soph-A-Port, Sophysa, France) was gradually advanced to the interpeduncular cistern. His port was placed in the subcutaneous tissue overlying his right upper chest (Figure 1). An external drug delivery system was connected to the port using a nondestructive needle. No procedural complications were encountered while inserting the pump.
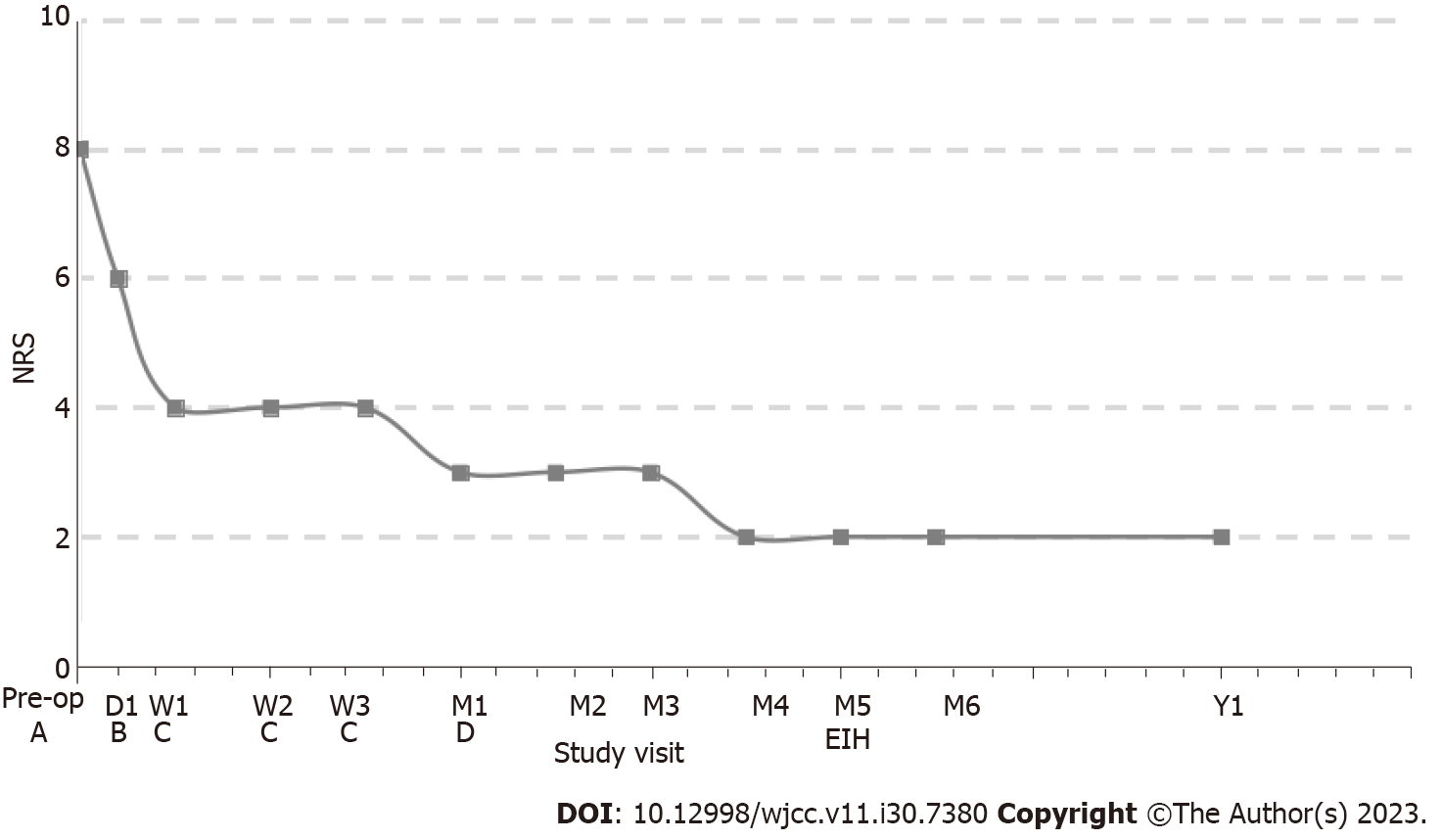
Subarachnoid infusion of hydromorphone starts at 1/300 of the daily opioid equivalent dose (oxycodone, 20 mg/d)[7]; therefore, continuous daily hydromorphone hydrochloride (Yichang Human-well Pharmaceutical Co. Ltd., China) was started at 0.064 mg/d, and gradually titrated up to 0.128 mg/d for improved pain control. For more efficient control of BTP, a bolus was added with 0.0003 mg each time and a 1-h lockout interval. The patient developed nausea and vomiting at the beginning of medication, which was gradually controlled after drug treatment. No sedation, confusion, or respiratory depression occurred. He was discharged with pain reduction, which was well maintained at the NRS pain score of 3 at rest and 5 at worst BTP. There were also fewer BTP episodes. Gradually adjust the patient’s continuous daily dose to 0.032 mg/d (NRS 1-2) after 3 mo of follow-up and stopped 5 mo later. He did not report pain or other discomfort at outpatient follow-up 6 mo and 1 year after stopping intracisternal hydromorphone (Figure 2).
With the increasing understanding of pain pathophysiology and intrathecal analgesia, implanted IDDS has been widely recognized worldwide for the treatment of various types of chronic intractable pain. IDDS can directly inject drugs into the cerebrospinal fluid (CSF), which has the advantages of higher selectivity, significantly lower doses of drugs, and fewer adverse effects[8]. However, in order to take advantage of this, proper patient selection and careful application of the therapy need to be exercised. To our knowledge, there are few reports of patients with craniofacial PHN with continuous subarachnoid injection of opioids. Intrathecal administration to treat intractable PHN has been proved effective in some studies. Previous studies have reported intrathecal injection of methylprednisolone with local anesthetic or midazolam for the treatment of PHN[9]. Several studies have reported intrathecal morphine infusion in the high neck segment for the treatment of cancer-related craniofacial pain[6,10]. For patients with non-cancer-related pain, intrathecal opioids are considered to have level 3 evidence, grade B recommendation, and strong consensus level[5]. Based on the above reasons, our case report focused on the effectiveness of interpeduncular cistern intrathecal targeted low-dose hydromorphone for intractable craniofacial PHN.
Our patient had severe unrelenting PHN in the cranial nerve V (trigeminal nerve) distribution, which impacted his quality of life. Despite aggressive therapies with large doses of oral analgesics and SGB, the patient experienced limited effect and he subsequently elected to accept implant of IDDS with the tip of intrathecal catheter placed at the interpeduncular cistern. After pump placement and initiation of intrathecal hydromorphone, his pain was significantly improved, demonstrating interpeduncular cistern intrathecal targeted drug delivery may be an effective method for treating PHN in the trigeminal nerve area.
This case proves that it is important to place the catheter tip in the corresponding position according to the patient’s pain level in order to achieve a good analgesic effect. The current concept of CSF flow dynamics emphasizes pulse flow and bidirectional oscillatory movement[11,12]. Previous studies have shown that intrathecal targeted medication delivery in IDDS is significantly limited by the pulse flow pattern of CSF, which involves continuous low-flow intrathecal infusion through small catheters so that the drug is confined to a few centimeters of the catheter tip (two or three vertebrae)[13,14].
The trigeminal nerve is a mixed nerve, which contains general somatosensory and special visceral movement of two kinds of fibers. Sensory fibers into the pons continue forward to the sensory, spinal trigeminal or midbrain nuclei[15,16]. According to the anatomical structure, the spinal trigeminal nucleus is a second-order neuron that transmits the pain signal centrally[17]. In spite of this, the tip of intrathecal catheter is always placed below the level of the trigeminal nerve root entry zone (where the target receptors are located) in the traditional way, thus possibly leading to insufficient analgesia. Its effect is attributed to the dense concentration of opioid receptors surrounding the brainstem and the CSF flow dynamics. The interpeduncular cistern intrathecal targeted drug delivery method for intractable PHN in the trigeminal nerve area could be more effective compared with the traditional approach.
Based on our previous experience, it is difficult to reach the high cervical region by inserting the catheter at the safe L2 level due to the lack of a suitable catheter, let alone the intracranial region. Cisterna magna puncture was considered because of its safety, but was abandoned because of difficulty in fixation. Therefore, we chose to puncture through C3-4 and place the catheter tip slowly upwards. The catheter must be advanced slowly into the cervical subarachnoid space under continuous X-ray to avoid damage to the tissues. Angle adjustment is important in the placement process. If the catheter encountered resistance, it was withdrawn and we adjusted the angle before the next attempt. The catheter tip had to be slowly and carefully passed through the C1 level.
While we did not observe any obvious complications related to the procedure in this case, potential complications such as arachnoiditis, fungal meningitis, respiratory depression, paresthesia, hemorrhage, surgical site infection, and low-pressure headache can occur in the perioperative period[18]. Thus, this intervention should be executed with care and only following thorough discussion. Patients should be informed of the benefits and potential adverse effects of treatment. Experimental studies of continuous intracisternal injection of opioids are warranted for this challenging-to-treat population and further research in the form of randomized control trials is needed.
The difficulties of the case mentioned above highlight the need for advances in intrathecal catheter design, access techniques, imaging, and greater understanding of pain pathways. Technical innovations include new catheters, its tip is soft and the body is strong for easier placement. Ultrasound-guided puncture appears to be a safe technique for placement of the catheter, while providing better visualization and no radiation exposure. We anticipate that this will occur in several concurrent phases, which will usher in an era in which the intracisternal space is recognized as a highly valued therapeutic target.
Interpeduncular cistern intrathecal infusion with low-dose hydromorphone by IDDS was an effective way to alleviate severe craniofacial PHN.
We are grateful to Xiao-Hong Li for editing the English text of a draft of this manuscript.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Higa K, Japan; Kokot A, Croatia S-Editor: Qu XL L-Editor: A P-Editor: Qu XL
| 1. | Johnson RW, Rice AS. Clinical practice. Postherpetic neuralgia. N Engl J Med. 2014;371:1526-1533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 336] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 2. | Massengill JS, Kittredge JL. Practical considerations in the pharmacological treatment of postherpetic neuralgia for the primary care provider. J Pain Res. 2014;7:125-132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 3. | Yang F, Yu S, Fan B, Liu Y, Chen YX, Kudel I, Concialdi K, DiBonaventura M, Hopps M, Hlavacek P, Cappelleri JC, Sadosky A, Parsons B, Udall M. The Epidemiology of Herpes Zoster and Postherpetic Neuralgia in China: Results from a Cross-Sectional Study. Pain Ther. 2019;8:249-259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 78] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 4. | Shrestha M, Chen A. Modalities in managing postherpetic neuralgia. Korean J Pain. 2018;31:235-243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 5. | Deer TR, Pope JE, Hayek SM, Bux A, Buchser E, Eldabe S, De Andrés JA, Erdek M, Patin D, Grider JS, Doleys DM, Jacobs MS, Yaksh TL, Poree L, Wallace MS, Prager J, Rauck R, DeLeon O, Diwan S, Falowski SM, Gazelka HM, Kim P, Leong M, Levy RM, McDowell G II, McRoberts P, Naidu R, Narouze S, Perruchoud C, Rosen SM, Rosenberg WS, Saulino M, Staats P, Stearns LJ, Willis D, Krames E, Huntoon M, Mekhail N. The Polyanalgesic Consensus Conference (PACC): Recommendations on Intrathecal Drug Infusion Systems Best Practices and Guidelines. Neuromodulation. 2017;20:96-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 179] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 6. | Zou D, Zhang W, Wang Y. Prepontine Cistern Intrathecal Targeted Drug Delivery for Cancer-Related Craniofacial Pain. Pain Med. 2021;22:3112-3114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 7. | Sylvester RK, Lindsay SM, Schauer C. The conversion challenge: from intrathecal to oral morphine. Am J Hosp Palliat Care. 2004;21:143-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 8. | Hayek SM, Hanes MC. Intrathecal therapy for chronic pain: current trends and future needs. Curr Pain Headache Rep. 2014;18:388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | Lin CS, Lin YC, Lao HC, Chen CC. Interventional Treatments for Postherpetic Neuralgia: A Systematic Review. Pain Physician. 2019;22:209-228. [PubMed] |
| 10. | Moman RN, Rogers JM, Pittelkow TP. High Cervical Intrathecal Targeted Drug Delivery: A Case Report of Refractory Oropharyngeal Cancer Pain. Case Rep Oncol Med. 2019;2019:2098921. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Henry-Feugeas MC, Idy-Peretti I, Baledent O, Poncelet-Didon A, Zannoli G, Bittoun J, Schouman-Claeys E. Origin of subarachnoid cerebrospinal fluid pulsations: a phase-contrast MR analysis. Magn Reson Imaging. 2000;18:387-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 74] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 12. | Friese S, Hamhaber U, Erb M, Kueker W, Klose U. The influence of pulse and respiration on spinal cerebrospinal fluid pulsation. Invest Radiol. 2004;39:120-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 60] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 13. | Bernards CM. Cerebrospinal fluid and spinal cord distribution of baclofen and bupivacaine during slow intrathecal infusion in pigs. Anesthesiology. 2006;105:169-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 108] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 14. | Flack SH, Anderson CM, Bernards C. Morphine distribution in the spinal cord after chronic infusion in pigs. Anesth Analg. 2011;112:460-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 15. | Okada S, Katagiri A, Saito H, Lee J, Ohara K, Iinuma T, Bereiter DA, Iwata K. Differential activation of ascending noxious pathways associated with trigeminal nerve injury. Pain. 2019;160:1342-1360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 16. | Reske-Nielsen E, Oster S, Pedersen B. Herpes zoster ophthalmicus and the mesencephalic nucleus. A neuropathological study. Acta Pathol Microbiol Immunol Scand A. 1986;94:263-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 17. | Fromm GH, Chattha AS, Terrence CF, Glass JD. Role of inhibitory mechanisms in trigeminal neuralgia. Neurology. 1981;31:683-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 68] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Lewis G. Intrathecal methylprednisolone for postherpetic neuralgia. N Engl J Med. 2001;344:1020; author reply 1021-1020; author reply 1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |