Published online Oct 26, 2023. doi: 10.12998/wjcc.v11.i30.7350
Peer-review started: August 17, 2023
First decision: August 30, 2023
Revised: September 10, 2023
Accepted: September 28, 2023
Article in press: September 28, 2023
Published online: October 26, 2023
Processing time: 68 Days and 18.2 Hours
Administering anti-osteoporotic agents to patients perioperatively is a widely accepted approach for improving bone fusion rates and reducing the risk of complications. The best anti-osteoporotic agents for spinal fusion surgery remain unclear.
To investigate the efficacy and safety of different anti-osteoporotic agents in spinal fusion surgery via network meta-analysis.
Searches were conducted in four electronic databases (PubMed, EMBASE, Web of Science, the Cochrane Library and China National Knowledge Infrastructure (CNKI) from inception to November 2022. Any studies that compared anti-osteoporotic agents vs placebo for spinal fusion surgery were included in this network meta-analysis. Outcomes included fusion rate, Oswestry disability index (ODI), and adverse events. Network meta-analysis was performed by R software with the gemtc package.
In total, 13 randomized controlled trials were included in this network meta-analysis. Only teriparatide (OR 3.2, 95%CI: 1.4 to 7.8) was more effective than placebo in increasing the fusion rate. The surface under the cumulative ranking curve (SUCRA) of teriparatide combined with denosumab was the highest (SUCRA, 90.9%), followed by teriparatide (SUCRA, 74.0%), zoledronic acid (SUCRA, 43.7%), alendronate (SUCRA, 41.1%) and risedronate (SUCRA, 35.0%). Teriparatide (MD -15, 95%CI: -28 to -2.7) and teriparatide combined with denosumab (MD -20, 95%CI: -40 to -0.43) were more effective than placebo in decreasing the ODI. The SUCRA of teriparatide combined with denosumab was highest (SUCRA, 90.8%), followed by teriparatide (SUCRA, 74.5%), alendronate (SURCA, 52.7), risedronate (SURCA, 52.1%), zoledronic acid (SURCA, 24.2%) and placebo (SURCA, 5.6%) for ODI. The adverse events were not different between groups.
This network meta-analysis suggests that teriparatide combined with denosumab and teriparatide alone significantly increase the fusion rate and decrease the ODI without increasing adverse events. Based on current evidence, teriparatide combined with denosumab or teriparatide alone is recommended to increase the fusion rate and to reduce ODI in spinal fusion patients.
Core Tip: This network meta-analysis suggests that teriparatide combined denosumab and teriparatide significantly increased the fusion rate, decreased Oswestry disability index (ODI) without increasing adverse events. Based on current evidence, teriparatide combined denosumab and teriparatide are recommended to increase fusion rate and to reduce ODI in spinal fusion patients. However, the overall quality of evidence is low, the overall certainty of the evidence synthesis is low. In the future, there is a need for more high-quality randomized controlled trials to reassess or confirm this conclusion.
- Citation: He XY, Chen HX, Zhao ZR. Efficacy and safety of different anti-osteoporotic drugs for the spinal fusion surgery: A network meta-analysis. World J Clin Cases 2023; 11(30): 7350-7362
- URL: https://www.wjgnet.com/2307-8960/full/v11/i30/7350.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i30.7350
Low back pain is one of the most frequent symptoms for which patients visit physicians around the world[1,2]. One frequently employed method for addressing degenerative lumbar conditions such as deformity, instability, lumbar stenosis, degenerative spondylolisthesis, and spinal trauma is spinal fusion surgery[3,4]. Pedicle screws, which are used to stabilize spinal instrumentation, are chosen according to their pullout strength and the bone mineral density in the spine[5,6]. Spinal fusion surgery is common in geriatrics, especially in aged women[7]. In general, spinal fusion patients are more likely to have low bone mass and osteoporosis[8,9]. Complications that have been reported in the surgical treatment of an osteoporotic spine using instrumentation include spinal instability, implant migration leading to pseudarthrosis, instrumentation failure, and other related issues[10,11].
The incidence of pseudoarthrosis following lumbar spine fusion can range from 5% to 35% and is notably higher in individuals who have undergone fusion across three or more spinal levels[12]. Pseudarthrosis may result in spine pain and poor functional outcomes after spinal fusion surgery[13]. Therefore, choosing anti-osteoporotic drugs to increase the fusion rate after spinal surgery is an important challenge for spinal surgeons.
Anti-osteoporosis drugs, including antiresorptive or anabolic drugs, as well as drugs with a mixed mechanism of action, are well accepted to increase the fusion rate[14,15]. Among many anti-osteoporotic medicines, teriparatide, bisphosphonate and denosumab are most commonly used in clinical practice. Teriparatide, the synthetic form of human parathyroid hormone (PTH) 1-34, is used to treat postmenopausal osteoporosis[10,16-19]. Teriparatide has an anabolic effect on osteoblasts, not only increasing bone mineral density and bone mass but also improving the microarchitecture of the skeleton[20]. Bisphosphonates are stable derivatives of inorganic pyrophosphate and potent antiresorptive agents[21]. The main bisphosphonates are alendronate, risedronate, ibandronate and zoledronic acid[22]. Bisphosphonates promote the apoptosis of osteoclasts, inhibit bone loss and increase bone density around the spine[23]. Although many studies have investigated the role of bisphosphonate administration after spinal fusion, the conclusions are still controversial. Denosumab is a fully human monoclonal antibody that binds receptor activator of the nuclear factor-κB ligand, thereby blocking its interaction with receptor activator of nuclear factor κB. Denosumab selectively inhibits osteoclastogenesis and has been approved by the United States Food and Drug Administration. Denosumab is well tolerated by patients, and it affects renal function less than other drugs[24].
While anti-osteoporotic medications have been recognized as effective for preventing bone loss during spinal fusion surgery, the most effective treatment regimen remains uncertain[25]. By utilizing Bayesian network meta-analysis, we indirectly compared therapies in cases where direct comparisons were not available, allowing for a more precise assessment of efficacy by combining both direct and indirect comparisons.
This study aimed to determine the effectiveness and safety of various anti-osteoporotic medications in the context of spinal surgery using network meta-analysis.
This network meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) statement. This study was registered through PROSPERO (PROSPERO Registration number: CRD42023445654).
Two independent reviewers (Xiaoyuan He and Zhirong Zhao) performed searches in four electronic databases [PubMed, EMBASE, Web of Science, the Cochrane Library and China National Knowledge Infrastructure (CNKI)] from inception to November 2022. Moreover, we manually searched related references to retrieve eligible studies. The search terms used were: “Alendronate”, “Clodronic Acid”, “Etidronic Acid”, “Ibandronic Acid”, “Pamidronate”, “Risedronic Acid”, “Technetium Tc 99m Medronate”, “Zoledronic Acid”, “"Diphosphonates"[Mesh]” OR ‘‘bisphosphonate” OR “” “Parathyroid Hormone”, “Teriparatide” AND “Spinal Fusion’’. More detailed information regarding the search strategy can be found in Supplementary material. Ethical approval was not required for this systematic review and network meta-analysis since no patient contact took place.
Studies were included in this review if they met all the following population/intervention/comparison/outcome criteria: (P) the study recruited patients undergoing spinal fusion; (I) it tested anti-osteoporosis medicine(s) (bisphosphonates, teriparatide, or denosumab); (C) it compared the drug(s) to a placebo; (O) its outcomes were fusion rate, Oswestry disability index (ODI) and/or adverse events; and (S) the study was a randomized controlled trial (RCT). The primary outcome of this meta-analysis was the fusion rate, which is predominantly influenced in the positive direction by the increase in bone mineral density induced by these anti-osteoporotic drugs. The secondary outcomes were the Oswestry disability index (ODI) and adverse events. The inclusion of ODI in our analysis helped evaluate dysfunction related to back pain. The study encompassed parallel-group randomized controlled trials, as well as first-phase crossover trials and multiarm trials. The exclusion criteria were as follows: (1) Case reports and comments; (2) studies with insufficient data; (3) reviews or meta-analyses; (4) studies with only case groups; and (5) no follow-up after discharge.
The revised Cochrane risk-of-bias tool for randomized trials was employed[26]. Risk of bias from five different domains was assessed: (1) Randomization process; (2) deviations from intended interventions; (3) missing outcome data; (4) measurement of outcome; and (5) selection of the reported result. Risk of bias is reported as ‘low risk of bias,’ ‘some concerns’ or ‘high risk of bias’. There are specific and clear instructions in this tool to help reviewers assess the risk of bias as "high", "low", or "unclear". Divergences were resolved by face-to-face discussion, or in case of persistent disagreement, a third experienced author was consulted.
Two authors (Chen HX and Zhao ZR) independently extracted all relevant general information from eligible studies using a standardized form in Microsoft Excel (Microsoft Excel for Windows 2011, Version 14.4.9, 2010; Microsoft Corp, Redmond, Wash). General characteristics of the studies included first author, publication year, location, surgical indication, numbers in the comparator groups and control, mean ages of the comparator and control groups, sex ratio, follow-up duration, dose of drugs and outcomes of interest (fusion rate, ODI and adverse events). To mitigate the effects of withdrawal bias, we prioritized the use of intention-to-treat analysis data whenever possible. In cases where outcome data were ambiguous, we reached out to the corresponding author via email in an effort to obtain the necessary information.
Network meta-analysis concerning the effects of the anti-osteoporosis drugs on fusion rate was performed by a random-effect model within a Bayesian framework, using packages "gemtc" and “rjags” of R software (version 3.5.1, https://www.r-project.org/). We ran the Markov chain Monte Carlo (MCMC) simulation with four chains for each model, using 500000 iterations, a burn-in of 20000 iterations and extraction of every 10th value (Sutton and Abrams[27]). Using the median values from the posterior distribution, we calculated the estimated outcomes (measured as mean differences or odds ratios) along with their corresponding 95% confidence intervals. If the 95% confidence intervals for the odds ratios did not encompass 1 or for the mean differences did not encompass 0, this indicated a statistically significant difference. A P value less than 0.05 was defined as statistically significant. The surface under the cumulative ranking curve (SUCRA) values were also calculated to rank different interventions. The larger the value of SURCA, the better the effect of the intervention. Heterogeneity was evaluated using the I2 test, and thresholds were defined as 50% when I2 was less than 50%, which indicated low heterogeneity. The global inconsistency was evaluated by comparing the fit of consistency and inconsistency models using the deviance information criterion (DIC), where a similar DIC of different models indicates good consistency. We utilized node-splitting analysis to evaluate local inconsistency, whereby a P value greater than 0.05 indicated that there was no significant inconsistency between the direct pairwise results and the indirect results.
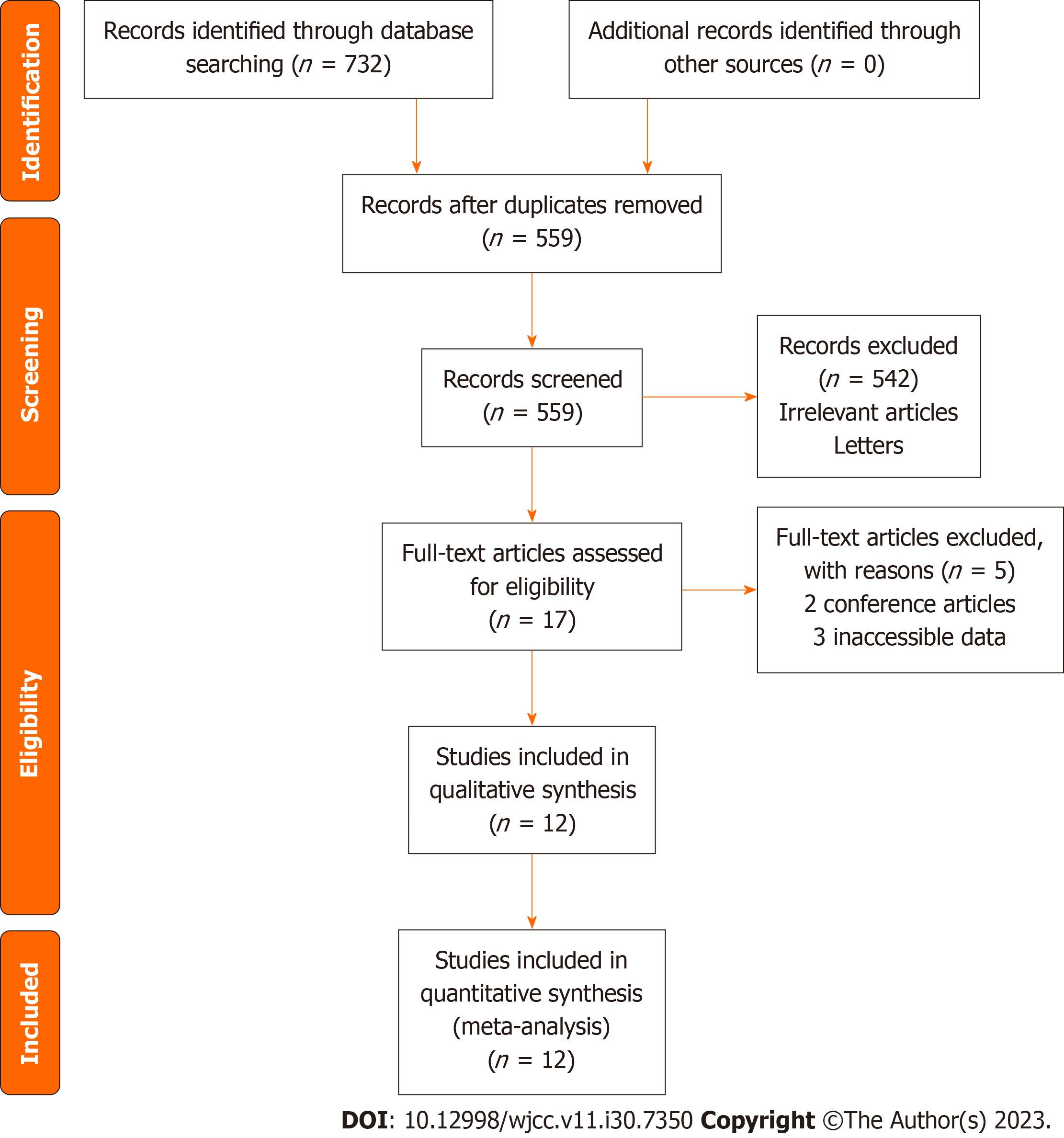
The initial search of four databases (PubMed, EMBASE, Web of Science, the Cochrane Library and CNKI) yielded 732 articles, 173 of which were excluded as duplicates. After reading the title and abstract, 542 articles were filtered out based on our inclusion and exclusion criteria. After reading the full texts manually, 5 articles were excluded for various reasons. In the end, 13 studies were included in this network meta-analysis (Figure 1)[28-41].
The general characteristics of the included RCTs can be seen in Table 1. We included 13 RCTs for analysis. These RCTs were published from 2011 to 2021. Four studies were done in China, six in Japan, one in Denmark, and the rest in Korea. We analyzed data from four studies reporting results comparing teriparatide vs placebo. One study compared alendronate vs placebo. Three studies compared zoledronic acid vs placebo for spinal fusion surgery. Only one study compared teriparatide combined with denosumab vs teriparatide alone for spinal fusion surgery. Two studies compared teriparatide vs alendronate. The dose, route and timing of administration of the anti-osteoporotic agents can be seen in Table 2.
| Ref. | Location | Study | Surgical indication | Comparator | Control | Number of patients | Age of patients (yr) | Sex (M/F) | Follow up | |||
| Comparator | Control | Comparator | Control | Comparator | Control | |||||||
| Jespersen et al[32], 2019 | Denmark | RCT | Spondylolisthesis | Teriparatide | Placebo | 41 | 46 | 71 | 70 | 11/30 | 7/39 | 12 mo |
| Sheng et al[37], 2018 | China | RCT | Spondylolisthesis HIVD, spinal stenosis | Zoledronic acid | Placebo | 28 | 28 | 60.7 | 63.1 | 7/21 | 10/18 | 12 mo |
| Ide et al[31], 2018 | Japan | RCT | Spinal stenosis | Teriparatide + denosumab | Teriparatide | 8 | 8 | 73.2 | 75.0 | 3/5 | 0/8 | 12 mo |
| Seki et al[36], 2017 | Japan | Prospective | Vertebral fracture | Teriparatide | Alendronate/risedronate | 33 | 25 | 72.5 | 71.5 | 0/33 | 0/25 | 24 mo |
| Ebata et al[30], 2017 | Japan | RCT | Lumbar degenerative disease | Teriparatide | Placebo | 36 | 38 | 72.6 | 70.4 | 0/36 | 0/38 | 6 mo |
| Cho et al[29], 2017 | Korea | Prospective | Spinal stenosis, spondylolisthesis | Teriparatide | Alendronate | 23 | 24 | 71.0 | 68.2 | 0/23 | 0/24 | 24 mo |
| Yagi et al[39], 2016 | Japan | Prospective | Posterior long instrumented fusion | Teriparatide | Placebo | 43 | 33 | 68.6 | 66.7 | 0/43 | 0/33 | 24 mo |
| Chen et al[28], 2016 | China | RCT | Spondylolisthesis | zoledronic acid | Placebo | 33 | 36 | 65 | 63 | 6/27 | 7/29 | 12 mo |
| Ohtori et al[35], 2013 | Japan | RCT | Spondylolisthesis with spinal stenosis | Teriparatide/Risedronate | Placebo | 20/20 | 20 | 78/75 | 73 | 0/20,0/20 | 0/22 | 12 mo |
| Li et al[33], 2012 | China | RCT | Non-specific | Zoledronic acid | Placebo | 28 | 25 | 63.63 | 63.83 | 13/28 | 16/25 | 12 mo |
| Nagahama et al[34], 2011 | Japan | RCT | Spondylolisthesis and spinal enosis | Alendronate | Placebo | 19 | 17 | 70.3 | 67.4 | 1/18 | 1/16 | 12 mo |
| Wang et al[40], 2021 | China | RCT | Transforaminal lumbar interbody fusion | Teriparatide | Zoledronic acid | 29 | 38 | 66.34 | 65.89 | 4/25 | 3/35 | 12 mo |
| Ref. | Drug | Dose | Route | Timing of administration |
| Jespersen et al[32], 2019 | Teriparatide | 20 μg | Subcutaneous | 90 d |
| Sheng et al[37], 2018 | Zoledronic acid | 5 mg | Intravenous | Intravenous single dose 3 d after surgery |
| Ide et al[31], 2018 | Teriparatide + denosumab | 60 mg | Subcutaneously | Administered at 2 and 8 mo following surgery |
| Teriparatide | 20 μg | Subcutaneous | Administered froma month before surgery to 12 mo after surgery | |
| Seki et al[36], 2017 | Teriparatide | 20 μg | Subcutaneous | Once a day starting 3 mo before surgery through 21 mo after surgery |
| Ebata et al[30], 2017 | Teriparatide | 56.5 μg | Subcutaneous | Once a week starting, 1 wk after surgery for a total of 6 mo |
| Cho et al[29], 2017 | Teriparatide | |||
| Yagi et al[39], 2016 | Teriparatide | 20 μg | Subcutaneous | Once a day from the day of surgery for a total of 18 mo |
| Chen et al[28], 2016 | zoledronic acid | 5 mg | Intravenous | Single dose 3 d after surgery |
| Ohtori et al[35], 2013 | Teriparatide/Risedronate | 20 μg | Subcutaneous | Once a day starting 2 mo before surgery through 10 mo after surgery |
| 2.5 mg | Oral | Once a day starting 2 mo before surgery through 10 mo after surgery | ||
| Li et al[33], 2012 | Zoledronic acid | 5 mg | Intravenous | 3 d after the surgery |
| Nagahama et al[34], 2011 | Alendronate | 35 mg | Oral | Not specified |
| Wang et al[40], 2021 | Teriparatide | 20 μg | Subcutaneously | Once daily and continuously for more than 6 mo starting from 1 d after surgery |
| Zoledronic acid | 5 mg | Intravenously | 15 min to 3 d after surgery |
Of the 13 studies, only four studies were rated as having a low risk of bias. Five studies were identified as having an unclear risk of bias. The remaining 4 studies were listed as having a high risk of bias. For the randomization process, 4 studies were listed as having a low risk of bias, and the other 9 studies were rated as having an unclear risk of bias. One was rated as having a high risk of bias for deviations from intended interventions, and 7 studies were listed as having an unclear risk of bias. The domain-specific and overall risk of bias of the individual studies can be seen in Table 3.
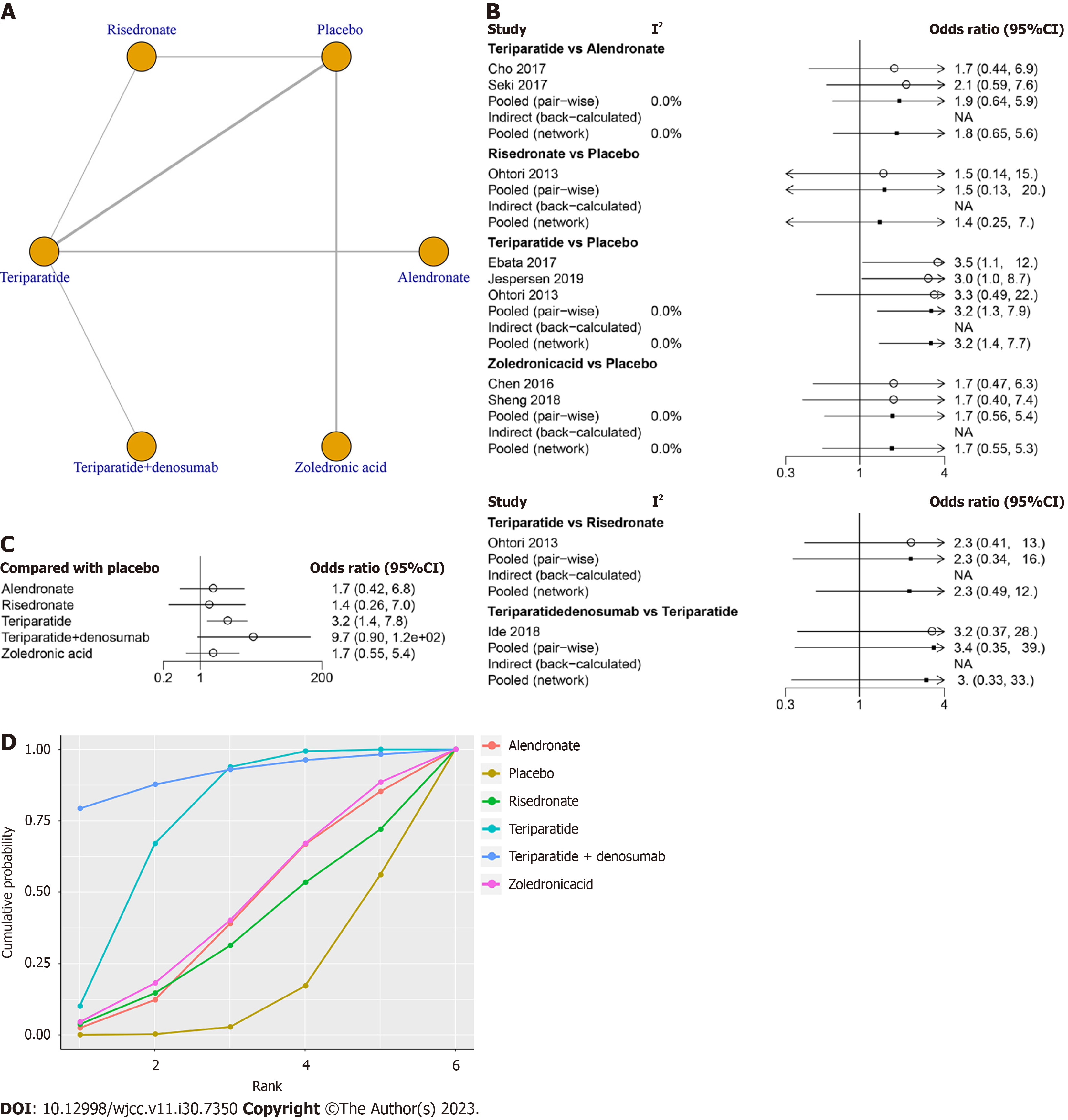
| Alendronate | 0.59 (0.15, 2.4) | 0.84 (0.11, 5.72) | 1.88 (0.64, 5.75) | 5.73 (0.48, 77.52) | 1 (0.17, 6.13) |
| 1.69 (0.42, 6.82) | Placebo | 1.42 (0.26, 7.05) | 3.17 (1.36, 7.77) | 9.66 (0.9, 121.89) | 1.69 (0.55, 5.37) |
| 1.19 (0.17, 8.76) | 0.7 (0.14, 3.89) | Risedronate | 2.24 (0.48, 12.08) | 6.85 (0.44, 123.81) | 1.2 (0.17, 9.35) |
| 0.53 (0.17, 1.57) | 0.32 (0.13, 0.74) | 0.45 (0.08, 2.1) | Teriparatide | 3.03 (0.33, 32.49) | 0.53 (0.13, 2.26) |
| 0.17 (0.01, 2.08) | 0.1 (0.01, 1.11) | 0.15 (0.01, 2.25) | 0.33 (0.03, 3.05) | Teriparatide + denosumab | 0.18 (0.01, 2.47) |
| 1 (0.16, 5.99) | 0.59 (0.19, 1.83) | 0.83 (0.11, 5.98) | 1.88 (0.44, 7.79) | 5.68 (0.4, 91.1) | Zoledronic acid |
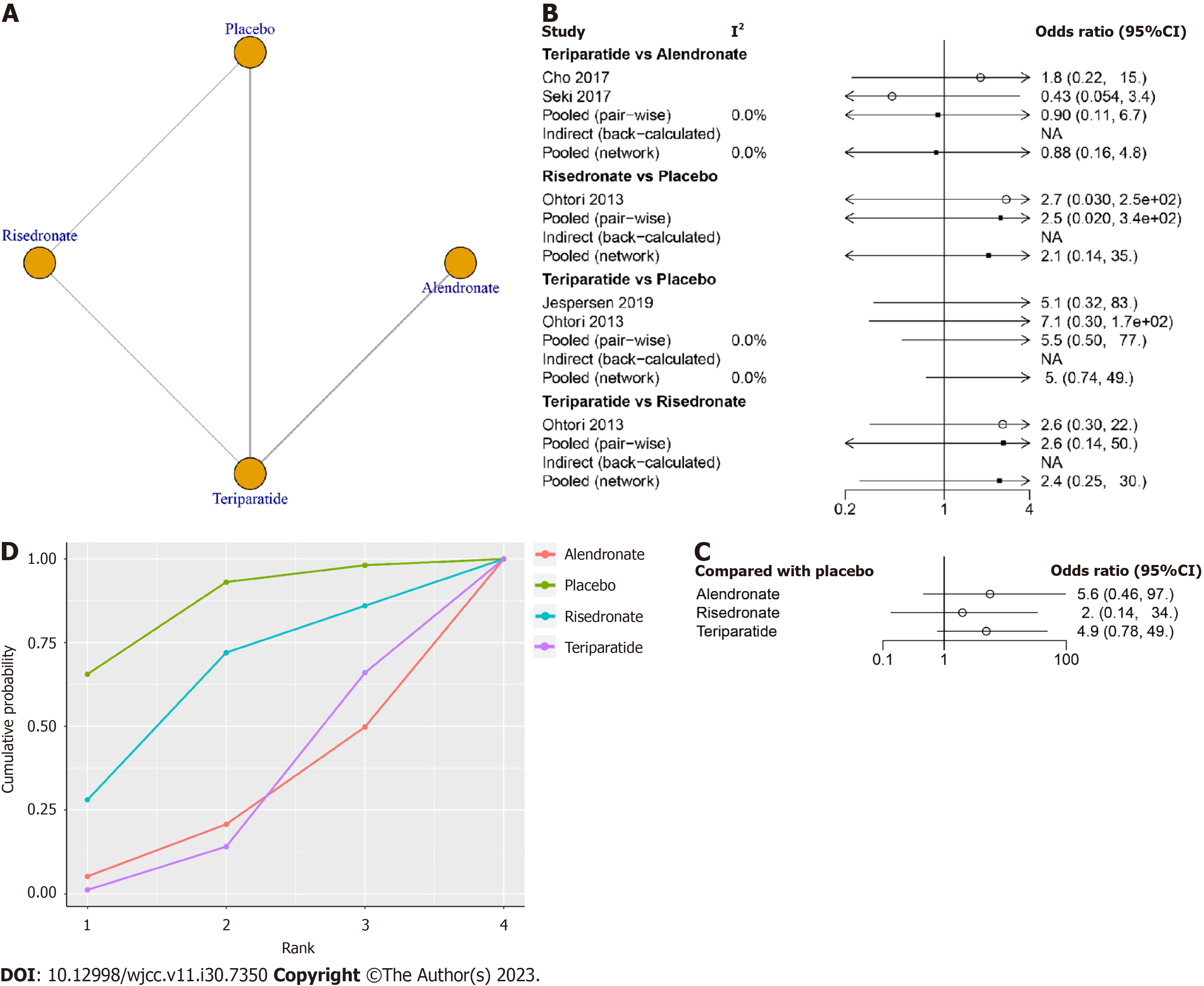
Ten studies involving 618 patients, including six treatments (risedronate, teriparatide, teriparatide combined with denosumab, zoledronic acid, alendronate and placebo), contributed to the clinical outcome of the fusion rate at final follow-up. The network structure diagrams in Figure 2A detail the direct comparisons between different drugs in the fusion rate. Network meta-analysis showed considerable heterogeneity, with global I2 = 0% (Figure 2B).
In the head-to-head comparison, only teriparatide (OR 3.2, 95%CI: 1.4 to 7.8, Figure 2C) was more effective than the placebo in increasing the fusion rate. There was no statistically significant difference between alendronate vs placebo, risedronate vs placebo, zoledronic acid vs placebo or teriparatide combined with denosumab vs placebo in terms of the fusion rate at final follow-up (P > 0.05, Table 3). The SUCRA was highest for teriparatide combined with denosumab (SUCRA, 90.9%), followed by teriparatide (SUCRA, 74.0%), zoledronic acid (SUCRA, 43.7%), alendronate (SUCRA, 41.1%) and risedronate (SUCRA, 35.0%, Figure 2D).
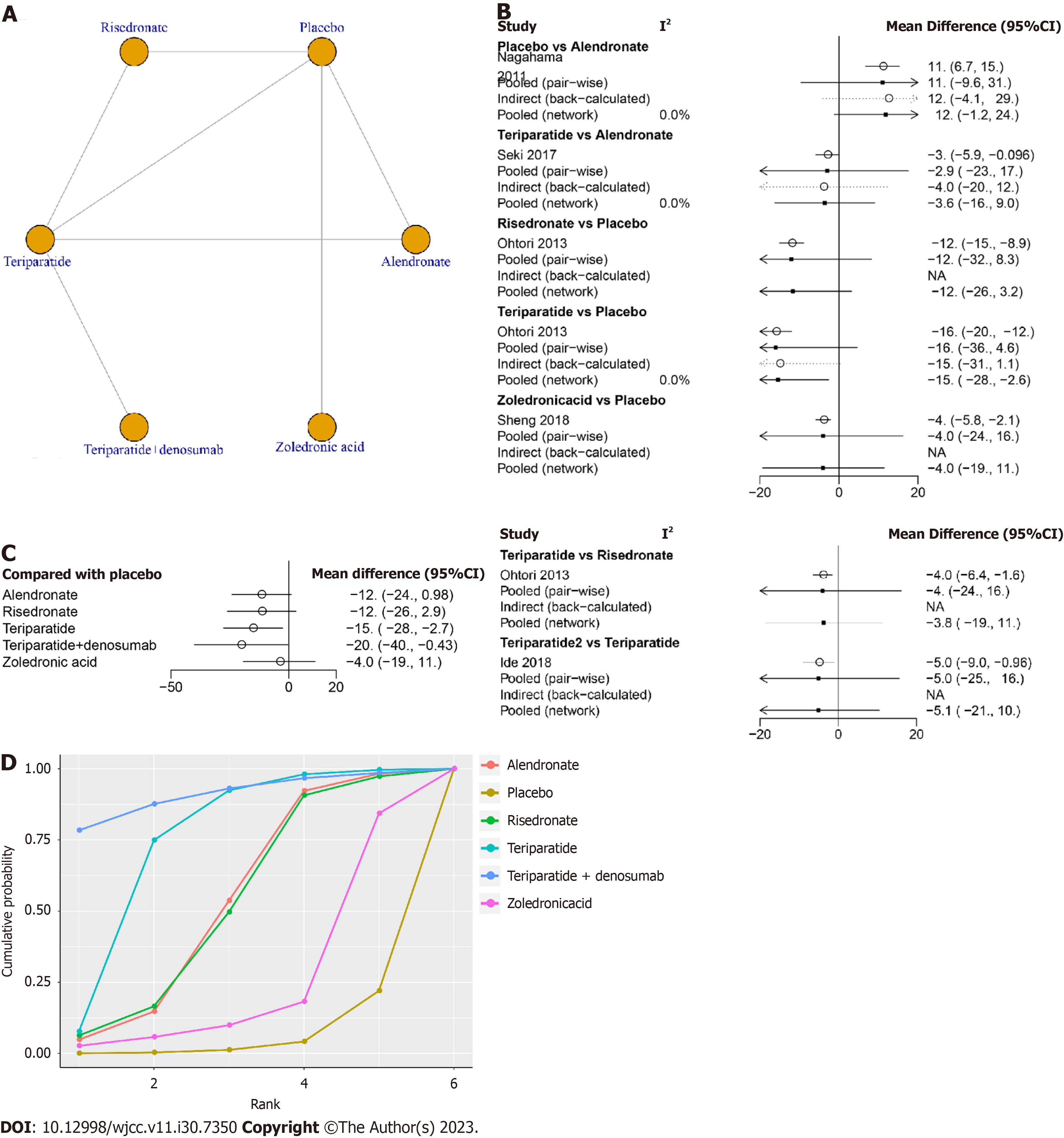
Five studies involving 226 patients, including six treatments (risedronate, teriparatide, teriparatide combined with denosumab, zoledronic acid, alendronate and placebo), reported the clinical outcome of the ODI at final follow-up. The network structure diagrams in Figure 3A detail the direct comparisons of ODI between different drugs. Network meta-analysis showed considerable heterogeneity, with global I2 = 0% (Figure 3B).
In the head-to-head comparison, teriparatide (MD -15, 95%CI: -28 to -2.7, Figure 3C) and teriparatide combined with denosumab (MD -20, 95%CI: -40 to -0.43, Figure 3C) were more effective than the placebo in decreasing the ODI. There was no statistically significant difference between other treatments and placebo in terms of the ODI at final follow-up (P > 0.05, Table 4).
| Alendronate | 11.85 (-0.98, 24.15) | 0.17 (-17.13, 17.05) | -3.57 (-16.04, 8.89) | -8.56 (-28.58, 11.16) | 7.85 (-12.25, 27.17) |
| -11.85 (-24.15, 0.98) | Placebo | -11.68 (-26.12, 2.94) | -15.43 (-27.71, -2.7) | -20.42 (-40.08, -0.43) | -4.01 (-19.31, 11.09) |
| -0.17 (-17.05, 17.13) | 11.68 (-2.94, 26.12) | Risedronate | -3.76 (-18.1, 10.98) | -8.74 (-29.92, 12.56) | 7.67 (-13.54, 28.7) |
| 3.57 (-8.89, 16.04) | 15.43 (2.7, 27.71) | 3.76 (-10.98, 18.1) | Teriparatide | -4.99 (-20.54, 10.47) | 11.44 (-8.49, 30.78) |
| 8.56 (-11.16, 28.58) | 20.42 (0.43, 40.08) | 8.74 (-12.56, 29.92) | 4.99 (-10.47, 20.54) | Teriparatide + denosumab | 16.43 (-8.94, 41.21) |
| -7.85 (-27.17, 12.25) | 4.01 (-11.09, 19.31) | -7.67 (-28.7, 13.54) | -11.44 (-30.78, 8.49) | -16.43 (-41.21, 8.94) | Zoledronic acid |
The SUCRA was highest for teriparatide combined with denosumab (SUCRA, 90.8%), followed by teriparatide (SUCRA, 74.5%), alendronate (SURCA, 52.7), risedronate (SURCA, 52.1%), zoledronic acid (SURCA, 24.2%) and placebo (SURCA, 5.6%, Figure 3D).
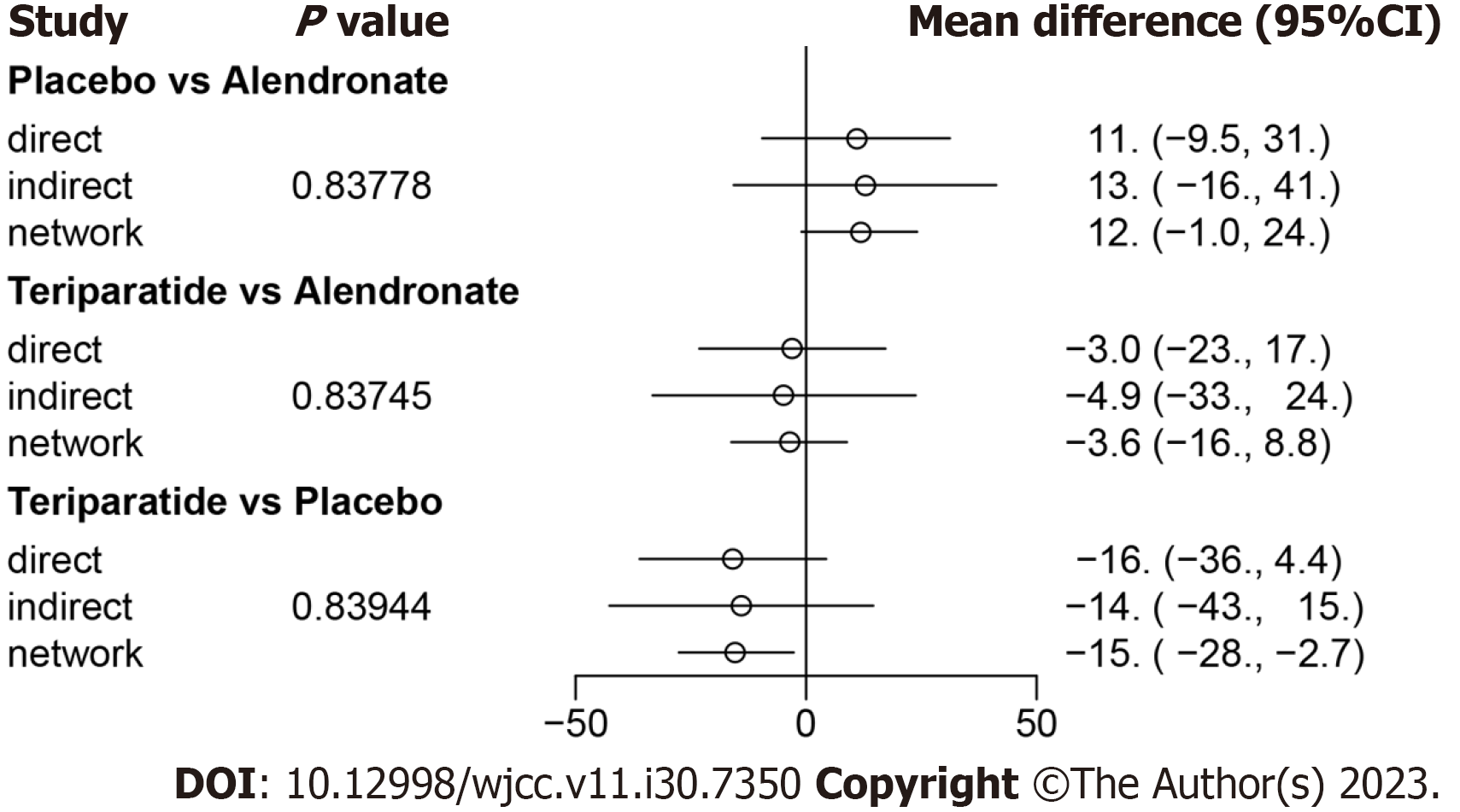
We used the node-splitting method and its Bayesian P value to report the inconsistency of our results. For ODI, the confidence intervals from direct and indirect evidence were generally consistent, with minor differences (all P > 0.05, Figure 4).
Four studies involving 252 patients, testing four treatments (risedronate, teriparatide, alendronate and placebo), contributed to the clinical outcome of the adverse events. The network structure diagrams in Figure 5A illustrate the direct comparisons of different drugs on adverse events. Network meta-analysis showed considerable heterogeneity, with global I2 = 0% (Figure 5B).
In the head-to-head comparison, there was no statistically significant difference between any anti-osteoporosis drugs and placebo in terms of adverse events (P > 0.05, Table 5, Figure 5C).
| Alendronate | 0.18 (0.01, 2.18) | 0.36 (0.02, 5.83) | 0.89 (0.16, 4.76) |
| 5.64 (0.46, 97.29) | Placebo | 2 (0.14, 33.59) | 4.91 (0.78, 49) |
| 2.8 (0.17, 56.64) | 0.5 (0.03, 7.4) | Risedronate | 2.46 (0.27, 30.06) |
| 1.13 (0.21, 6.23) | 0.2 (0.02, 1.28) | 0.41 (0.03, 3.74) | Teriparatide |
The SUCRA of teriparatide combined with denosumab was highest (SUCRA, 85.6%), followed by risedronate (SUCRA, 62.0%), teriparatide (SURCA, 27.1%) and alendronate (SURCA, 25.3%, Figure 5D).
This is the first network meta-analysis comparing different anti-osteoporosis drugs for spinal fusion surgery patients. Our network meta-analysis included 13 RCTs and compared different anti-osteoporosis drugs on fusion rate, ODI and adverse events after spinal fusion surgery. A total of 592 patients were treated with 6 therapeutic methods, including risedronate, teriparatide, teriparatide combined with denosumab, zoledronic acid, alendronate and placebo.
Teriparatide combined with denosumab and teriparatide alone ranked as the most and second most preferable anti-osteoporosis drug, with higher fusion rates and lower ODIs. Moreover, adverse events did not differ among these groups. These results may help orthopedic surgeons select anti-osteoporosis drugs for spinal fusion surgery patients. In comparison to prior meta-analyses, a key advantage of this network meta-analysis lies in its thorough search strategy and its analysis of the safety and effectiveness of various pharmacological treatments across a larger network of studies and sample size. Additionally, the study features a strong design that allows for the ranking of treatments based on their effects on the desired outcome.
Interestingly, no significant treatment effect of bisphosphonates (risedronate, zoledronic acid and alendronate) was observed on the spinal fusion rate. Previously, a pairwise meta-analysis compared bisphosphonate and teriparatide use in thoracolumbar spinal fusion[42]. They revealed that bisphosphonates had no effects on the spinal fusion rate compared with the control. In contrast, some researchers reached the opposite conclusion about bisphosphonates for the fusion rate in spinal fusion surgery patients. Mei et al[23] conducted an updated meta-analysis and found that postoperative bisphosphonates did not significantly alter the fusion rate after lumbar spinal fusion. Govindarajan et al[43] conducted a meta-analysis and demonstrated the independent benefits of bisphosphonate therapy in accelerating the fusion rate after spinal surgery. These two meta-analyses had common drawbacks of combining these bisphosphonates as a pooled group for analysis. In this network meta-analysis, we separated these bisphosphonates for analysis and ranked their effects.
The United Kingdom National Institute for Clinical Excellence guidelines recommend teriparatide as an alternative treatment in the prevention of osteoporotic fragility fractures in postmenopausal women[44]. According to the American College of Physicians, clinicians should consider denosumab as a secondary pharmacological option for reducing fracture risk in postmenopausal women with primary osteoporosis who are unable to take bisphosphonates due to contraindications or adverse effects[45]. From our network meta-analysis, we recommend teriparatide combined with denosumab as the first choice for increasing the fusion rate. Only one study compared teriparatide combined with denosumab vs teriparatide with a small sample size. Therefore, care should be taken when interpreting these results.
This study does have several limitations that need to be considered when interpreting the findings. First, the major concern of this network meta-analysis is the inclusion of drugs with different doses and treatment durations, which lessens the robustness and reliability of the results and conclusions. Second, subgroup analysis was not done due to the number of included studies. Future studies could compare subgroups of fusion level, drug dose, and drug duration. Third, potential confounding factors (e.g., smoking status, obesity, and initial osteoporotic status) were not accounted for and might influence the results. In addition, a wide range of mean ages, the prevalence of females and Asians, and follow-up time data increased the heterogeneity between studies.
This network meta-analysis suggests that teriparatide combined with denosumab and teriparatide alone significantly can increase the fusion rate and decreased the ODI without increasing adverse events. Based on current evidence, teriparatide combined with denosumab or teriparatide alone is recommended to increase the fusion rate and to reduce the ODI in spinal fusion patients. However, the overall quality of evidence is low, and the overall certainty of the synthesized evidence is low. There is a need for more high-quality RCTs to reassess or confirm this conclusion.
Administering anti-osteoporotic agents to patients perioperatively is a widely accepted approach for improving bone fusion rates and reducing the risk of complications. The best anti-osteoporotic agents for spinal fusion surgery remain unclear.
This network meta-analysis suggests that teriparatide combined with denosumab and teriparatide alone significantly can increase the fusion rate and decreased the Oswestry disability index (ODI) without increasing adverse events.
The purpose of this study was to investigate the efficacy and safety of different anti-osteoporotic agents in spinal fusion surgery via network meta-analysis.
Searches were conducted in four electronic databases (PubMed, EMBASE, Web of Science, the Cochrane Library and China National Knowledge Infrastructure (CNKI) from inception to November 2022. Any studies that compared anti-osteoporotic agents vs placebo for spinal fusion surgery were included in this network meta-analysis. Outcomes included fusion rate, ODI, and adverse events. Network meta-analysis was performed by R software with the gemtc package.
In total, 13 randomized controlled trials were included in this network meta-analysis. Only teriparatide (OR 3.2, 95%CI: 1.4 to 7.8) was more effective than placebo in increasing the fusion rate. The surface under the cumulative ranking curve (SUCRA) of teriparatide combined with denosumab was the highest (SUCRA, 90.9%), followed by teriparatide (SUCRA, 74.0%), zoledronic acid (SUCRA, 43.7%), alendronate (SUCRA, 41.1%) and risedronate (SUCRA, 35.0%). Teriparatide (MD -15, 95%CI: -28 to -2.7) and teriparatide combined with denosumab (MD -20, 95%CI: -40 to -0.43) were more effective than placebo in decreasing the ODI. The SUCRA of teriparatide combined with denosumab was highest (SUCRA, 90.8%), followed by teriparatide (SUCRA, 74.5%), alendronate (SURCA, 52.7), risedronate (SURCA, 52.1%), zoledronic acid (SURCA, 24.2%) and placebo (SURCA, 5.6%) for ODI. The adverse events were not different between groups.
This network meta-analysis suggests that teriparatide combined with denosumab and teriparatide alone significantly increase the fusion rate and decrease the ODI without increasing adverse events. Based on current evidence, teriparatide combined with denosumab or teriparatide alone is recommended to increase the fusion rate and to reduce ODI in spinal fusion patients.
Teriparatide combined with denosumab or teriparatide alone is recommended to increase the fusion rate and to reduce ODI in spinal fusion patients.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Orthopedics
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ma X, China S-Editor: Liu JH L-Editor: A P-Editor: Xu ZH
| 1. | Knezevic NN, Candido KD, Vlaeyen JWS, Van Zundert J, Cohen SP. Low back pain. Lancet. 2021;398:78-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 673] [Article Influence: 168.3] [Reference Citation Analysis (0)] |
| 2. | Urits I, Burshtein A, Sharma M, Testa L, Gold PA, Orhurhu V, Viswanath O, Jones MR, Sidransky MA, Spektor B, Kaye AD. Low Back Pain, a Comprehensive Review: Pathophysiology, Diagnosis, and Treatment. Curr Pain Headache Rep. 2019;23:23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 281] [Article Influence: 46.8] [Reference Citation Analysis (0)] |
| 3. | Lu VM, Kerezoudis P, Gilder HE, McCutcheon BA, Phan K, Bydon M. Minimally Invasive Surgery Versus Open Surgery Spinal Fusion for Spondylolisthesis: A Systematic Review and Meta-analysis. Spine (Phila Pa 1976). 2017;42:E177-E185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 59] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 4. | Cai YF, Tian TZ, Chen LY, Liu BX, Zhou JP, Shi M, Liang HD. The effect of platelet-rich plasma on the fusion rate and clinical outcome of spinal fusion surgery: A systematic review and meta-analysis. PLoS One. 2020;15:e0243204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Mu X, Kim SW, Uhl E, Schöller K. The effects of lumbar fusion and non-fusion surgery on the development of Modic changes. J Orthop Surg Res. 2022;17:89. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 6. | Kurucan E, Bernstein DN, Ying M, Li Y, Menga EN, Sponseller PD, Mesfin A. Trends in spinal deformity surgery in Marfan syndrome. Spine J. 2019;19:1934-1940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Migliorini F, Giorgino R, Hildebrand F, Spiezia F, Peretti GM, Alessandri-Bonetti M, Eschweiler J, Maffulli N. Fragility Fractures: Risk Factors and Management in the Elderly. Medicina (Kaunas). 2021;57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 124] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 8. | Gupta A, Cha T, Schwab J, Fogel H, Tobert D, Razi AE, Hecht A, Bono CM, Hershman S. Osteoporosis increases the likelihood of revision surgery following a long spinal fusion for adult spinal deformity. Spine J. 2021;21:134-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 47] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 9. | Zhang W, Li L, Zhou X, Li K, Liu C, Lin X, Lubisi N, Chen J, Si H. Concurrent Treatment with Vitamin K2 and D3 on Spine Fusion in Patients with Osteoporosis-Associated Lumbar Degenerative Disorders. Spine (Phila Pa 1976). 2022;47:352-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 10. | Chaudhary N, Lee JS, Wu JY, Tharin S. Evidence for Use of Teriparatide in Spinal Fusion Surgery in Osteoporotic Patients. World Neurosurg. 2017;100:551-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 11. | Lubelski D, Choma TJ, Steinmetz MP, Harrop JS, Mroz TE. Perioperative Medical Management of Spine Surgery Patients With Osteoporosis. Neurosurgery. 2015;77 Suppl 4:S92-S97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 12. | Kang T, Park SY, Hong SH, Lee JH, Lee SH, Park JH. Bone union after spinal fusion surgery using local bone in long-term bisphosphonate users: a prospective comparative study. Arch Osteoporos. 2019;14:74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 13. | Hirsch BP, Unnanuntana A, Cunningham ME, Lane JM. The effect of therapies for osteoporosis on spine fusion: a systematic review. Spine J. 2013;13:190-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 14. | Wang M, Meng X, Li Y, Feng Y, Chang Z, Hai Y. Effects of anti-osteoporosis treatment in the elderly with anterior cervical discectomy and fusion. Acta Orthop Traumatol Turc. 2016;50:186-190. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 15. | Migliorini F, Colarossi G, Eschweiler J, Oliva F, Driessen A, Maffulli N. Antiresorptive treatments for corticosteroid-induced osteoporosis: a Bayesian network meta-analysis. Br Med Bull. 2022;143:46-56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 78] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 16. | Wanderman N, Alvi M, Yolcu Y, Carlson B, Sebastian A, Bydon M, Freedman B. Is Teriparatide Beneficial to Spinal Fusion Surgery in the Older Patient?: A Narrative Review. Clin Spine Surg. 2019;32:182-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Migliorini F, Maffulli N, Colarossi G, Eschweiler J, Tingart M, Betsch M. Effect of drugs on bone mineral density in postmenopausal osteoporosis: a Bayesian network meta-analysis. J Orthop Surg Res. 2021;16:533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 117] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 18. | Migliorini F, Maffulli N, Spiezia F, Peretti GM, Tingart M, Giorgino R. Potential of biomarkers during pharmacological therapy setting for postmenopausal osteoporosis: a systematic review. J Orthop Surg Res. 2021;16:351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 89] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 19. | Migliorini F, Maffulli N, Spiezia F, Tingart M, Maria PG, Riccardo G. Biomarkers as therapy monitoring for postmenopausal osteoporosis: a systematic review. J Orthop Surg Res. 2021;16:318. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 96] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 20. | Ming N, Cheng JT, Rui YF, Chan KM, Kuhstoss S, Ma YL, Sato M, Wang Y, Li G. Dose-dependent enhancement of spinal fusion in rats with teriparatide (PTH[1-34]). Spine (Phila Pa 1976). 2012;37:1275-1282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 21. | Liu WB, Zhao WT, Shen P, Zhang FJ. The effects of bisphosphonates on osteoporotic patients after lumbar fusion: a meta-analysis. Drug Des Devel Ther. 2018;12:2233-2240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 22. | Park YS, Kim HS, Baek SW, Kong DY, Ryu JA. The effect of zoledronic acid on the volume of the fusion-mass in lumbar spinal fusion. Clin Orthop Surg. 2013;5:292-297. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 23. | Mei J, Song X, Guan X, Wu D, Wang J, Liu Q. Postoperative bisphosphonate do not significantly alter the fusion rate after lumbar spinal fusion: a meta-analysis. J Orthop Surg Res. 2021;16:284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 24. | Tani S, Ishikawa K, Kudo Y, Tsuchiya K, Matsuoka A, Maruyama H, Emori H, Yamamura R, Hayakawa C, Sekimizu M, Oshita Y, Ozawa T, Shirahata T, Nagai T, Toyone T, Inagaki K. The effect of denosumab on pedicle screw fixation: a prospective 2-year longitudinal study using finite element analysis. J Orthop Surg Res. 2021;16:219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 25. | Migliorini F, Colarossi G, Baroncini A, Eschweiler J, Tingart M, Maffulli N. Pharmacological Management of Postmenopausal Osteoporosis: a Level I Evidence Based - Expert Opinion. Expert Rev Clin Pharmacol. 2021;14:105-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 90] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 26. | Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge SM, Emberson JR, Hernán MA, Hopewell S, Hróbjartsson A, Junqueira DR, Jüni P, Kirkham JJ, Lasserson T, Li T, McAleenan A, Reeves BC, Shepperd S, Shrier I, Stewart LA, Tilling K, White IR, Whiting PF, Higgins JPT. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6581] [Cited by in RCA: 15259] [Article Influence: 2543.2] [Reference Citation Analysis (0)] |
| 27. | Sutton AJ, Abrams KR. Bayesian methods in meta-analysis and evidence synthesis. Stat Methods Med Res. 2001;10:277-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 353] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 28. | Chen F, Dai Z, Kang Y, Lv G, Keller ET, Jiang Y. Effects of zoledronic acid on bone fusion in osteoporotic patients after lumbar fusion. Osteoporos Int. 2016;27:1469-1476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 29. | Cho PG, Ji GY, Shin DA, Ha Y, Yoon DH, Kim KN. An effect comparison of teriparatide and bisphosphonate on posterior lumbar interbody fusion in patients with osteoporosis: a prospective cohort study and preliminary data. Eur Spine J. 2017;26:691-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 30. | Ebata S, Takahashi J, Hasegawa T, Mukaiyama K, Isogai Y, Ohba T, Shibata Y, Ojima T, Yamagata Z, Matsuyama Y, Haro H. Role of Weekly Teriparatide Administration in Osseous Union Enhancement within Six Months After Posterior or Transforaminal Lumbar Interbody Fusion for Osteoporosis-Associated Lumbar Degenerative Disorders: A Multicenter, Prospective Randomized Study. J Bone Joint Surg Am. 2017;99:365-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 106] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 31. | Ide M, Yamada K, Kaneko K, Sekiya T, Kanai K, Higashi T, Saito T. Combined teriparatide and denosumab therapy accelerates spinal fusion following posterior lumbar interbody fusion. Orthop Traumatol Surg Res. 2018;104:1043-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 32. | Jespersen AB, Andresen ADK, Jacobsen MK, Andersen MØ, Carreon LY. Does Systemic Administration of Parathyroid Hormone After Noninstrumented Spinal Fusion Surgery Improve Fusion Rates and Fusion Mass in Elderly Patients Compared to Placebo in Patients With Degenerative Lumbar Spondylolisthesis? Spine (Phila Pa 1976). 2019;44:157-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 33. | Li C, Wang HR, Li XL, Zhou XG, Dong J. The relation between zoledronic acid infusion and interbody fusion in patients undergoing transforaminal lumbar interbody fusion surgery. Acta Neurochir (Wien). 2012;154:731-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 34. | Nagahama K, Kanayama M, Togawa D, Hashimoto T, Minami A. Does alendronate disturb the healing process of posterior lumbar interbody fusion? A prospective randomized trial. J Neurosurg Spine. 2011;14:500-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 105] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 35. | Ohtori S, Inoue G, Orita S, Yamauchi K, Eguchi Y, Ochiai N, Kishida S, Kuniyoshi K, Aoki Y, Nakamura J, Ishikawa T, Miyagi M, Kamoda H, Suzuki M, Kubota G, Sakuma Y, Oikawa Y, Inage K, Sainoh T, Takaso M, Toyone T, Takahashi K. Comparison of teriparatide and bisphosphonate treatment to reduce pedicle screw loosening after lumbar spinal fusion surgery in postmenopausal women with osteoporosis from a bone quality perspective. Spine (Phila Pa 1976). 2013;38:E487-E492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 206] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 36. | Seki S, Hirano N, Kawaguchi Y, Nakano M, Yasuda T, Suzuki K, Watanabe K, Makino H, Kanamori M, Kimura T. Teriparatide versus low-dose bisphosphonates before and after surgery for adult spinal deformity in female Japanese patients with osteoporosis. Eur Spine J. 2017;26:2121-2127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 37. | Sheng J, Da L, Zheng W, Wu H, Xu W, Ren Y, Chen Y. Zoledronic acid combined with bone cement augmented pedicle screw for osteoporotic lumbar fusion. Medical Journal of Chinese People's Liberation Army. 43:1044-1048. [DOI] [Full Text] |
| 38. | Ushirozako H, Hasegawa T, Ebata S, Oba H, Ohba T, Mukaiyama K, Isogai Y, Okada E, Ojima T, Takahashi J, Haro H, Matsuyama Y. Weekly Teriparatide Administration and Preoperative Anterior Slippage of the Cranial Vertebra Next to Fusion Segment < 2 mm Promote Osseous Union After Posterior Lumbar Interbody Fusion. Spine (Phila Pa 1976). 2019;44:E288-E297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 39. | Yagi M, Ohne H, Konomi T, Fujiyoshi K, Kaneko S, Komiyama T, Takemitsu M, Yato Y, Machida M, Asazuma T. Teriparatide improves volumetric bone mineral density and fine bone structure in the UIV+1 vertebra, and reduces bone failure type PJK after surgery for adult spinal deformity. Osteoporos Int. 2016;27:3495-3502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 96] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 40. | Wang Z, Zhuang C, Chen W, Li Z, Li J, Lin H, Dong J. The Effect of Daily Teriparatide versus One-Time Annually Zoledronic Acid Administration After Transforaminal Lumbar Interbody Fusion in Osteoporotic Patients. Clin Interv Aging. 2021;16:1789-1799. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 41. | Xiong Y, Li L, Liu P, Zhou B, Kang Y, Wang G. Effect of Teriparatide Versus Zoledronate on Posterior Lumbar Interbody Fusion in Postmenopausal Women with Osteoporosis. World Neurosurg. 2022;167:e1310-e1316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Reference Citation Analysis (0)] |
| 42. | Buerba RA, Sharma A, Ziino C, Arzeno A, Ajiboye RM. Bisphosphonate and Teriparatide Use in Thoracolumbar Spinal Fusion: A Systematic Review and Meta-analysis of Comparative Studies. Spine (Phila Pa 1976). 2018;43:E1014-E1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 43. | Govindarajan V, Diaz A, Perez-Roman RJ, Burks SS, Wang MY, Levi AD. Osteoporosis treatment in patients undergoing spinal fusion: a systematic review and meta-analysis. Neurosurg Focus. 2021;50:E9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 44. | Gregson CL, Armstrong DJ, Bowden J, Cooper C, Edwards J, Gittoes NJL, Harvey N, Kanis J, Leyland S, Low R, McCloskey E, Moss K, Parker J, Paskins Z, Poole K, Reid DM, Stone M, Thomson J, Vine N, Compston J. UK clinical guideline for the prevention and treatment of osteoporosis. Arch Osteoporos. 2022;17:58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 310] [Cited by in RCA: 286] [Article Influence: 95.3] [Reference Citation Analysis (0)] |
| 45. | Qaseem A, Hicks LA, Etxeandia-Ikobaltzeta I, Shamliyan T, Cooney TG; Clinical Guidelines Committee of the American College of Physicians, Cross JT Jr, Fitterman N, Lin JS, Maroto M, Obley AJ, Tice JA, Tufte JE. Pharmacologic Treatment of Primary Osteoporosis or Low Bone Mass to Prevent Fractures in Adults: A Living Clinical Guideline From the American College of Physicians. Ann Intern Med. 2023;176:224-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 101] [Article Influence: 50.5] [Reference Citation Analysis (0)] |