Published online Oct 26, 2023. doi: 10.12998/wjcc.v11.i30.7337
Peer-review started: July 27, 2023
First decision: September 26, 2023
Revised: September 26, 2023
Accepted: October 8, 2023
Article in press: October 8, 2023
Published online: October 26, 2023
Processing time: 90 Days and 5.3 Hours
Corneal neovascularization (CoNV) is the second major cause of blindness. Vascular endothelial growth factor (VEGF) inhibitors, e.g., bevacizumab, have been used to prevent CoNV.
We conducted an updated systematic review and meta-analysis of clinical trials to examine the efficacy and safety of anti-VEGF in CoNV.
A literature search was conducted using three electronic databases. Mean difference (MD), standard mean difference (SMD), and relative risk (RR) are used to estimate the effect size.
Nine randomized controlled and three non-randomized trials were obtained. The pooled results demonstrated a significant reduction of CoNV area/Length (SMD = -1.17, 95%CI: -1.58 to -0.75), best corrected visual acuity (MD = -0.54, 95%CI: -0.91 to -0.17), and graft rejection (RR = 0.44, 95%CI: 0.24 to 0.8) and failure (RR = 0.39, 95%CI: 0.19 to 0.78) rates in the anti-VEGF group than the placebo group. A non-significant reduction of the epithelial defect was also observed in the bevacizumab group compared with the placebo (RR = 0.56, 95%CI: 0.30 to 1.06). Compared with a placebo, the unsynthesizable trials also support that bevacizumab improves visual acuity, CoNV, graft rejection, and failure rates. Trials reporting other comparisons revealed the superiority of combined remedy with bevacizumab compared to other treatments in reducing CoNV.
Anti-VEGF agents, mainly bevacizumab, are an effective and safe treatment for CoNV of all causes and prevent corneal graft rejection and failure in corneal transplantation.
Core Tip: Vascular endothelial growth factor (VEGF) inhibitor is one of the pharmacological options for treating corneal neovascularization (CoNV) - the second major cause of blindness. The present study conducted a systematic review and meta-analysis support the use of Bevacizumab in the treatment of CoNV. The results of our study supports that anti-VEGF agents are effective in reducing CoNV, best-corrected visual acuity, and graft rejection/failure rate. In addition, our study found evidence supporting the improvement of visual acuity which was not significant in the previous systematic review and meta-analysis.
- Citation: Lai SC, Loh EW, Chiou DI, Hong CT. Efficacy and safety of anti-vascular endothelial growth factor agents on corneal neovascularization: A meta-analysis. World J Clin Cases 2023; 11(30): 7337-7349
- URL: https://www.wjgnet.com/2307-8960/full/v11/i30/7337.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i30.7337
Corneal neovascularization (CoNV) is a condition of pathological vascular ingrowth into the cornea from the limbus, causing the avascular structure to become non-transparent and further markedly threaten the visual acuity. CoNV may be induced by infection, chemical injury, burn, trauma, autoimmune problems, post-corneal surgery, contact lens wearing, and other factors leading to inflammation. Skobe et al[1] indicated that CoNV is the second cause of blindness worldwide. Lee et al[2] reported that CoNV develops in an estimated 1.4 million patients in the United States annually, and 12% of these cases are associated with decreased visual acuity. Lasagni Vitar et al[3] conducted a 14-year retrospective study reviewing 13493 charts in Italy and found that 10.4% of the patients had CoNV, and severe CoNV (three or four of the quadrants) was a significant predictor of low visual acuity. CoNV also reduces the immune privilege of the cornea, which increases the rejection rate of corneal transplantation[4,5].
Various treatment approaches, including anti-inflammatory drugs (e.g., steroids and immunomodulators), laser ablation, photodynamic therapy (PDT), diathermy, and ocular surface restoration, have been used in CoNV management. These approaches are not without problems. Topical steroid use is associated with multiple adverse effects, such as glaucoma and cataracts. A few studies show that PDT is effective and safe for CoNV treatment[6-8], but the technique is time-consuming and relatively expensive. Another effective drug for CoNV is the vascular endothelial growth factor (VEGF) inhibitor. VEGF inhibitors prevent CoNV by blocking the VEGF pathways that promote the survival, proliferation, and migration of vascular endothelial cells that causes neovascularization[9]. Bevacizumab (Avastin) is the most used anti-VEGF drug - a recombinant humanized monoclonal immunoglobulin that binds to VEGF-A, one of the VEGF isoforms in humans. A systematic review and meta-analysis in 2013 that included seven human and 18 experimental animal studies concluded that bevacizumab significantly reduced CoNV[10]. Several randomized controlled trials (RCTs) and non-randomized trials (NRTs) examining the efficacy of anti-VEGF in CoNV have been published in the past few years. In this study, we conducted an updated systematic review and meta-analysis of clinical trials to examine the efficacy and safety of anti-VEGF in CoNV.
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines when reporting the research findings. PROSPERO, an online international prospective register of systematic reviews curated by the National Health Service, United Kingdom, has accepted our review protocol (file number: CRD42022368008).
This study followed the Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines. Relevant studies published before October 2022 were identified by systematic search using PubMed, EMBASE, and Cochrane Library databases. A combination of keywords ‘anti-vascular endothelial growth factor’ OR ‘bevacizumab’ OR ‘ranibizumab’ OR ‘aflibercept’ and ‘corneal neovascularization’ in the form of medical subject headings were used in the search. We also examined the reference sections of relevant papers to identify eligible studies. All articles were retrieved, reviewed, and selected by two reviewers (D-IC and S-CL) separately. Discrepancies were resolved through discussion and consultation with the senior reviewer (E-WL). No language restrictions were applied.
This study included all RCTs and NRTs that compared the efficacy and safety of anti-VEGF drugs in treating or preventing CoNV induced by all kinds of causes, including infections, autoimmune problems, corneal surgeries, chemical burn, contact lens wearing, allergic eye diseases, lipid keratopathy, and exposure keratopathy. We excluded the records if they were case reports, case series, prospective single-arm trials, retrospective studies, review or commentary articles, conference abstracts, trial protocols, or studies using duplicate samples.
We used two methods for assessing the risk of bias in the current study. We assessed non-randomized trials according to the Cochrane Methodology of Risk of Bias in Non-Randomized Studies of Interventions (ROBINS-I)[11] and RCTs according to the revised Cochrane risk-of-bias tool for randomized trials (RoB 2.0, released 15 March 2019)[12]. The ROBINS-I included the following seven domains: (D1) confounding bias, (D2) selection of participant bias, (D3) classification of intervention bias, (D4) deviations from intended intervention bias, (D5) missing data bias, (D6) outcome measurement bias, and (D7) selection of reporting bias. The Rob 2.0 included the following five domains: (D1) bias arising from the randomization process, (D2) deviation from intended interventions, (D3) missing outcome data, (D4) measurement of the outcome, and (D5) selection of the reported result. Two reviewers (D-IC and S-CL) completed the risk-of-bias assessment independently. Disagreements were resolved through discussion and consultation with the same senior reviewer (E-WL).
Information on study designs, inclusion and exclusion criteria, study population characteristics (e.g., age, gender, baseline pathology of the cornea), intervention used (type of treatment, dosage, and frequency), pre- and post-treatment parameters, and complications were extracted. The primary outcomes included: (1) Change in the length or area of CoNV, (2) change in visual acuity (VA) or best corrected visual acuity (BCVA), and (3) graft rejection rate or overall survival rate. For the length or area of CoNV, we pooled the pre- and post-treatment data separately, as well as the area of reduction as reported by the authors. The same reviewers carried out the above procedures.
Continuous outcome data were analyzed using the standard mean difference (SMD) or mean difference (MD) if data was reported in the same unit, and dichotomous outcome data were analyzed using relative risk (RR). The precision of each effect size was reported as a 95% confidence interval (95%CI). Cochran’s Q test was conducted, and the I2 was calculated to evaluate the statistical heterogeneity across trials. We assigned levels of heterogeneity, namely low, moderate, and high, to I2 values of 25%–50%, 51%–75%, and 76%–100%, respectively, for ease of reading. We used the random-effects model[13,14] in meta-analyses, and Review Manager (version 5.4; The Cochrane Collaboration, 2020, the Nordic Cochrane Centre, Copenhagen, Denmark) was used to conduct the analyses.
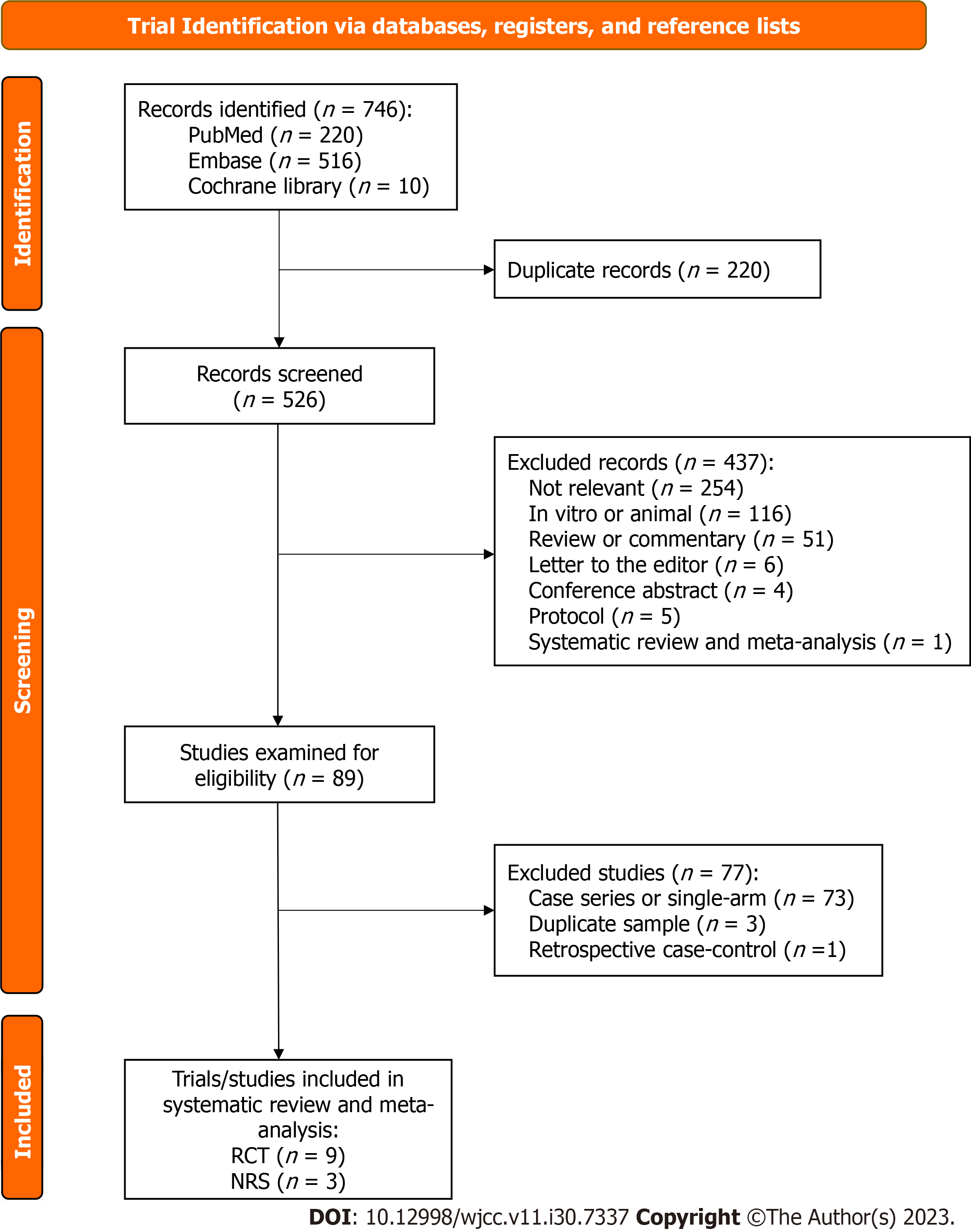
Figure 1 illustrates the flowchart of screening and selection of trials. The initial search identified 746 records. After removing 220 duplicates, 526 records were screened by titles and abstracts. A total of 254 irrelevant records, 116 cell or animal studies, 51 review or commentary articles, six letters, four conference abstracts, five clinical trial protocols, and one systematic review and meta-analysis were excluded. With careful examination of the contents, we removed 73 case reports/series or single-arm studies, three studies using duplicate samples, and one retrospective case-control study. Finally, we included nine RCTs[15-23] and three NRTs[24-26].
Table 1 summarizes the characteristics of the included RCTs and NRTs. The sample size of these studies ranged from 7 to 92 patients. Most trials reported the age and gender information of the patients. The follow-up period ranged from one month to three years. The inclusion criteria of these trials varied. Four RCTs recruited patients with CoNV of different pathologies, four RCTs and two NRTs recruited patients undergoing high-risk transplantations, and one RCT and one NRT recruited patients undergoing recurrent pterygium surgery. The dosage and the frequency of the anti-VEGF drugs also differed.
| Study | Inclusion criteria | Size (M/F), age by yr | Group: No. | F/U by month |
| Randomized controlled trials | ||||
| Bhatti et al[15] | Undergoing high-risk corneal transplantation with CoNV | 81 (62/19), 52.1 ± 5.5 | Topi B: 40, Placebo: 41 | 7.1 (2-8) |
| Dohlman et al[16] | Age > 18 yr, undergoing high-risk PK, defined as CoNV in 1 or more quadrants ≥ 2 mm from the limbus, or extension of CoNV to the graft-host junction in a previously failed graft | 92 (49/43), 62.55 ± 16.5 | SC + Topi B: 48, Placebo: 44 | 12 |
| Fasciani et al[17] | Age > 18 yr, scheduled for high-risk transplantation due to marked CN in post-herpetic leucoma and in re-grafting for repeated rejections | 27 (16/11), 44.7 ± 14.5 | SC B: 14, Placebo: 13 | 26.1 ± 5.7 |
| Hamdan et al[18] | CoNV caused by ocular surface disorders including fungal keratitis and PK | 7 (4/3), 69.7 ± 15.4 | SC B: 2, PDT: 2, Combined: 3 | 6 |
| Kim et al[19] | Chronic CoNV greater than 6 mo | 16 (9/7), 51.1 ± 15.6 | SC B: 8, SC R: 8 | 1 |
| Li et al[20] | CoNV induced by PK after 3 mo | 19 (12/7), 38 | Placebo: 5, SC B: 5, SC B + TA: 5, SC TA: 5 | 36 |
| Ozgurhan et al[21] | Age > 18 yr, undergoing recurrent pterygium excision with conjunctival autograft transplantation | 44 (10/34), 49.5 ± 21.1 | Topi B: 22, Placebo: 22 | 6 |
| Petsoglou et al[22] | Age > 18 yr, presence of progressive CoNV, no epithelial defect. Progressive CoNV with a minimum radial ingrowth of vessels 2 mm from the limbus in the interval of 2 wk to 2 mo | 30 (15/15), 45.7 ± 19.2 | SC B: 15, Placebo: 15 | 3 |
| You et al[23] | CoNV that did not improve after treatment with 1% prednisolone acetate eyedrops instilled QID for at least 1 mo | 29 (18/11), 54.0 ± 12.4 | SC B 1.25mg: 7, SC B 2.5 mg: 15, SC B 5 mg: 7 | 3 |
| Non-randomized studies | ||||
| Huang et al[24] | Age > 18 yr, with diffuse CoNV and opaque cornea due to chemical burns with a complete limbal deficiency; a minimal 6m interval from initial injury to surgery; a posterior cornea with at least 50 mm thickness with a normal reflectivity in an ultrasound biomicroscopy and a smooth endothelial surface in optical coherence tomography | 39 (33/6), 29.4 ± 12.4 | SC B: 26, Placebo: 13 | 14.3±2.2 |
| Hurmeric et al[25] | Age > 18 yr, with pterygium recurrence less than 6m between the diagnosed recurrence and presentation | 9 (7/2), 56 (39-69) | SC R ×1: 5, SC R ×3: 4 | 6 |
| Trufanov et al[26] | Undergoing high-risk transplantation with corneal opacifications of various etiology complicated with CoNV | 56 (NI/NI), 51.1 ± 13.6 | SC A: 27, SC A + laser: 14, Placebo: 15 | 24.5 ± 4.9 |
The upper part of Table 2 summarizes the assessment results of risk of bias for RCTs, and the lower part summarizes the assessment results for NRTs. For RCTs, two trials were of high risk in the bias arising from the randomization process (D1) because they did not conceal the allocation sequence[15,20], five trials were of some concerns because they had imbalance baselines or did not report the allocation sequences[17-19,21,23], and the remaining two trials were of low risk. In the bias due to deviations from intended interventions (D2), three trials were of some concerns because two of them were open labeled without information on deviation[15,17], and one did not report information on blinding or deviation[21]. The remaining six trials were of low risk. In the bias due to missing outcome data (D3), one trial was of high risk due to missing data without explanations[15], two trials were of some concerns because no information of missing data was described[17,20], and the remaining six trials were of low risk. In the bias in the measurement of the outcome (D4), all trials were of low risk. In the bias in the selection of the reported result (D5), one trial was of high risk because the results were reported incompletely[15], and the remaining eight trials were of low risk. For the overall risk, two RCTs were of low risk[16,22], four RCTs were of some concerns[18,19,21,23], and three RCTs had a high risk[15,17,20].
| RoB 2.0 for randomized controlled trials | ||||||||
| D1 | D2 | D3 | D4 | D5 | All | |||
| Bhatti et al[15] | H1 | SC3 | H5 | L | H7 | H | ||
| Dohlman et al[16] | L | L | L | L | L | L | ||
| Fasciani et al[17] | SC2 | SC3 | SC6 | L | L | H | ||
| Hamdan et al[18] | SC2 | L | L | L | L | SC | ||
| Kim et al[19] | SC2 | L | L | L | L | SC | ||
| Li et al[20] | H1 | L | SC6 | L | L | H | ||
| Ozgurhan et al[21] | SC2 | SC4 | L | L | L | SC | ||
| Petsoglou et al[22] | L | L | L | L | L | L | ||
| You et al[23] | SC2 | L | L | L | L | SC | ||
| D1: Bias arising from the randomization process | ||||||||
| D2: Bias due to deviations from intended interventions | ||||||||
| D3: Bias due to missing outcome data | ||||||||
| D4: Bias in measurement of the outcome | ||||||||
| D5: Bias in selection of the reported result | ||||||||
| ROBINS-I for non-randomized studies | ||||||||
| D1 | D2 | D3 | D4 | D5 | D6 | D7 | All | |
| Huang et al[24] | L | L | L | L | M6 | L | L | M |
| Hurmeric et al[25] | M8 | L | L | L | L | L | S9 | S |
| Trufanov et al[26] | M8 | L | L | L | M6 | L | S9 | S |
| D1: Bias due to confounding | ||||||||
| D2: Bias in selection of participants into the study | ||||||||
| D3: Bias in classification of interventions | ||||||||
| D4: Bias due to deviations from intended interventions | ||||||||
| D5: Bias due to missing data | ||||||||
| D6: Bias in measurement of outcomes | ||||||||
| D7: Bias in selection of the reported result | ||||||||
For NRTs, two studies were of moderate risk in the bias due to confounding (D1) due to the lack of baseline information or appropriate analysis to control the confounding[25,26], two trials were of moderate risk in the bias due to missing data (D5) because they did not report the missing data[24,26], and two trials were of serious risk in the bias in the selection of the reported result (D7) because statistical analyses were not conducted in these trials[25,26]. All three NRTs were of low risk in the bias in the selection of participants into the study (D2), bias in the classification of interventions (D3), bias due to deviations from intended interventions (D4), and bias in the measurement of outcomes D6. For the overall risk, one NRT had a moderate risk[24], whereas the other two NRTs had a serious risk[25,26].
Because a large proportion of included trials/studies of our study could not be synthesized, we summarized all findings in Table 3 for overview and pooled those which were synthesizable. Among the 12 trials included, one compared different types of anti-VEGF (bevacizumab to ranibizumab)[19], one compared different doses of bevacizumab[23], and one compared different frequencies of ranibizumab[25]. We described the review results in the last subsection for ease of understanding.
| Trial/Study | Target | Comparison | Brief conclusion | Overall RoB |
| Efficacy of reducing CoNV | ||||
| Hamdan et al[18] | All pathologies | SC B vs PDT vs combined | B = PDT = combined | SC |
| Ozgurhan et al[21] | Pterygium | Topi B vs placebo | B > placebo | SC |
| Bhatti et al[15] | Transplant | Topi B vs placebo | B > placebo | H |
| Dohlman et al[16] | Transplant | SC + Topi B vs placebo | B > placebo | L |
| Li et al[20] | Transplant | Placebo vs SC B vs SC B + TA vs SC TA | B = combined > TA = placebo | H |
| Petsoglou et al[22] | All pathologies | SC B vs placebo | B > placebo | L |
| Kim et al[19] | All pathologies | SC B vs SC R | B > R | SC |
| You et al[23] | All pathologies | SC B 1.25 mg vs SC B 2.5 mg vs SC B 5 mg | 1.25 mg < 2.5 mg = 5 mg | SC |
| Hurmeric et al[25] | Pterygium | SC R ×1 vs SC R ×3 | R ×1 = R ×3 (Statistical analysis not conducted) | S |
| Pooled results of three trials[16,20,22] showed B > placebo on reducing CoNV | ||||
| Efficacy of improving BCVA/VA | ||||
| Li et al[20] | Transplant | Placebo vs SC B vs SC B + TA vs SC TA | B > pre-treatment (No statistical detail) | H |
| Bhatti et al[15] | Transplant | Topi B vs placebo | B > placebo | H |
| Dohlman et al[16] | Transplant | SC + Topi B vs placebo | B = placebo | L |
| Huang et al[24] | Transplant | SC B vs placebo | B > placebo | M |
| Petsoglou et al[22] | All pathologies | SC B vs placebo | B = placebo | L |
| Kim et al[19] | All pathologies | SC B vs SC R | B = R = pre-treatment | SC |
| You et al[23] | All pathologies | SC B 1.25 mg vs 2.5 mg vs 5 mg | 1.25 mg = 2.5 mg = 5 mg = pre-treatment | SC |
| Pooled results of two trials[22,24] showed B > placebo on improving VA | ||||
| Efficacy of reducing graft rejection/failure | ||||
| Li et al[20] | Transplant | Placebo vs SC B vs SC B + TA vs SC TA | B, C > pre-treatment (No statistical detail) | H |
| Dohlman et al[16] | Transplant | SC + Topi B vs placebo | B = placebo | L |
| Fasciani et al[17] | Transplant | SC B vs placebo | B > placebo | H |
| Huang et al[24] | Transplant | SC B vs placebo | B > placebo | M |
| Trufanov et al[26] | Transplant | SC A vs SC A + laser vs placebo | A = A + laser > placebo (statistical analysis not conducted) | S |
| Pooled results of four trials[16,17,24,26] showed B > placebo on reducing rejection/failure risks | ||||
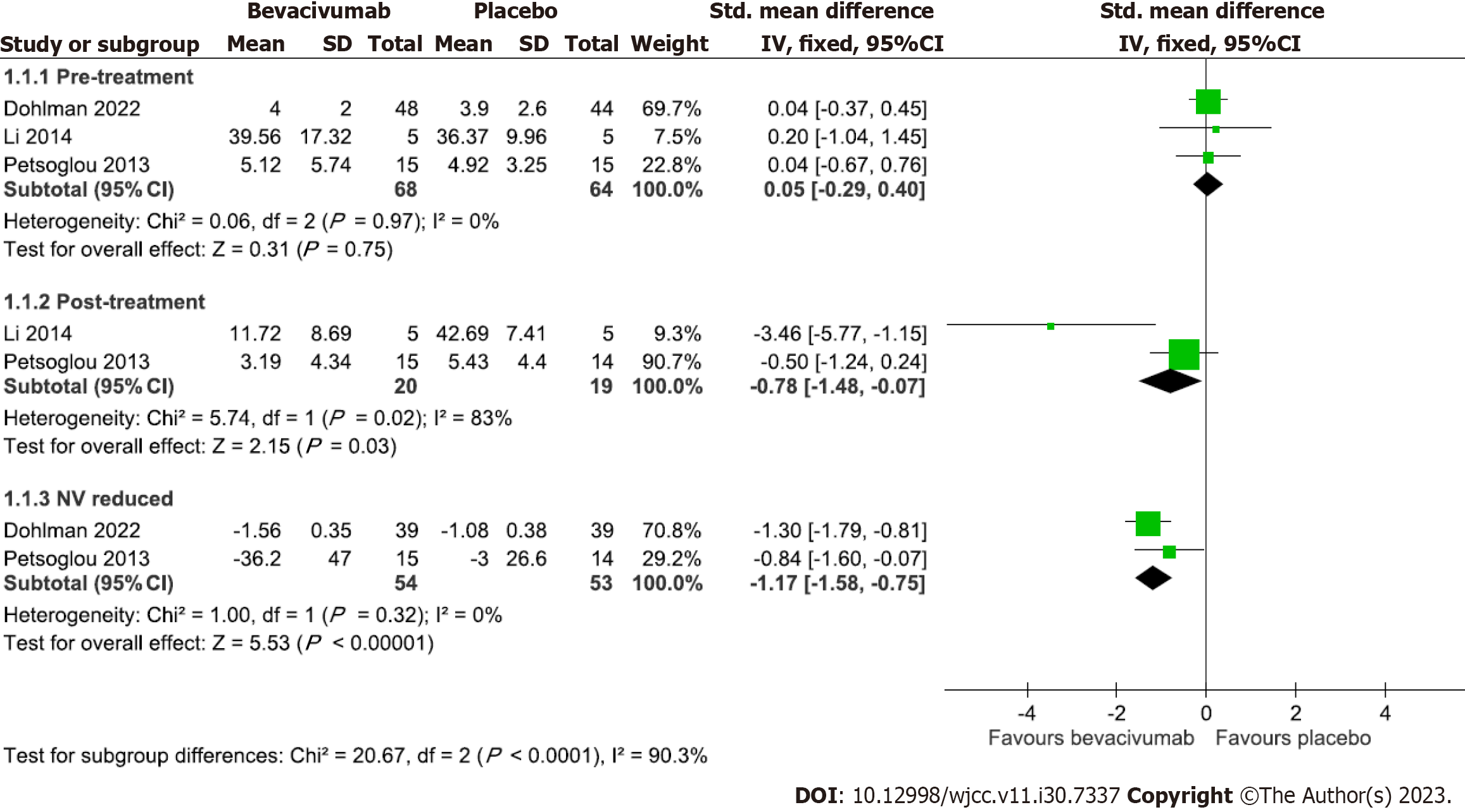
Five trials reported the comparison of bevacizumab on CoNV with a placebo[15,16,20-22]. Three trials reported pre-treatment, reduced, and/or post-treatment CoNV area/Length at 3 mo[22], 1 year[16], and 3 years[20], respectively, were used in the meta-analysis (Figure 2). The pooled results demonstrated no significant difference in post-treatment CoNV area between the bevacizumab group and the placebo group (SMD = -1.77, 95%CI: -4.65 to 1.11) with high heterogeneity across trials (P = 0.02, I2 = 83%), but a significant reduction of CoNV area/Length in the bevacizumab group than the placebo group (SMD = -1.17, 95%CI: -1.58 to -0.75) without heterogeneity across trials (P = 0.32, I2 = 0%). Among the two trials that cannot be pooled, Ozgurhan et al[21] compared the number of patients having recurrent CoNV in the bevacizumab group with the placebo group at 1 mo, 2 mo, 3 mo, and 6 mo, and concluded that bevacizumab significantly reduced the number of patients with recurrent CoNV; Bhatti et al[15] reported CoNV area in 4 wk and 24 wk after receiving corneal transplantation, and a significant reduction of CoNV area was reported without providing mean and standard deviation.
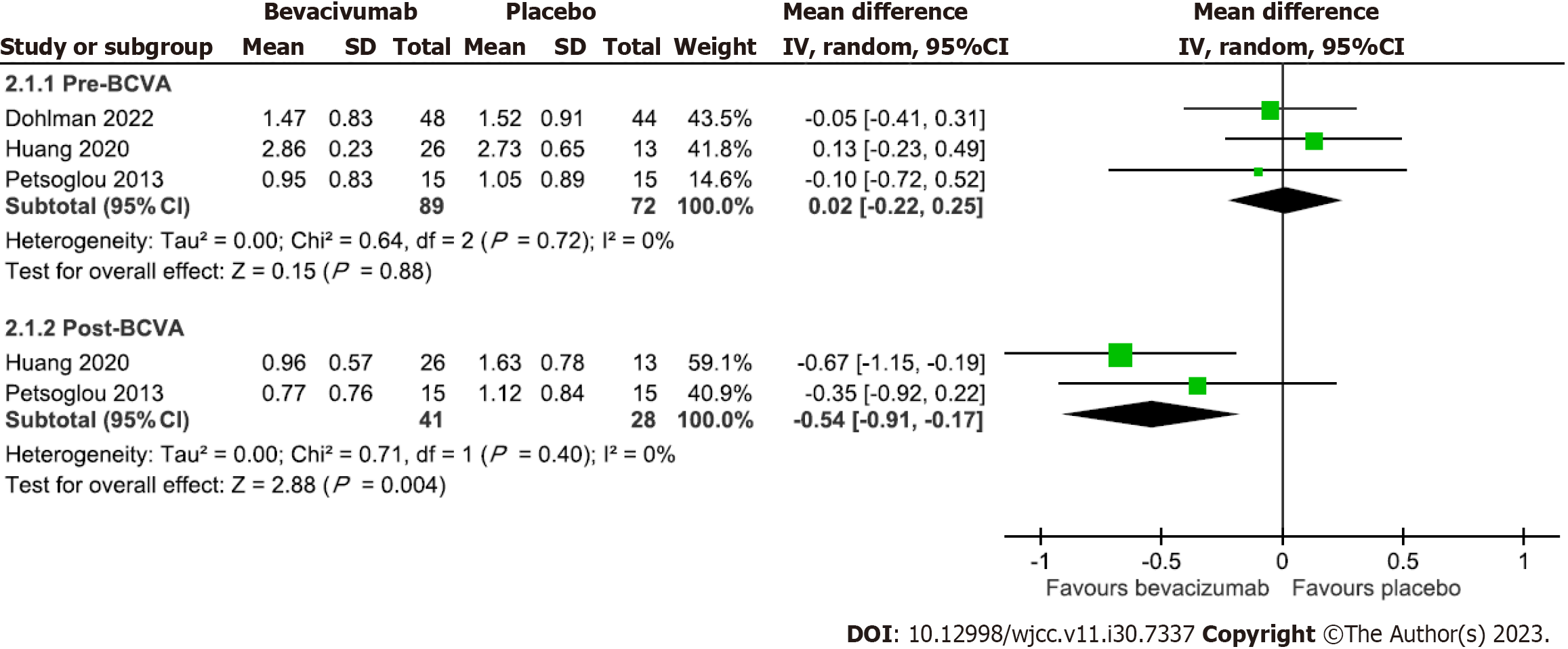
Five trials compared the effect of bevacizumab on BCVA with a placebo[15,16,20,22,24]. Two of them reported that the mean pre-treatment and post-treatment BCVA data at the follow-up endpoint were used in the meta-analysis[22,24]. Figure 3 demonstrates the pooled effect of bevacizumab on BCVA. The results revealed a significantly better BCVA outcome in the bevacizumab group (MD = -0.54, 95%CI: -0.91 to -0.17) than in the placebo group without heterogeneity across trials (P = 0.4, I2 = 0%). The results of the remaining three trials that cannot be pooled are mentioned below. Among them, Li et al[20] reported partial VA improvement without providing statistical details. Bhatti et al[15] and Dohlman et al[16] reported the comparisons in four VA ranges. Bhatti et al[15] reported a significantly better VA in the bevacizumab group without providing the pre-treatment VA data, while Dohlman et al[16] reported pre-treatment VA and post-treatment VA range at 4, 8, 16, 26, and 52 wk and found no significant VA difference between the two groups at any time point.
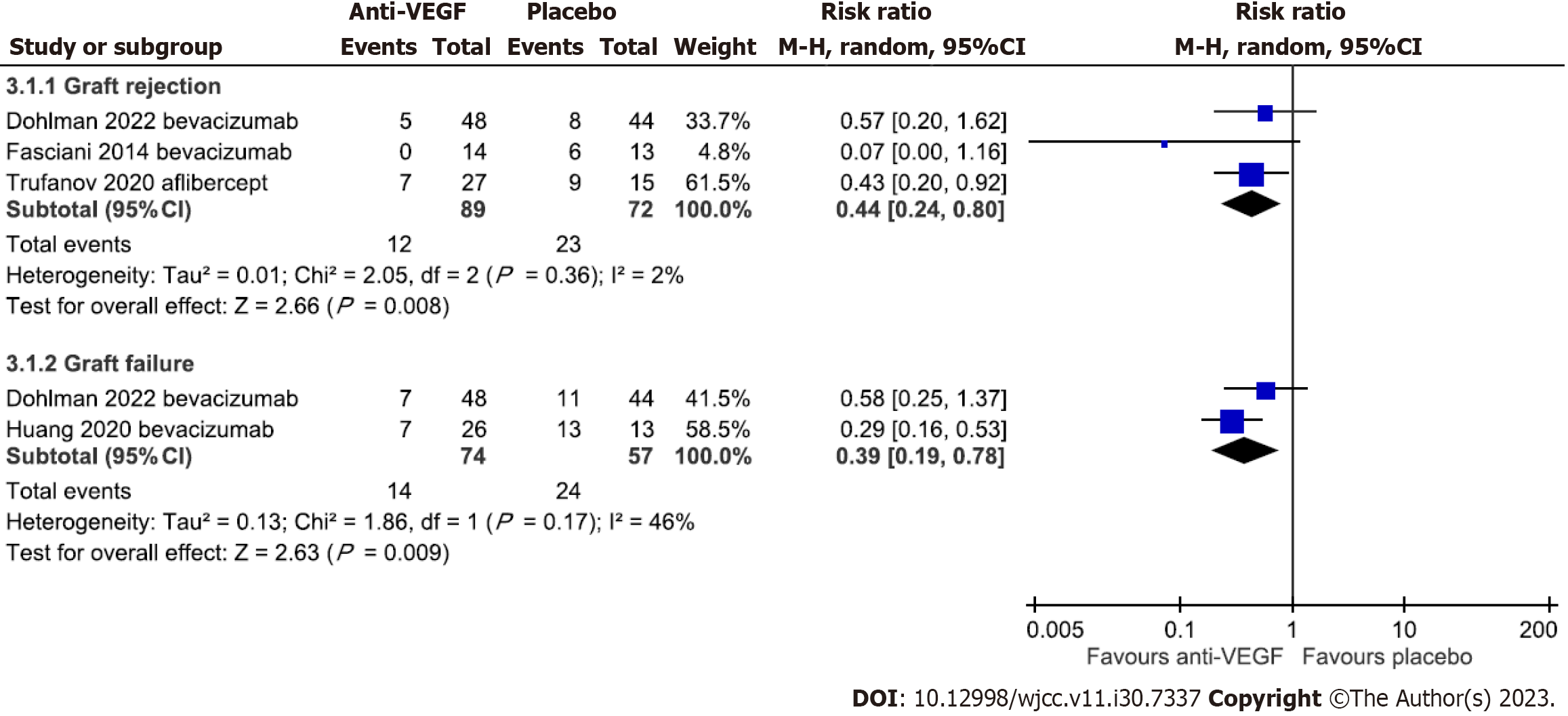
Five trials compared the effect of anti-VEGF drugs with placebo on graft rejection and/or overall survival rates after corneal transplantation[16,17,20,24,26]. Li et al[20] indicated graft rejection decreased in groups using bevacizumab, but the rejection and failure rate details were not reported. The remaining four trials with mean follow-up duration ranging from 14.3 ± 2.2 to 26.1 ± 5.7 mo were used in the meta-analysis (Figure 4)[16,17,24,26]. The result revealed significantly lower graft rejection (RR = 0.44, 95%CI: 0.24 to 0.8) and failure (RR = 0.39, 95%CI: 0.19 to 0.78) rates in the anti-VEGF group compared with placebo, with low heterogeneity across trials (P = 0.36, I2 = 2%; P = 0.17, I2 = 46% respectively).
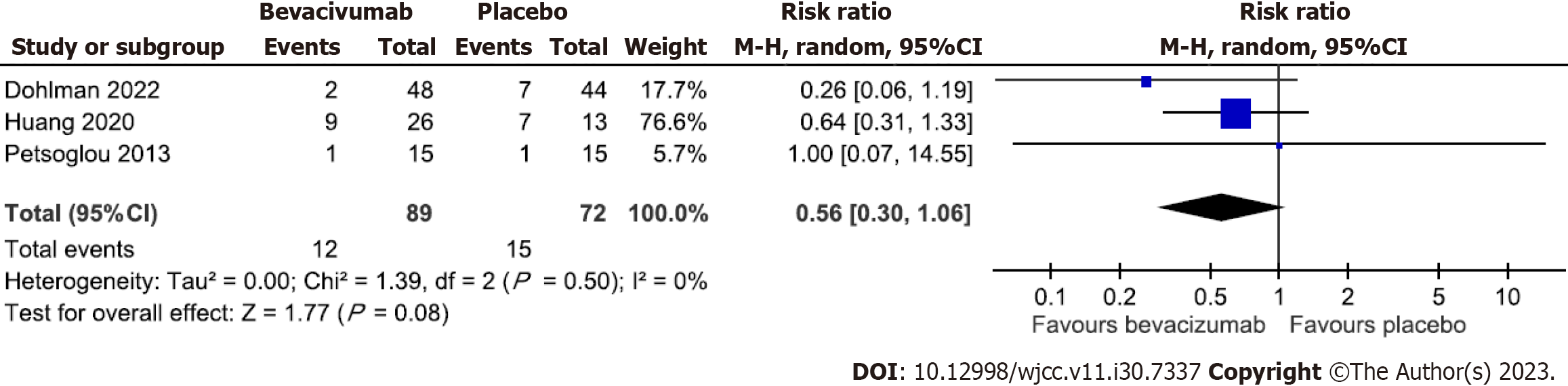
Two of the 12 trials included in this study did not report adverse event information[15,20]. Dohlman et al[16] reported that one patient developed atrial fibrillation in the bevacizumab group. However, it was not considered related to the treatment. For local adverse events, Kim et al[19] reported persistent epithelial defects with corneal melting after the bevacizumab injection in two patients with limbal stem cell deficiency. The other three trials also reported corneal epithelial defects. However, most patients healed after treatments using local antibiotics, artificial tears, and bandage contact lens[16,22,24]. Other minor local adverse events such as foreign body sensation, pain, subconjunctival hemorrhage, photophobia, and tearing were noted[16,17,21-24]. Because bevacizumab may harm epithelial healing, we pooled the three trials that compared bevacizumab with placebo with epithelial defect events reported for meta-analysis[16,22,24]. Figure 5 demonstrates the pooled effect of bevacizumab on the risk of developing epithelial defects. The results revealed a non-significant reduction of the risk of developing epithelial defect in the bevacizumab group compared with placebo (RR = 0.56, 95%CI: 0.30 to 1.06) and without heterogeneity across trials (P = 0.50, I2 = 0%).
This subsection describes the comparisons of different types of anti-VEGF agents, different dosages of bevacizumab, and different frequencies of ranibizumab on CoNV. Li et al[20] and Hamdan et al[18] compared the efficacy bevacizumab with other types of treatment. Li et al[20] compared bevacizumab with triamcinolone acetonide and combined therapy of bevacizumab and triamcinolone acetonide and found that both bevacizumab and combined therapy groups significantly reduced CoNV compared with the triamcinolone acetonide group. Hamdan et al[18] compared bevacizumab with PDT and combined therapy of bevacizumab and PDT with a follow-up period of six months; the combined therapy showed a non-significant tendency toward greater efficacy than single monotherapies in reducing CoNV. Kim et al[19] compared the efficacy of bevacizumab with ranibizumab in a 1-month follow-up period. They found that the average decrease of CoNV area was significantly greater in the bevacizumab group than in the ranibizumab group, with a mean change of VA not showing a significant improvement in either group. You et al[23] compared the efficacy of bevacizumab at different dosages (1.25 mg/0.05 mL, 2.5 mg/0.1 mL, and 5.0 mg/0.2 mL, subconjunctival route) in a 3-mo follow-up period. They found that 2.5 mg and 5 mg of bevacizumab reduced the CoNV area significantly, but no VA improvement differences between groups. Hurmeric et al[25] compared the effect of ranibizumab in different frequencies (once or every two weeks for three times) and revealed no prominent role of recurrent injections over a single injection in reducing CoNV.
We found that anti-VEGF agents significantly reduced CoNV, BCVA, and graft rejection/failure rate compared with placebo. There was a non-significant trend toward reduction of the risk of developing corneal epithelial defects in the bevacizumab group compared with placebo. Also, combined remedies with bevacizumab have better efficacy in reducing CoNV compared with single other treatments. Per the previous systematic review and meta-analysis by Papathanassiou et al[10], we demonstrated that anti-VEGF agents significantly reduce CoNV in corneal transplantation, recurrent pterygium, and other pathologies. While Papathanassiou et al[10] did not find evidence supporting the efficacy of bevacizumab in improving VA, we demonstrated some evidence supporting the efficacy of bevacizumab in improving VA.
Among the included trials, one RCT revealed that bevacizumab is more effective than steroids in reducing CoNV[20]. Also, Kim et al[19] found that the average reduction of CoNV area was significantly greater in the bevacizumab group than in the ranibizumab group. Ranibizumab is a lower molecular weight anti-VEGF agent compared with bevacizumab, and it is supposed to have a better penetration capacity than bevacizumab. Also, ranibizumab was reported to be more potent than bevacizumab and might provide better VEGF inhibition compared with bevacizumab[27]. However, it did not show better efficacy in reducing CoNV, according to Kim et al[19]. This might be caused by underdosing of ranibizumab. An ideal dose of ranibizumab needs further studies for clarification.
The effect of anti-VEGF drugs on VA appears to be affected by pre-treatment disease status. We noticed that those trials with worse pre-treatment VA tended to have a relatively noticeable VA change, and those with relatively better pre-treatment VA tended to have an unchanged post-treatment VA. Nevertheless, though not statistically significant, anti-VEGF drugs demonstrated a tendency to improve VA in patients with CoNV. For high-risk corneal transplantation, our study showed that anti-VEGF agents significantly decrease the risk of graft rejection and failure, possibly through the reduction of inflammatory responses, which is crucial to graft failure[28,29]. Also, the reduction of NV of the host may decrease the exposure of graft antigens to the immune system and further reduce the rejection risk. Chong et al[4] outlined the comprehensive immunologic mechanisms involved in corneal transplant rejection and indicated that both the afferent (allosensitization) and efferent (rejection) arms of the alloimmune response are enhanced in the presence of NV. One previous meta-analysis also concluded that graft failure and rejection risk elevate along with the increase of corneal quadrants affected by neovascularization before the formation of keratoplasty[30].
Several animal studies indicated that bevacizumab delayed epithelial healing[31-33]. One included trial of our study reported two cases with persistent epithelial defects and corneal melting after the bevacizumab injection[19]. The authors suggested that bevacizumab should be carefully used, especially in patients with limbal stem cell deficiency. A case report described a patient with corneal melting after topical bevacizumab use for penetrating keratoplasty[34]; another case report described a patient with corneal thinning after intrastromal injection of bevacizumab for idiopathic lipid keratopathy[35]. However, our study showed no significant difference between bevacizumab and placebo groups in the risk of developing epithelial defects. Indeed, more patients reported having epithelial defects in the placebo group than in the bevacizumab group. Accordingly, bevacizumab is generally safe for CoNV treatment.
Various routes, dosages, and frequencies of anti-VEGF agents are used for CoNV treatment. Previous experimental studies suggested that, although topically applied bevacizumab has limited capacity to penetrate the intact corneal epithelium, bevacizumab can penetrate the neovascularized cornea after topical application[36], and both topical and subconjunctival routes of administration could effectively decrease CoNV[37,38]. The included trials of our study used both administration routes mentioned above, and they appeared to show similar efficacy. Our study could not reveal a solid conclusion for this aspect due to the limited data available. Further trials are still needed to seek the ideal administration of anti-VEGF agents in CoNV. Lastly, one of the limitations of the present study was the failure of providing evidence about the cost-effectiveness of different formulation of anti-VEGF agents, since no study included conducting this analysis.
While most of the meta-analyses showed no evidence of heterogeneity, two analyses demonstrated low and high levels of heterogeneity, which might arise from the clinical variety of the sample. First, different diagnostic criteria were used in the included trials. Second, the drug administration route, dosage, and frequency were inconsistent across trials. Third, the time points of outcome measurement differed across trials. This study consists of two main limitations. First, we included a limited number of RCTs, and the sample size per treatment group was small. Thus, the statistical power is weak. Second, several trials reported fragmented outcome information and thus increased the risk of bias.
The treatment for CoNV is an issue of debate in efficacy, safety, and cost. Bevacizumab is a relatively cheap anti-VEGF agent. In this study, we found evidence demonstrating that anti-VEGF agents, mainly bevacizumab, are an effective and safe treatment for CoNV. Although the effect of improving VA remains ambiguous, anti-VEGF agents reduce CoNV of all causes and prevent the corneal graft from rejection and failure in corneal transplantation patients. However, the most appropriate dosage and route of administration remain uncertain. Also, the number of human trials or studies for anti-VEGF drugs other than bevacizumab is limited. Additional trials and studies with larger sample sizes are needed to clarify these issues.
Corneal neovascularization (CoNV) is a condition of pathological vascular ingrowth into the cornea from the limbus, causing the avascular structure to become non-transparent and further markedly threaten the visual acuity. Various treatment approaches, including anti-inflammatory drugs (e.g., steroids and immunomodulators), laser ablation, photodynamic therapy, diathermy, and ocular surface restoration, have been used in CoNV management. These approaches are not without problems.
Several randomized controlled trials and non-randomized trials examining the efficacy of anti-vascular endothelial growth factor (VEGF) in CoNV have been published in the past few years.
We conducted an updated systematic review and meta-analysis of clinical trials to examine the efficacy and safety of anti-VEGF in CoNV.
Relevant studies published before October 2022 were identified by systematic search using PubMed, EMBASE, and Cochrane Library databases.
In this study, we found evidence demonstrating that anti-VEGF agents, mainly bevacizumab, are an effective and safe treatment for CoNV.
Anti-VEGF agents significantly reduced CoNV, BCVA, and graft rejection/failure rate compared with placebo. There was a non-significant trend toward reduction of the risk of developing corneal epithelial defects in the bevacizumab group compared with placebo. Also, combined remedies with bevacizumab have better efficacy in reducing CoNV compared with single other treatments.
Anti-VEGF agents reduce CoNV of all causes and prevent the corneal graft from rejection and failure in corneal transplantation patients. However, the most appropriate dosage and route of administration remain uncertain. Also, the number of human trials or studies for anti-VEGF drugs other than bevacizumab is limited. Additional trials and studies with larger sample sizes are needed to clarify these issues.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Ophthalmology
Country/Territory of origin: Taiwan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Morya AK, India S-Editor: Liu JH L-Editor: A P-Editor: Zhao S
| 1. | Skobe M, Dana R. Blocking the path of lymphatic vessels. Nat Med. 2009;15:993-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (1)] |
| 2. | Lee P, Wang CC, Adamis AP. Ocular neovascularization: an epidemiologic review. Surv Ophthalmol. 1998;43:245-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 322] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 3. | Lasagni Vitar RM, Triolo G, Fonteyne P, Acuti Martellucci C, Manzoli L, Rama P, Ferrari G. Epidemiology of Corneal Neovascularization and Its Impact on Visual Acuity and Sensitivity: A 14-Year Retrospective Study. Front Med (Lausanne). 2021;8:733538. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (1)] |
| 4. | Chong EM, Dana MR. Graft failure IV. Immunologic mechanisms of corneal transplant rejection. Int Ophthalmol. 2008;28:209-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 77] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 5. | Dana MR, Streilein JW. Loss and restoration of immune privilege in eyes with corneal neovascularization. Invest Ophthalmol Vis Sci. 1996;37:2485-2494. [PubMed] |
| 6. | Ahmed Hassan A, Foad Ghoneim D, Abdelraheem El-Dib A, Abdelkawi Ahmed S, Abdel-Salam AM. Photothrombosis of corneal neovascularization by photodynamic therapy utilizing verteporfin and diode laser. J Lasers Med Sci. 2013;4:131-139. [PubMed] |
| 7. | Al-Torbak AA. Photodynamic therapy with verteporfin for corneal neovascularization. Middle East Afr J Ophthalmol. 2012;19:185-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Díaz-Dávalos CD, Carrasco-Quiroz A, Rivera-Díez D. [Neovascularization corneal regression in patients treated with photodynamic therapy with verteporfin]. Rev Med Inst Mex Seguro Soc. 2016;54:164-169. [PubMed] |
| 9. | Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004;25:581-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2468] [Cited by in RCA: 2570] [Article Influence: 122.4] [Reference Citation Analysis (0)] |
| 10. | Papathanassiou M, Theodoropoulou S, Analitis A, Tzonou A, Theodossiadis PG. Vascular endothelial growth factor inhibitors for treatment of corneal neovascularization: a meta-analysis. Cornea. 2013;32:435-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 11. | Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I, Carpenter JR, Chan AW, Churchill R, Deeks JJ, Hróbjartsson A, Kirkham J, Jüni P, Loke YK, Pigott TD, Ramsay CR, Regidor D, Rothstein HR, Sandhu L, Santaguida PL, Schünemann HJ, Shea B, Shrier I, Tugwell P, Turner L, Valentine JC, Waddington H, Waters E, Wells GA, Whiting PF, Higgins JP. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7683] [Cited by in RCA: 10864] [Article Influence: 1207.1] [Reference Citation Analysis (2)] |
| 12. | Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge SM, Emberson JR, Hernán MA, Hopewell S, Hróbjartsson A, Junqueira DR, Jüni P, Kirkham JJ, Lasserson T, Li T, McAleenan A, Reeves BC, Shepperd S, Shrier I, Stewart LA, Tilling K, White IR, Whiting PF, Higgins JPT. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6581] [Cited by in RCA: 15260] [Article Influence: 2543.3] [Reference Citation Analysis (0)] |
| 13. | DerSimonian R, Laird N. Meta-analysis in clinical trials revisited. Contemp Clin Trials. 2015;45:139-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1231] [Cited by in RCA: 1927] [Article Influence: 192.7] [Reference Citation Analysis (0)] |
| 14. | Nikolakopoulou A, Mavridis D, Salanti G. Demystifying fixed and random effects meta-analysis. Evid Based Ment Health. 2014;17:53-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 100] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 15. | Bhatti N, Qidwai U, Hussain M, Kazi A. Efficacy of topical bevacizumab in high-risk corneal transplant survival. Pak J Med Sci. 2013;29:519-522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Dohlman TH, McSoley M, Amparo F, Carreno-Galeano T, Wang M, Dastjerdi M, Singh RB, Coco G, Di Zazzo A, Shikari H, Saboo U, Sippel K, Ciralsky J, Yoo SH, Sticca M, Wakamatsu TH, Murthy S, Hamrah P, Jurkunas U, Ciolino JB, Gomes JAP, Perez VL, Yin J, Dana R. Bevacizumab in High-Risk Corneal Transplantation: A Pilot Multicenter Prospective Randomized Control Trial. Ophthalmology. 2022;129:865-879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 17. | Fasciani R, Mosca L, Giannico MI, Ambrogio SA, Balestrazzi E. Subconjunctival and/or intrastromal bevacizumab injections as preconditioning therapy to promote corneal graft survival. Int Ophthalmol. 2015;35:221-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 18. | Hamdan J, Boulze M, Aziz A, Alessi G, Hoffart L. [Corneal neovascularisation treatments compared: Subconjunctival bevacizumab injections and/or photodynamic therapy]. J Fr Ophtalmol. 2015;38:924-933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Kim JH, Seo HW, Han HC, Lee JH, Choi SK, Lee D. The effect of bevacizumab versus ranibizumab in the treatment of corneal neovascularization: a preliminary study. Korean J Ophthalmol. 2013;27:235-242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Li L, Liang YL and Li YC. [Prevent effect of Bevacizumab and Triamcinolone acetonide on corneal neovascularization induced by penetrating keratoplasty]. Guoji Yanke Zazhi. 2014;14:2016-2018. [DOI] [Full Text] |
| 21. | Ozgurhan EB, Agca A, Kara N, Yuksel K, Demircan A, Demirok A. Topical application of bevacizumab as an adjunct to recurrent pterygium surgery. Cornea. 2013;32:835-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 22. | Petsoglou C, Balaggan KS, Dart JK, Bunce C, Xing W, Ali RR, Tuft SJ. Subconjunctival bevacizumab induces regression of corneal neovascularisation: a pilot randomised placebo-controlled double-masked trial. Br J Ophthalmol. 2013;97:28-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 23. | You IC, Kang IS, Lee SH, Yoon KC. Therapeutic effect of subconjunctival injection of bevacizumab in the treatment of corneal neovascularization. Acta Ophthalmol. 2009;87:653-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 57] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 24. | Huang ST, Zhou T, Yang YX, Zhou BB, Yin XF, Zhou SY. Early Application of Bevacizumab After Sclerocorneal Grafting for Patients With Severe Late-Stage Ocular Chemical Burns. Cornea. 2020;39:754-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 25. | Hurmeric V, Vaddavalli P, Galor A, Perez VL, Roman JS, Yoo SH. Single and multiple injections of subconjunctival ranibizumab for early, recurrent pterygium. Clin Ophthalmol. 2013;7:467-473. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 26. | Trufanov SV, Malozhen SA, Krakhmaleva DA, Surnina ZV, Pivin EA, Kasparova EA. [Antiangiogenic therapy in high-risk keratoplasty]. Vestn Oftalmol. 2020;136:11-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 27. | Chen Y, Wiesmann C, Fuh G, Li B, Christinger HW, McKay P, de Vos AM, Lowman HB. Selection and analysis of an optimized anti-VEGF antibody: crystal structure of an affinity-matured Fab in complex with antigen. J Mol Biol. 1999;293:865-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 346] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 28. | Amano S, Rohan R, Kuroki M, Tolentino M and Adamis AP. Requirement for vascular endothelial growth factor in wound- and inflammation-related corneal neovascularization. Invest Ophthalmol Vis Sci. 1998;39:18-22. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 29. | Cursiefen C, Chen L, Borges LP, Jackson D, Cao J, Radziejewski C, D'Amore PA, Dana MR, Wiegand SJ, Streilein JW. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J Clin Invest. 2004;113:1040-1050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 658] [Cited by in RCA: 798] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 30. | Bachmann B, Taylor RS, Cursiefen C. Corneal neovascularization as a risk factor for graft failure and rejection after keratoplasty: an evidence-based meta-analysis. Ophthalmology. 2010;117:1300-5.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 196] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 31. | Kim EC, Lee WS, Kim MS. The inhibitory effects of bevacizumab eye drops on NGF expression and corneal wound healing in rats. Invest Ophthalmol Vis Sci. 2010;51:4569-4573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 32. | Kim TI, Chung JL, Hong JP, Min K, Seo KY, Kim EK. Bevacizumab application delays epithelial healing in rabbit cornea. Invest Ophthalmol Vis Sci. 2009;50:4653-4659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 69] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 33. | Dong M, Di G, Zhang X, Zhou Q, Shi W. Subconjunctival Bevacizumab Injection Impairs Corneal Innervations and Epithelial Wound Healing in Mice. Invest Ophthalmol Vis Sci. 2017;58:1469-1477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 34. | Galor A, Yoo SH. Corneal Melt While Using Topical Bevacizumab Eye Drops. Ophthalmic Surg Lasers Imaging. 2010;1-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 35. | Sun KJ, Jun AS, Bohm K, Daroszewski D, Jabbour S. Corneal thinning following bevacizumab intrastromal injection for the treatment of idiopathic lipid keratopathy. Am J Ophthalmol Case Rep. 2022;27:101618. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 36. | Dastjerdi MH, Sadrai Z, Saban DR, Zhang Q, Dana R. Corneal penetration of topical and subconjunctival bevacizumab. Invest Ophthalmol Vis Sci. 2011;52:8718-8723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 92] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 37. | Ozdemir O, Altintas O, Altintas L, Ozkan B, Akdag C and Yuksel N. Comparison of the effects of subconjunctival and topical anti-VEGF therapy (bevacizumab) on experimental corneal neovascularization. Arq Bras Oftalmol. 2014;77:209-213. [RCA] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 38. | Hashemian MN, Z-Mehrjardi H, Moghimi S, Tahvildari M, Mojazi-Amiri H. Prevention of corneal neovascularization: comparison of different doses of subconjunctival bevacizumab with its topical form in experimental rats. Ophthalmic Res. 2011;46:50-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |