Published online Oct 26, 2023. doi: 10.12998/wjcc.v11.i30.7318
Peer-review started: August 9, 2023
First decision: September 13, 2023
Revised: September 17, 2023
Accepted: September 28, 2023
Article in press: September 28, 2023
Published online: October 26, 2023
Processing time: 77 Days and 4.7 Hours
The evidence from observational studies has been inconclusive on the causal relationship between cheese intake and gestational hypertension or diabetes.
To determine whether cheese consumption was causally related to hypertension and diabetes during pregnancy.
This was a two-sample Mendelian randomized (MR) study. Summary-level genetic data for cheese intake was exposure and corresponding outcome data for gestational hypertension and gestational diabetes were extracted from the IEU OpenGWAS database. MR analysis was conducted using inverse variance weighting. For sensitivity analyses, MR-Egger regression, weighted median, weighted mode, and leave-one-out methods were conducted. A fixed-effect model was used to meta-analyze two sample MR estimates. The traits of gestational hypertension were pregnancy hypertension (123579 individuals) and oedema, proteinuria and hypertensive disorders in pregnancy, childbirth and the puerperium (123579 individuals), and traits of gestational diabetes were gestational diabetes (123579 individuals) and diabetes mellitus in pregnancy (116363 individuals), respectively.
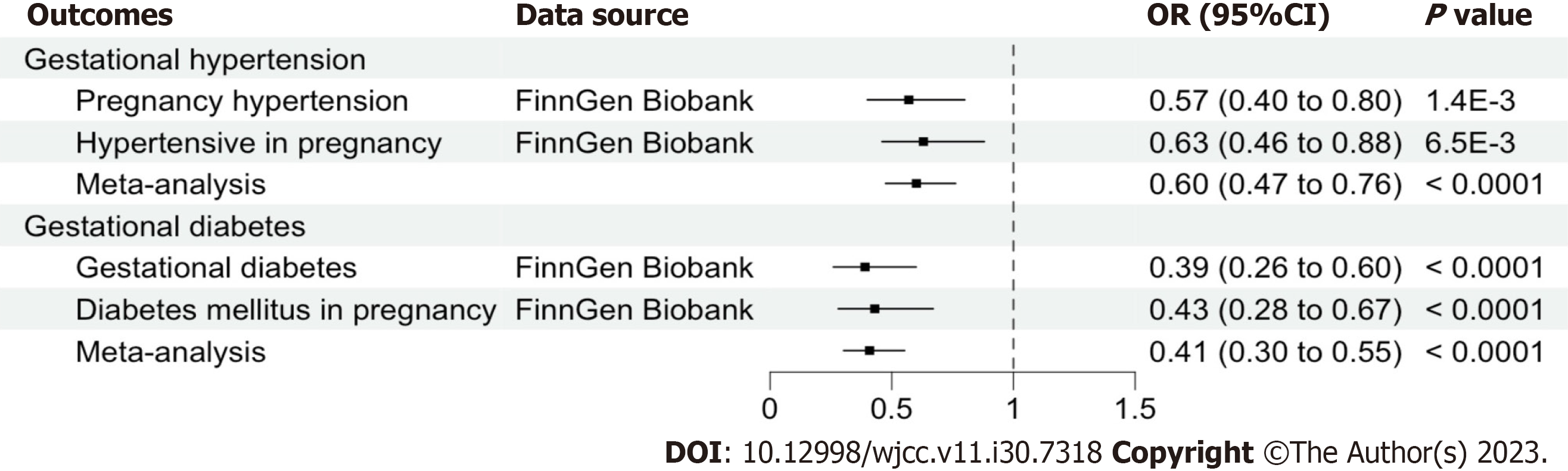
Cheese intake per standard deviation increase has causally reduced the risks of gestational hypertension [odds ratio (OR) = 0.60, 95% confidence interval (CI): 0.47-0.76, P < 0.001] and gestational diabetes (OR = 0.41, 95%CI: 0.30-0.55, P < 0.001) in inverse variance weighted analysis. Sensitivity analysis showed no heterogeneity (all P > 0.05) nor horizontal pleiotropy (all P > 0.05) in the relationship between cheese intake and gestational hypertension, but heterogeneity presented (all P < 0.05) in relation to gestational diabetes in the two-sample MR analysis.
Cheese intake was inversely associated with gestational hypertension and gestational diabetes in MR analysis, suggesting that cheese consumption may be beneficial in preventing hypertension and diabetes during pregnancy.
Core Tip: Gestational hypertension and gestational diabetes were associated with an increased risk of complications for both the mother and fetus during pregnancy. We found that cheese intake was inversely associated with gestational hypertension and gestational diabetes in Mendelian randomization analysis, suggesting that cheese consumption may be beneficial in preventing hypertension and diabetes during pregnancy. These findings suggested that dietary interventions, especially increasing cheese intake, may be effective in the prevention gestational hypertension and gestational diabetes, and should be promoted in more regions.
- Citation: Zhong T, Huang YQ, Wang GM. Causal relationship association of cheese intake with gestational hypertension and diabetes result from a Mendelian randomization study. World J Clin Cases 2023; 11(30): 7318-7328
- URL: https://www.wjgnet.com/2307-8960/full/v11/i30/7318.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i30.7318
Gestational hypertension and gestational diabetes were associated with an increased risk of complications for both the mother and fetus during pregnancy, and also been associated with an increased risk of several long-term health outcomes in pregnant women and intermediate outcomes in their children[1-5]. Although gestational hypertension and gestational diabetes posed a huge public problem to society, effective prevention has remained a major challenge in most countries, and measures were very limited. In recent years, some studies have found that dietary modification including cheese-containing dietary pattern might play an important role in the prevention and alleviate of hypertension and diabetes during pregnancy, and this method could be implemented in maternity care[6-11]. Although several previous studies have found a link between cheese consumption and reduced risk of pregnancy-related complications, most findings were based on observational findings. However, observational studies lacked of randomization, made it difficult to effectively control for confounding, making causal relationship difficult to be established.
In recent years, the introduction of Mendelian randomized (MR) has provided an effective way to make causal inferences in observational studies, and the public release of a large amount of genome-wide association studies (GWAS) summary data has contributed to the flourishing of two-sample MR[12]. MR studies are performed by using instrumental variable (IV) analysis of genetic variation using randomization during meiosis and conception, providing unbiased and unconfounded estimates[12]. Briefly, MR is a technique that can detect and estimate phenotypic causal effects unbiasedly[13]. With the popularity of GWAS and GWAS meta-analysis, MR has become an effective and feasible method to study causal relationships[14]. Two-sample MR is a method for estimating the causal effect of exposure on prognosis using only summary statistics from the GWAS, in which genetic variant-exposure factor association data and genetic variant-disease prognosis association data from two independent samples with similar distribution characteristics were used[15,16]. In this study, we have performed a two-sample MR study based on public GWAS data to analyse whether cheese intake was associated with gestational hypertension or gestational diabetes.
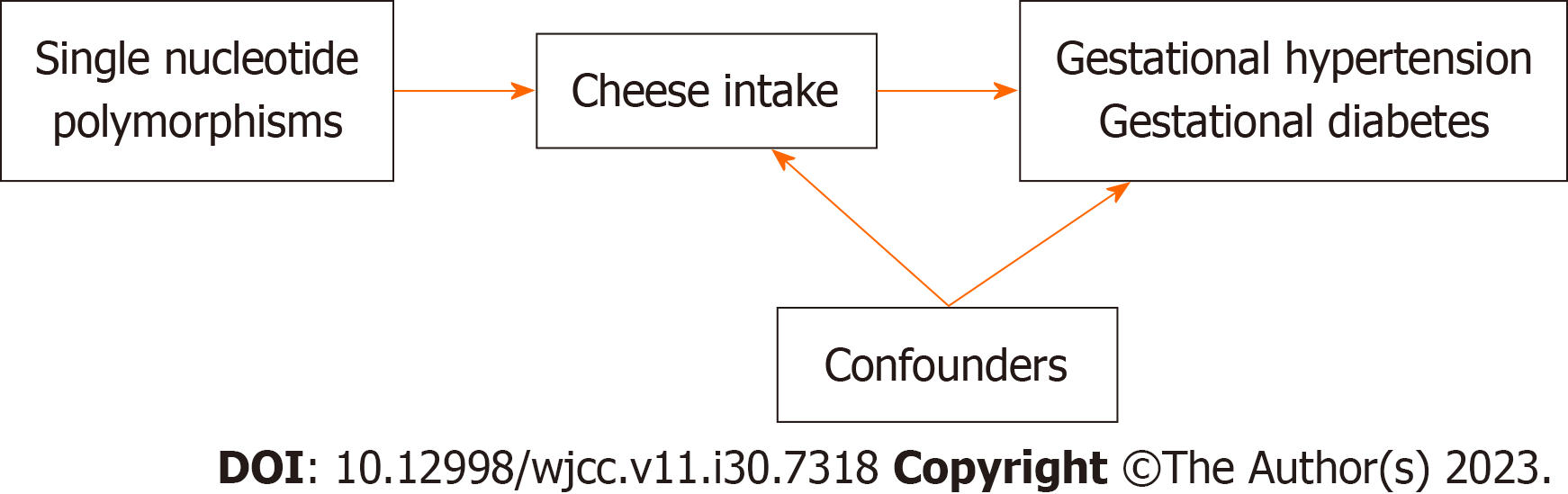
In this study, we conducted a two-sample MR analysis to explore the causal association of cheese intake with gestational hypertension and gestational diabetes in the summary level GWAS dataset, as well as the risk of gestational hypertension and gestational diabetes with single nucleotide polymorphisms (SNPs) defined as IVs (Figure 1). While all the pooled data in the present study was obtained from publicly available datasets that obtained relevant ethical approval and participant consent, the design and analysis of this study was also approved by the Ethics Committee of Guangdong Provincial People’s Hospital (KY-Q-2021-244-01).
Summary-level genetic data for cheese intake as exposure and corresponding outcome data for gestational hypertension and gestational diabetes was extracted from the IEU OpenGWAS database (https://gwas.mrcieu.ac.uk/). The traits of gestational hypertension were characterized by gestational hypertension (123579 cases, 7686 cases/control 115893 cases) and gestational edema, proteinuria and hypertensive disorders (123579 cases, 8844 cases/control 114735 cases); gestational diabetes mellitus was characterized by gestational diabetes (123579 cases, 5687 cases/control 117892 cases) and gestational diabetes mellitus (116363 cases, 6033 cases/control 110330 cases), respectively. All the above SNPs associated with cheese intake were found in UK Biobank, and with gestational hypertension and gestational diabetes were both found in FinnGen Biobank. The detailed descriptions of included traits were summarized in Table 1. All data was downloaded and analyzed on May 20, 2023.
| Exposure | Trait | ID | Number of SNPs | Population | Sample size | Case/control | Database | Consortium | Year |
| Cheese intake | Cheese intake | ukb-b-1489 | 9851867 | European | 451486 | NA | UK Biobank | MRC-IEU | 2018 |
| Outcomes | |||||||||
| Gestational hypertension | Pregnancy hypertension | finn-b-O15_HYPTENSPREG | 16379784 | European | 123579 | 7686/115893 | FinnGen Biobank | NA | 2021 |
| Oedema, proteinuria and hypertensive disorders in pregnancy, childbirth and the puerperium | finn-b-O15_OEDEM_PROTUR_HYPERT | 16379784 | European | 123579 | 8844/114735 | FinnGen Biobank | NA | 2021 | |
| Gestational diabetes | Gestational diabetes (for exclusion) | finn-b-GEST_DIABETES | 16379784 | European | 123579 | 5687/117892 | FinnGen Biobank | NA | 2021 |
| Diabetes mellitus in pregnancy | finn-b-O15_PREG_DM | 16379684 | European | 116363 | 6033/110330 | FinnGen Biobank | NA | 2021 |
The analytical strategy for this study was based on the strengthening the reporting of observational studies in epidemiology using MR statement[17]. For all SNPs instrumental selection, genetic variants related to exposure factors with a genome-wide significant level (P < 5 × 10-8), not in linkage disequilibrium (LD) at a threshold of R2 < 0.001. If a particular exposure SNP was not present in an outcome dataset, proxy SNPs should be used instead through LD tagging with minimum LD R2 value > 0.8. The causality was tested primarily by using the inverse variance weighting (IVW) method. To test for robustness, we performed sensitivity analyses using the MR-Egger regression, weighted median, and weighted mode methods. Weighted mode methods were conducted by leave one-out sensitivity analysis, in which the effect of each single instrument on the overall effect was shown in the plot of the leave-one-out analysis. Heterogeneity tests were performed using IVW and MR-Egger methods. To test for heterogeneity, we used the Cochrane Q statistic. The MR-Egger regression method could detect horizontal pleiotropy. The MR-Egger intercept was non-zero with statistical significance (P < 0.05) if possible horizontal pleiotropy of IVs existed. Scatter plots, forest plots, and funnel plots were used to present the results, And the effect of each SNP was presented in a forest plot. The MR estimates for exposure and outcomes were presented as odds ratios (OR) with corresponding 95% confidence intervals (CI). In addition, as outcomes were from different datasets, the MR outcomes were pooled meta-analysis by using a mixed-effects model to obtain an overall causal estimate, assuming no between-method heterogeneity. MR analysis was performed by using the R software (version 4.0.3, http://www.r-project.org) and the R package “TwoSampleMR” version 0.5.5 (https://mrcieu.github.io/TwoSampleMR/).
We selected two subtype traits from FinnGen Biobank database with a genotype of gestational diabetes (finn-b-GEST_DIABETES and finn-b-O15_PREG_DM), gestational hypertension (finn-b-O15_HYPTENSPREG and finn-b-O15_OEDEM_PROTUR_HYPERT) and one group with a trait of cheese intake from UK Biobank database (ukb-b-1489). All participants were of European ethnicity. The numbers of SNPs related to cheese intake were 9851867, and those for gestational hypertension and gestational diabetes were both 16379784. The detailed descriptions of included traits were summarized in Table 1.
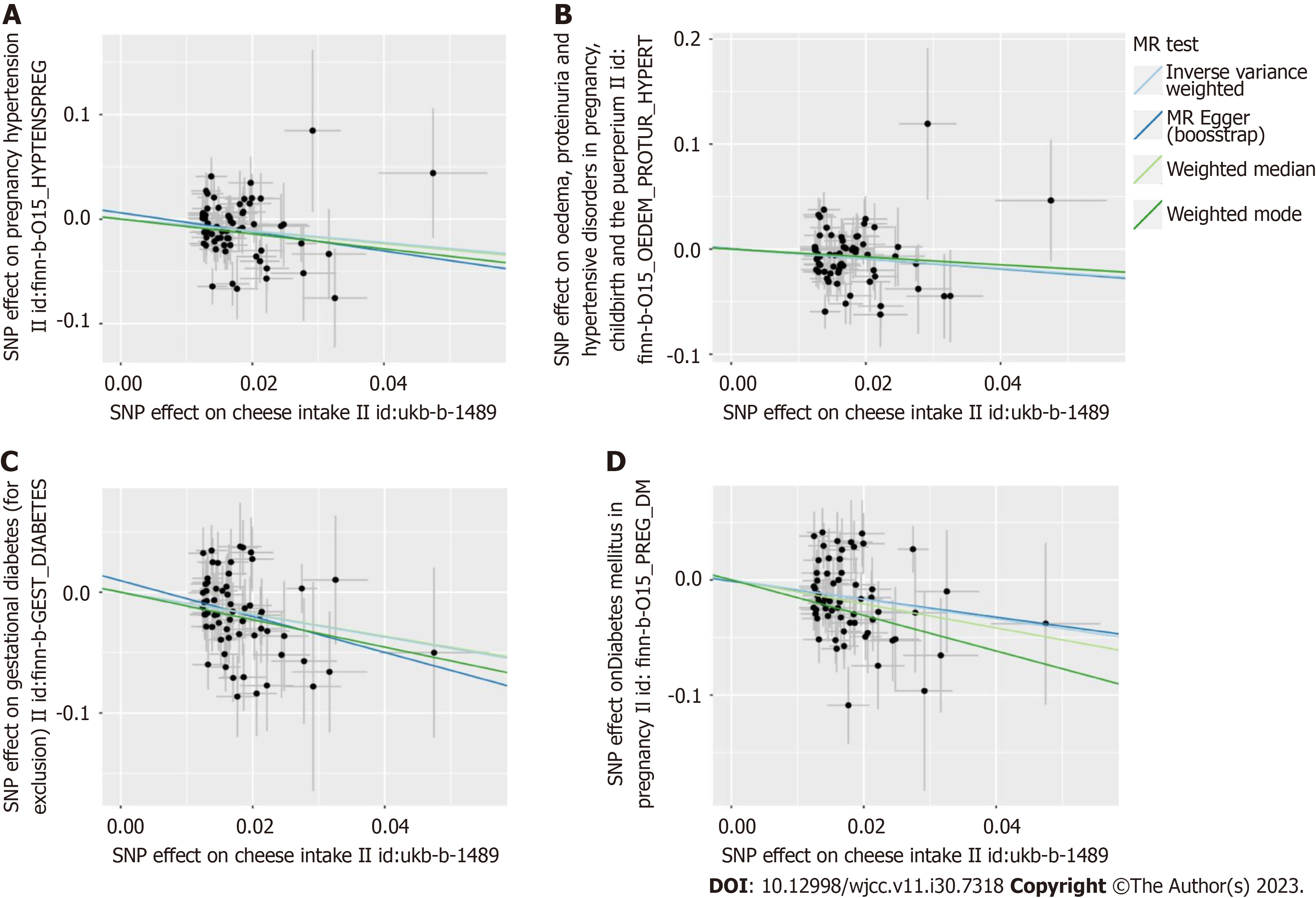
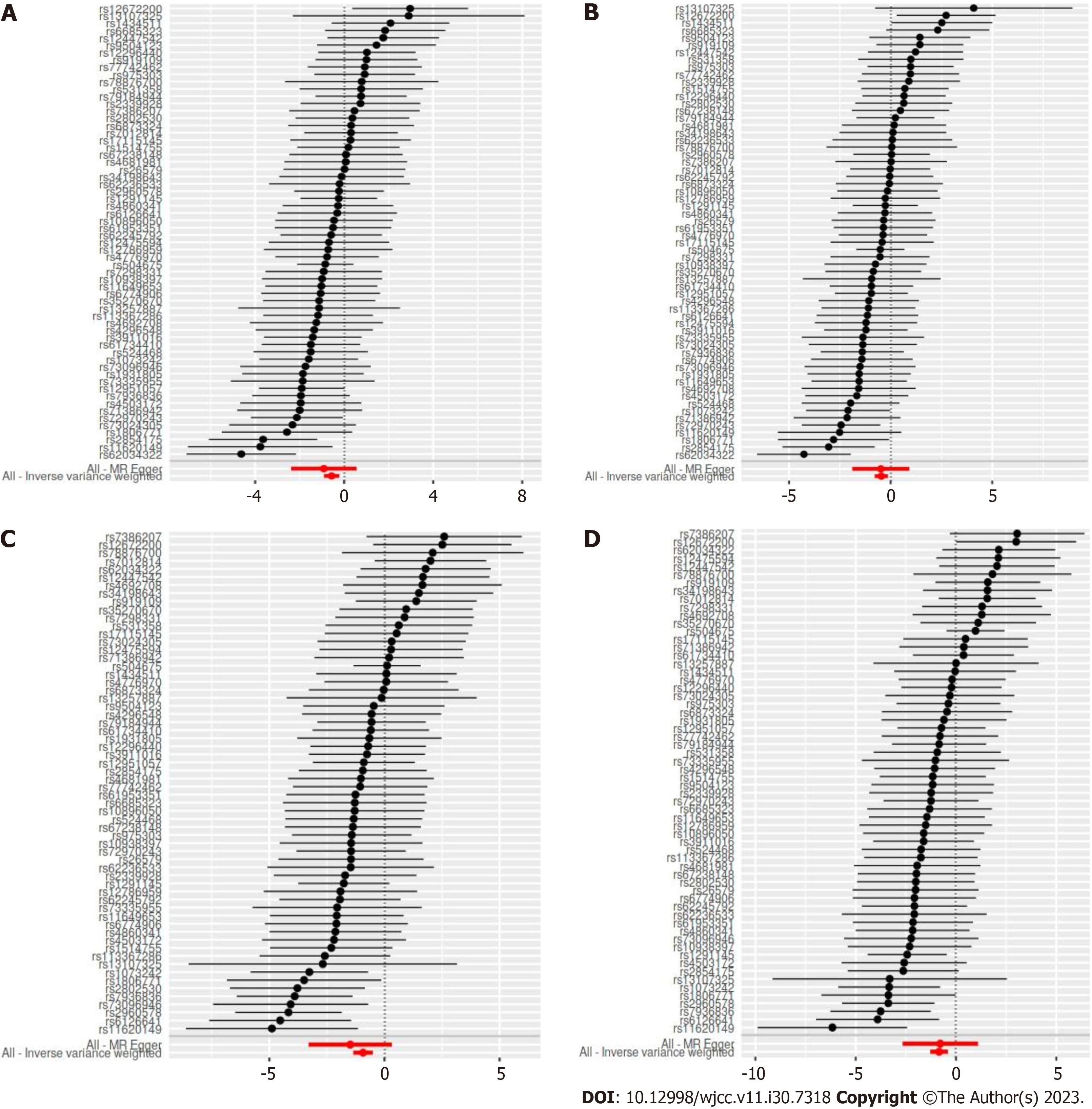
The two-sample MR estimates for the association of cheese intake with gestational hypertension and gestational diabetes were summarized in Table 2. The MR analyses on the result of the IVW method showed an inversely causal effect of genetically predicted cheese intake with pregnancy hypertension (OR = 0.57, 95%CI: 0.40-0.80; P = 1.377e-3), oedema, proteinuria and hypertensive disorders in pregnancy, childbirth and the puerperium (OR = 0.63, 95%CI: 0.46-0.88; P = 0.006), gestational diabetes (OR = 0.39, 95%CI: 0.26-0.60; P = 1.822e-5) and diabetes mellitus in pregnancy (OR = 0.43, 95%CI: 0.28-0.67; P = 1.718e-4) by using 62 SNPs as the instruments, respectively. The weighted median estimator showed consistent result when the study outcomes were pregnancy hypertension (OR = 0.55, 95%CI: 0.34-0.90; P = 0.017), gestational diabetes (OR = 0.40, 95%CI: 0.23-0.71; P = 1.507e-3) and diabetes mellitus in pregnancy (OR = 0.35, 95%CI: 0.20-0.62; P = 2.998e-4), but not consistent of oedema, proteinuria and hypertensive disorders in pregnancy, childbirth and the puerperium (OR = 0.69, 95%CI: 0.45-1.05; P = 0.083). In addition, although this negative correlation could also be observed in other methods including MR Egger and Weighted mode, it was not statistically significant (all P > 0.05) (Table 2). A meta-analysis using fixed-effect IVW models showed that genetically predicted cheese intake was inversely associated with the risk of gestational hypertension (OR = 0.60, 95%CI: 0.47-0.76; P < 0.0001) and gestational diabetes (OR = 0.41, 95%CI: 0.30-0.55; P < 0.0001) (Figure 2). The SNP effects on the outcome were plotted against SNP effects on the exposure were displayed in Figure 3.
| Outcomes | Trait | Number of instruments | Method | OR | 95%CI | P value | Heterogeneity | Pleiotropy | ||
| Q statistic | P value | Intercept | P value | |||||||
| Gestational hypertension | Pregnancy hypertension | 62 | MR Egger | 0.40 | 0.09-1.75 | 0.229 | 73.04 | 0.120 | 0.006 | 0.634 |
| 62 | Weighted median | 0.55 | 0.34-0.90 | 0.017 | ||||||
| 62 | Inverse variance weighted | 0.57 | 0.40-0.80 | 1.377e-3 | 73.31 | 0.134 | ||||
| 62 | Weighted mode | 0.49 | 0.20-1.19 | 0.121 | ||||||
| Oedema, proteinuria and hypertensive disorders in pregnancy, childbirth and the puerperium | 62 | MR Egger | 0.62 | 0.15-2.52 | 0.505 | 76.44 | 0.075 | 4.3e-4 | 0.971 | |
| 62 | Weighted median | 0.69 | 0.45-1.05 | 0.083 | ||||||
| 62 | Inverse variance weighted | 0.63 | 0.46-0.88 | 0.006 | 76.44 | 0.088 | ||||
| 62 | Weighted mode | 0.69 | 0.28-1.68 | 0.416 | ||||||
| Gestational diabetes | Gestational diabetes (for exclusion) | 62 | MR Egger | 0.23 | 0.04-1.38 | 0.113 | 83.73 | 0.023 | 9.5 e-3 | 0.537 |
| 62 | Weighted median | 0.40 | 0.23-0.71 | 1.507e-3 | ||||||
| 62 | Inverse variance weighted | 0.39 | 0.26-0.60 | 1.822e-5 | 84.26 | 0.025 | ||||
| 62 | Weighted mode | 0.32 | 0.10-1.03 | 0.062 | ||||||
| Diabetes mellitus in pregnancy | 62 | MR Egger | 0.46 | 0.07-3.00 | 0.418 | 91.39 | 5.586e-3 | -0.001 | 0.948 | |
| 62 | Weighted median | 0.35 | 0.20-0.62 | 2.998e-4 | ||||||
| 62 | Inverse variance weighted | 0.43 | 0.28-0.67 | 1.718 e-4 | 91.4 | 7.089e-3 | ||||
| 62 | Weighted mode | 0.21 | 0.04-1.20 | 0.085 | ||||||
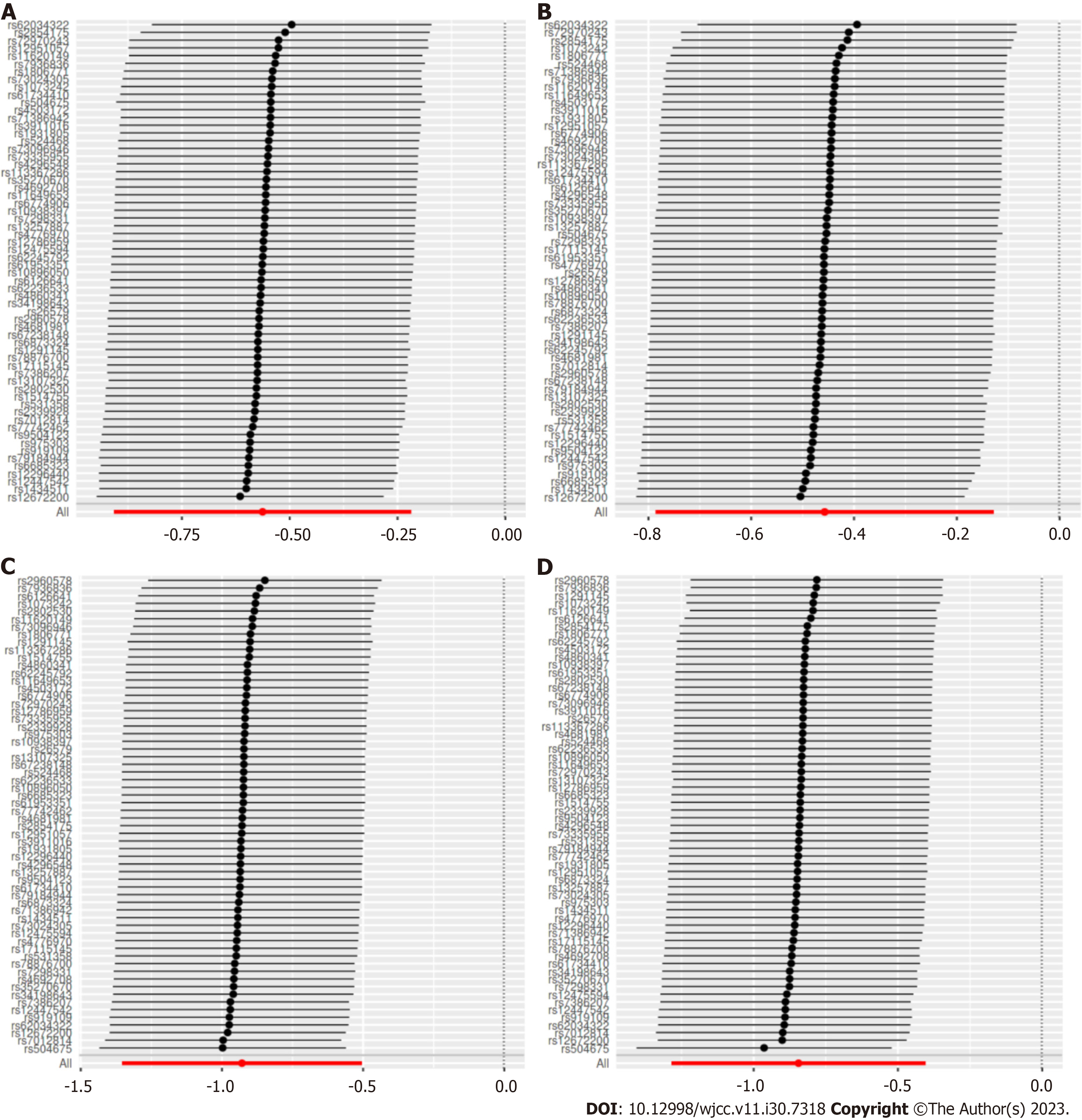
MR-Egger intercept tests were used to test for pleiotropy, indicating that there was no horizontal pleiotropy existed on the association between cheese intake and gestational hypertension or gestational diabetes (all P > 0.05). Heterogeneity tests demonstrated that there was no heterogeneity between cheese intake and gestational hypertension (all P > 0.05), but heterogeneity was observed in the Q test analysis on the association between cheese intake and gestational diabetes (all P < 0.05) (Table 2). Funnel plots can show the directional horizontal pleiotropy of IVs by drawing a single Wald ratio for each SNP. Sensitivity analyses using the leave-one-out analysis demonstrated that there was significant association of cheese intake with gestational hypertension or gestational diabetes (Figure 4). The overall estimates of MR effect size, calculated by IVW showed significant associations of cheese intake with gestational hypertension or gestational diabetes, but the MR-Egger method was not statistically significant despite finding also a negative correlation (Figure 5).
In this two-sample MR study, based on pooled statistics from a large GWAS, we clarified that cheese intake could reduce the risk of hypertension and diabetes during pregnancy. A pooled meta-analysis based on the results of the IVW method, using a fixed effects model to calculate the overall causal effect, showed that cheese intake was significantly and inversely associated with gestational hypertension and gestational diabetes. Heterogeneity and horizontal polymorphism in gestational hypertension were not found in the two-sample MR analysis, but heterogeneity in gestational diabetes was present.
Our results were similar to those of some previous observational or small sample randomised controlled trials (RCTs) that cheese intake might prevent gestational hypertension and gestational diabetes during pregnancy. Chinese intake in Canadian and Iranian women prior to pregnancy have been shown to be an important intervention target to reduce the likelihood of pregnancy-related complications, such as gestational hypertension[11,18]. According to a study of pregnant United States women, consuming more cheese before and during pregnancy could alleviate or reduce gestational diabetes risk[6,7]. Taking cheese during pregnancy might lead to modest dietary improvements in pregnant women at high risk of gestational diabetes, according to the Finnish gestational diabetes prevention study[8,9,19]. A small sample RCT study suggested that an increased intake of low-fat but not regular-fat cheese between pre-pregnancy and early pregnancy was associated with a lower risk of gestational diabetes in high-risk women[20]. However, despite an association between cheese consumption and gestational diabetes, a study in Singapore did not find a significant association[21]. Previous results differed from the present results mainly because some potential confounding variables were not adequately adjusted. However, in this study, the influence of confounding variables on the results was overcome through the two-sample MR study. In addition, although this study found heterogeneity in SNPs among gestational diabetes, this may be due to part of gestational diabetes itself existed gene mutation including pregnancy in women with monogenic diabetes or maternal genetic characteristics. Previous studies suggested that monogenic diabetes was an underdiagnosed type of diabetes mellitus, which could be harmful in pregnancy and was easily misdiagnosed as gestational diabetes[22-24]. However, the present study does not distinguish whether pregnant women have monogenic diabetes, missing data on monogenic diabetes was also an important shortcoming of this study.
In addition, this study found no horizontal pleiotropy in SNPs, but some of the sensitivity analysis results were not statistically significant, which may be due to the presence of other pleiotropy bias in some SNPs. However, a meta-analysis of fixed effects models showed that cheese intake was inversely associated with gestational diabetes and gestational hypertension with no heterogeneity. The fixed-effect meta-analysis model used an inverse-variance weight (variance of the observed effect size) and assumed that all studies share a single common effect and, as a result, all of the variance in observed effect sizes was attributable to sampling error[25]. These analytical methods, such as MR analysis, leave-one-out sensitivity analysis and pooled analysis of fixed effects models ensured the reliability of the results in the present MR study, indicating that cheese consumption could help preventing gestational diabetes and hypertension, but warranted further research to understand underlying pathophysiology of gestational diabetes and hypertension associated with dietary behaviors during pregnancy, such as increased cheese intake.
The possible mechanisms linking cheese intake to a reduced risk of hypertension and diabetes in pregnancy were unclear and we speculated that there were several possible reasons for this. Firstly, cheese was rich in calcium, and previous meta-analysis studies have found that calcium supplementation in pregnancy has been associated with a reduced risk of pregnancy-induced hypertension and diabetes, but further high-quality evidence was needed[26,27]. Secondly, cheese contained lots of lactic acid bacteria, which were healthy for the body and could maintain intestinal flora balance[28]. It was important to note that intestinal flora imbalance was an important cause of gestational hypertension and gestational diabetes during pregnancy[29-31]. Thirdly, the benefits of eating cheese could counteract the negative effects of its high saturated fat content. This was because cheese not only contained nutrients such as protein, calcium, iron, zinc, vitamins A, B1, B2 and folic acid, but also probiotics, which would reduce the state of inflammation and oxidative stress in the body[32,33]. Inflammation and oxidative stress were both closely related to gestational hypertension and gestational diabetes[34,35]. In addition, cheese also contained conjugated linoleic acid, an unsaturated fatty acid that could raise the level of high density lipoprotein cholesterol and lower the level of low density lipoprotein cholesterol[36]. Previous studies have shown that specific lipid biomarkers in early pregnancy may be associated with gestational hypertension and gestational diabetes[37,38]. Finally, animal experiments have shown the consumption of goat milk cheese could prevent obesity, insulin resistance, inflammation, and hepatic steatosis while on a high-fat diet induced obesity in mice[39].
There were several strengths to the current study. On the one hand, it was the first study to investigate the causal relationship between cheese consumption and gestational diabetes and hypertension through a MR analysis. On the other hand, a key strength of MR analysis was the use of randomly allocated genetic variants to help overcome environmental confounding, which was analogous to randomization of treatment allocation in clinical trials. In addition, a two-sample MR design and high-quality GWAS summary statistics based on a large sample size was also an advantage of this study. Finally, to ensure the stability of the results, MR-Egger regression tests were performed, and no pleiotropy were found. Of course, several limitations of the present MR study should also be considered. Firstly, it should be noted that the study population was all of European ancestry, so the results cannot be generalized to other races. Secondly, self-reported information contributed to some of history, so recalled bias cannot be eliminated. Thirdly, although IVW, MR-Egger regression, weighted median and weighted mode methods found an inverse association of cheese intake with gestational diabetes and gestational hypertension in MR analysis, the results of some methods were not statistically significant. Fourthly, even though we found some heterogeneity in gestational diabetes, our fixed-effect meta-analysis found that cheese intake was significantly associated with gestational diabetes according to IVW results. Fifthly, genetic poly
The present study indicated an inversely genetic causal association of cheese intake prior to pregnancy with pregnancy complications such as gestational hypertension and gestational diabetes during pregnancy, which suggesting that the consumption of cheese during pregnancy may contribute to the prevention of gestational hypertension and gestational diabetes. These findings suggested that dietary interventions, especially increasing cheese intake, may be effective in the prevention gestational hypertension and gestational diabetes, and should be promoted in more regions.
Evidence from observational studies has not been able to establish a causal association of cheese intake with hypertension or diabetes during pregnancy.
Eating cheese during pregnancy may help prevent hypertension and diabetes.
The objective was to determine whether cheese consumption was causally related to hypertension and diabetes during pregnancy.
A Mendelian randomised (MR) study with two samples was conducted. The IEU OpenGWAS database’s corresponding outcome data for gestational diabetes and gestational hypertension were taken out, and summary-level genetic information for cheese consumption was exposed. MR analysis was performed by using inverse variance weighting as the main method. Methods used for sensitivity studies included MR-Egger regression, weighted median, weighted mode, and leave-one-out techniques. The meta-analysis of two sample MR estimations was performed using a fixed-effect model. The characteristics of gestational diabetes were gestational diabetes (123579 individuals) and diabetes mellitus in pregnancy (116363 individuals), whereas the characteristics of gestational hypertension were pregnancy hypertension (123579 individuals) and oedema, proteinuria, and hypertensive disorders in pregnancy, childbirth, and the puerperium (123579 individuals).
Inverse variance weighted analysis has shown a causal relationship between cheese consumption per standard deviation increase and the risks of gestational hypertension and gestational diabetes (odds ratio = 0.41, 95% confidence interval: 0.30-0.55, P < 0.001). The two-sample MR analysis of the relationship between cheese intake and gestational hypertension revealed no heterogeneity (all P > 0.05) or horizontal pleiotropy, but there was heterogeneity (all P > 0.05) in relation to gestational diabetes.
In this MR analysis, cheese consumption was found to be inversely related to gestational hypertension and gestational diabetes, implying that cheese intake may be beneficial in preventing gestational hypertension and gestational diabetes.
These findings indicated that dietary interventions, particularly increasing cheese consumption, could be effective in preventing hypertension and diabetes during pregnancy, and should be promoted in more areas.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Vorobjova T, Estonia S-Editor: Wang JJ L-Editor: A P-Editor: Xu ZH
| 1. | US Preventive Services Task Force, Davidson KW, Barry MJ, Mangione CM, Cabana M, Caughey AB, Davis EM, Donahue KE, Doubeni CA, Kubik M, Li L, Ogedegbe G, Pbert L, Silverstein M, Stevermer J, Tseng CW, Wong JB. Screening for Gestational Diabetes: US Preventive Services Task Force Recommendation Statement. JAMA. 2021;326:531-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 74] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 2. | Magee LA, Brown MA, Hall DR, Gupte S, Hennessy A, Karumanchi SA, Kenny LC, McCarthy F, Myers J, Poon LC, Rana S, Saito S, Staff AC, Tsigas E, von Dadelszen P. The 2021 International Society for the Study of Hypertension in Pregnancy classification, diagnosis & management recommendations for international practice. Pregnancy Hypertens. 2022;27:148-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 439] [Article Influence: 146.3] [Reference Citation Analysis (0)] |
| 3. | Magee LA, Smith GN, Bloch C, Côté AM, Jain V, Nerenberg K, von Dadelszen P, Helewa M, Rey E. Guideline No. 426: Hypertensive Disorders of Pregnancy: Diagnosis, Prediction, Prevention, and Management. J Obstet Gynaecol Can. 2022;44:547-571.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 106] [Article Influence: 35.3] [Reference Citation Analysis (1)] |
| 4. | Garovic VD, Dechend R, Easterling T, Karumanchi SA, McMurtry Baird S, Magee LA, Rana S, Vermunt JV, August P; American Heart Association Council on Hypertension; Council on the Kidney in Cardiovascular Disease, Kidney in Heart Disease Science Committee; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Lifestyle and Cardiometabolic Health; Council on Peripheral Vascular Disease; and Stroke Council. Hypertension in Pregnancy: Diagnosis, Blood Pressure Goals, and Pharmacotherapy: A Scientific Statement From the American Heart Association. Hypertension. 2022;79:e21-e41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 255] [Article Influence: 85.0] [Reference Citation Analysis (0)] |
| 5. | McIntyre HD, Catalano P, Zhang C, Desoye G, Mathiesen ER, Damm P. Gestational diabetes mellitus. Nat Rev Dis Primers. 2019;5:47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 487] [Cited by in RCA: 977] [Article Influence: 162.8] [Reference Citation Analysis (1)] |
| 6. | Hinkle SN, Li M, Grewal J, Yisahak SF, Grobman WA, Newman RB, Wing DA, Grantz KL, Zhang C. Changes in Diet and Exercise in Pregnant Women after Diagnosis with Gestational Diabetes: Findings from a Longitudinal Prospective Cohort Study. J Acad Nutr Diet. 2021;121:2419-2428.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 7. | Shin D, Lee KW, Song WO. Dietary Patterns during Pregnancy Are Associated with Risk of Gestational Diabetes Mellitus. Nutrients. 2015;7:9369-9382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 105] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 8. | Niinistö S, Takkinen HM, Uusitalo L, Rautanen J, Nevalainen J, Kenward MG, Lumia M, Simell O, Veijola R, Ilonen J, Knip M, Virtanen SM. Maternal dietary fatty acid intake during pregnancy and the risk of preclinical and clinical type 1 diabetes in the offspring. Br J Nutr. 2014;111:895-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Kinnunen TI, Puhkala J, Raitanen J, Ahonen S, Aittasalo M, Virtanen SM, Luoto R. Effects of dietary counselling on food habits and dietary intake of Finnish pregnant women at increased risk for gestational diabetes - a secondary analysis of a cluster-randomized controlled trial. Matern Child Nutr. 2014;10:184-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | Meinilä J, Valkama A, Koivusalo SB, Stach-Lempinen B, Rönö K, Lindström J, Kautiainen H, Eriksson JG, Erkkola M. Is improvement in the Healthy Food Intake Index (HFII) related to a lower risk for gestational diabetes? Br J Nutr. 2017;117:1103-1109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Hajianfar H, Esmaillzadeh A, Feizi A, Shahshahan Z, Azadbakht L. Major Maternal Dietary Patterns during Early Pregnancy and Their Association with Neonatal Anthropometric Measurement. Biomed Res Int. 2018;2018:4692193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Davey Smith G, Hemani G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet. 2014;23:R89-R98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2280] [Cited by in RCA: 2881] [Article Influence: 261.9] [Reference Citation Analysis (0)] |
| 13. | Bowden J, Holmes MV. Meta-analysis and Mendelian randomization: A review. Res Synth Methods. 2019;10:486-496. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 461] [Cited by in RCA: 986] [Article Influence: 164.3] [Reference Citation Analysis (0)] |
| 14. | Emdin CA, Khera AV, Kathiresan S. Mendelian Randomization. JAMA. 2017;318:1925-1926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 500] [Cited by in RCA: 2137] [Article Influence: 267.1] [Reference Citation Analysis (0)] |
| 15. | Zheng J, Baird D, Borges MC, Bowden J, Hemani G, Haycock P, Evans DM, Smith GD. Recent Developments in Mendelian Randomization Studies. Curr Epidemiol Rep. 2017;4:330-345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 734] [Cited by in RCA: 722] [Article Influence: 90.3] [Reference Citation Analysis (0)] |
| 16. | Gkatzionis A, Burgess S, Newcombe PJ. Statistical methods for cis-Mendelian randomization with two-sample summary-level data. Genet Epidemiol. 2023;47:3-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 67] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 17. | Skrivankova VW, Richmond RC, Woolf BAR, Yarmolinsky J, Davies NM, Swanson SA, VanderWeele TJ, Higgins JPT, Timpson NJ, Dimou N, Langenberg C, Golub RM, Loder EW, Gallo V, Tybjaerg-Hansen A, Davey Smith G, Egger M, Richards JB. Strengthening the Reporting of Observational Studies in Epidemiology Using Mendelian Randomization: The STROBE-MR Statement. JAMA. 2021;326:1614-1621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 828] [Cited by in RCA: 2009] [Article Influence: 502.3] [Reference Citation Analysis (0)] |
| 18. | Jarman M, Mathe N, Ramazani F, Pakseresht M, Robson PJ, Johnson ST, Bell RC; APrON and ENRICH study teams. Dietary Patterns Prior to Pregnancy and Associations with Pregnancy Complications. Nutrients. 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 19. | Valkama A, Koivusalo S, Lindström J, Meinilä J, Kautiainen H, Stach-Lempinen B, Rönö K, Klemetti M, Pöyhönen-Alho M, Tiitinen A, Huvinen E, Laivuori H, Andersson S, Roine R, Eriksson JG. The effect of dietary counselling on food intakes in pregnant women at risk for gestational diabetes: a secondary analysis of a randomised controlled trial RADIEL. Eur J Clin Nutr. 2016;70:912-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Valkama AJ, Meinilä J, Koivusalo S, Lindström J, Rönö K, Stach-Lempinen B, Kautiainen H, Eriksson JG. The effect of pre-pregnancy lifestyle counselling on food intakes and association between food intakes and gestational diabetes in high-risk women: results from a randomised controlled trial. J Hum Nutr Diet. 2018;31:301-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | de Seymour J, Chia A, Colega M, Jones B, McKenzie E, Shirong C, Godfrey K, Kwek K, Saw SM, Conlon C, Chong YS, Baker P, Chong MF. Maternal Dietary Patterns and Gestational Diabetes Mellitus in a Multi-Ethnic Asian Cohort: The GUSTO Study. Nutrients. 2016;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 22. | Lima Ferreira J, Voss G, Sá Couto A, Príncipe RM. Monogenic diabetes caused by GCK gene mutation is misdiagnosed as gestational diabetes - A multicenter study in Portugal. Diabetes Metab Syndr. 2021;15:102259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 23. | Chakera AJ, Spyer G, Vincent N, Ellard S, Hattersley AT, Dunne FP. The 0.1% of the population with glucokinase monogenic diabetes can be recognized by clinical characteristics in pregnancy: the Atlantic Diabetes in Pregnancy cohort. Diabetes Care. 2014;37:1230-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 102] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 24. | Timsit J, Ciangura C, Dubois-Laforgue D, Saint-Martin C, Bellanne-Chantelot C. Pregnancy in Women With Monogenic Diabetes due to Pathogenic Variants of the Glucokinase Gene: Lessons and Challenges. Front Endocrinol (Lausanne). 2021;12:802423. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 25. | Kanters S. Fixed- and Random-Effects Models. Methods Mol Biol. 2022;2345:41-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 68] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 26. | Khaing W, Vallibhakara SA, Tantrakul V, Vallibhakara O, Rattanasiri S, McEvoy M, Attia J, Thakkinstian A. Calcium and Vitamin D Supplementation for Prevention of Preeclampsia: A Systematic Review and Network Meta-Analysis. Nutrients. 2017;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 77] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 27. | Hofmeyr GJ, Lawrie TA, Atallah ÁN, Torloni MR. Calcium supplementation during pregnancy for preventing hypertensive disorders and related problems. Cochrane Database Syst Rev. 2018;10:CD001059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 132] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 28. | Settanni L, Moschetti G. Non-starter lactic acid bacteria used to improve cheese quality and provide health benefits. Food Microbiol. 2010;27:691-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 273] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 29. | Ponzo V, Fedele D, Goitre I, Leone F, Lezo A, Monzeglio C, Finocchiaro C, Ghigo E, Bo S. Diet-Gut Microbiota Interactions and Gestational Diabetes Mellitus (GDM). Nutrients. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 110] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 30. | Lin H, Chen J, Ma S, An R, Li X, Tan H. The Association between Gut Microbiome and Pregnancy-Induced Hypertension: A Nested Case-Control Study. Nutrients. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 31. | Ahmadian E, Rahbar Saadat Y, Hosseiniyan Khatibi SM, Nariman-Saleh-Fam Z, Bastami M, Zununi Vahed F, Ardalan M, Zununi Vahed S. Pre-Eclampsia: Microbiota possibly playing a role. Pharmacol Res. 2020;155:104692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 32. | Thorning TK, Bertram HC, Bonjour JP, de Groot L, Dupont D, Feeney E, Ipsen R, Lecerf JM, Mackie A, McKinley MC, Michalski MC, Rémond D, Risérus U, Soedamah-Muthu SS, Tholstrup T, Weaver C, Astrup A, Givens I. Whole dairy matrix or single nutrients in assessment of health effects: current evidence and knowledge gaps. Am J Clin Nutr. 2017;105:1033-1045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 244] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 33. | Chen GC, Wang Y, Tong X, Szeto IMY, Smit G, Li ZN, Qin LQ. Cheese consumption and risk of cardiovascular disease: a meta-analysis of prospective studies. Eur J Nutr. 2017;56:2565-2575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 82] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 34. | Phoswa WN, Khaliq OP. The Role of Oxidative Stress in Hypertensive Disorders of Pregnancy (Preeclampsia, Gestational Hypertension) and Metabolic Disorder of Pregnancy (Gestational Diabetes Mellitus). Oxid Med Cell Longev. 2021;2021:5581570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 101] [Article Influence: 25.3] [Reference Citation Analysis (1)] |
| 35. | Pantham P, Aye IL, Powell TL. Inflammation in maternal obesity and gestational diabetes mellitus. Placenta. 2015;36:709-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 417] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 36. | Wang Y, Jones PJ. Dietary conjugated linoleic acid and body composition. Am J Clin Nutr. 2004;79:1153S-1158S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 97] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 37. | Jin WY, Lin SL, Hou RL, Chen XY, Han T, Jin Y, Tang L, Zhu ZW, Zhao ZY. Associations between maternal lipid profile and pregnancy complications and perinatal outcomes: a population-based study from China. BMC Pregnancy Childbirth. 2016;16:60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 196] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 38. | Wang Y, Shi D, Chen L. Lipid profile and cytokines in hypertension of pregnancy: A comparison of preeclampsia therapies. J Clin Hypertens (Greenwich). 2018;20:394-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 39. | Delgadillo-Puga C, Cuchillo-Hilario M. Reviewing the Benefits of Grazing/Browsing Semiarid Rangeland Feed Resources and the Transference of Bioactivity and Pro-Healthy Properties to Goat Milk and Cheese: Obesity, Insulin Resistance, Inflammation and Hepatic Steatosis Prevention. Animals (Basel). 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |