Published online Oct 26, 2023. doi: 10.12998/wjcc.v11.i30.7277
Peer-review started: August 3, 2023
First decision: August 16, 2023
Revised: August 24, 2023
Accepted: September 28, 2023
Article in press: September 28, 2023
Published online: October 26, 2023
Processing time: 82 Days and 23.6 Hours
Chronic wounds that fail to progress through normal phases of healing present a significant healthcare burden owing to prolonged treatment and associated costs. Traditional wound care typically involves regular dressing changes, which can be painful. Recent approaches have explored the use of lidocaine to manage pain and red-light irradiation (RLI), known for its anti-inflammatory and cell proliferation effects, to potentially enhance wound healing.
To investigate the therapeutic efficacy of lidocaine wet compression (LWC) combined with RLI for chronic wounds.
We enrolled 150 patients with chronic wounds from the Wound and Ostomy Outpatient Clinic of the Second Hospital of Anhui Medical University from April to September 2022. The wounds were treated with dressing changes. The patients were randomly assigned to the control and experimental groups using a random number table and given the same first dressing change (2% LWC for 5 min and routine dressing change). From the second dressing change, in addition to 2% LWC for 5 min and routine dressing change, the experimental group received RLI, whereas the control group continued to receive the same LWC and dressing change. The first and second dressing changes were performed on days 1 and 2, respectively. The third dressing change was performed 3 d after the second change. The frequency of subsequent dressing changes was determined based on wound exudation and pain. Pain during the first three dressing changes was evaluated in both groups. The average number of dressing changes within 28 d and the degree of wound healing on day 28 were also recorded.
During the initial dressing change, no noticeable differences were observed in the pain levels experienced by the two groups, indicating similar pain tolerance. However, during the second and third dressing changes, the experimental group reported significantly less pain than the control group. Furthermore, over 28 d, the experimental group required fewer dressing changes than the control group.
Notably, the effectiveness of wound healing on the 28th day was significantly higher in the experimental group than that of in the control group. The combination of LWC and RLI was effective in reducing early-stage pain, pro
Core Tip: The combination of lidocaine wet compression (LWC) and red-light irradiation (RLI) has shown promising results for the treatment of chronic wounds. A study involving 150 patients demonstrated that the addition of RLI to LWC resulted in reduced pain during dressing changes, improved wound healing, decreased the frequency of dressing changes, and enhanced overall quality of life. During the second and third dressing changes, the experimental group experienced significantly less pain than the control group. Over 28 d, the experimental group required fewer dressing changes on average and achieved higher effectiveness in wound healing by day 28. This combination therapy has the potential to provide effective relief to patients with chronic wounds and improve their outcomes.
- Citation: Bao MZ, Zhou LB, Zhao L, Zhang H, Li Y, Yang L, Tai AT. Efficacy of lidocaine wet compress combined with red-light irradiation for chronic wounds. World J Clin Cases 2023; 11(30): 7277-7283
- URL: https://www.wjgnet.com/2307-8960/full/v11/i30/7277.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i30.7277
Chronic wounds are defined as wounds that fail to progress through a normal, orderly, and timely sequence of repair due to the failure of normal, orderly, and timely repair of wounds because of internal and external factors[1]. These wounds represent a substantial financial burden, accounting for the largest direct medical expenses of all human skin diseases, with an estimated annual cost of $9.7 billion in the United States alone[2]. Like renal and cardiac diseases, chronic wounds affect the patients’ quality of life[3]. Approximately 77% of patients with chronic wounds experience wound pain[4], which triggers various negative psychological reactions and results in adverse neuroendocrine responses. This slows wound healing and increases medical expenses, thereby reducing the patients’ quality of life[5]. The degree of pain in these patients is related to their emotions and diseases, as well as to some procedures involved in dressing changes, such as removing old dressings, wound cleaning, covering new dressings, and bandaging[6].
Both lidocaine wet compression (LWC) and red-light irradiation (RLI) can reduce pain and enhance healing in patients with chronic wounds[7,8]. However, the combined effects of LWC and RLI (LWC+RLI) on pain relief in these patients have not been extensively studied. This study compared the effects of LWC alone and LWC+RLI on pain relief in 150 patients with chronic wounds. This study aims to provide a scientific foundation for future treatment strategies for clinical wound pain.
This study adopted objective sampling to select 150 patients with chronic wounds admitted to the Wound Ostomy Outpatient Clinic of the Second Hospital of Anhui Medical University between April and September 2022 as study participants. We included patients with wounds meeting the definition of chronic wounds[1] and with a wound area of ≥ 4 cm2. Patients signed an informed consent form to participate in the study and cooperated with the study requirements. The patients were aged > 18 years, had no known allergy to lidocaine, and lived in the same city as the hospital from which they received regular treatment. Patients who received less than three treatments; had severe organ failure, cancer, and immunodeficiency disorders; had cognitive or mental disorders; used painkillers during treatment; and withdrew from the study for various reasons. The 150 patients were randomly divided into control and experimental groups at a 1:1 ratio according to a random number table (generated by the SPSS19.0 software package) combined with the envelope method. Patients were observed from days 1 to 28 of treatment.
Dressing changes in the control group: During dressing change in the control group, the old dressing was removed and the wound was rinsed with normal saline. Next, 2% LWC was applied for 5 min, and the wound and surrounding skin were disinfected using cotton balls soaked in 0.5% povidone iodine[9]. Surgical debridement was performed if necessary. Finally, the wound was covered with sterile dressing and bandaged. All patients were given health education to ensure good protein intake, actively improve their nutritional intake, and maintain personal and local wound hygiene. Dressing changes and health education were completed by nurse specialists trained in wound and ostomy care at the hospital and had obtained the corresponding qualifications.
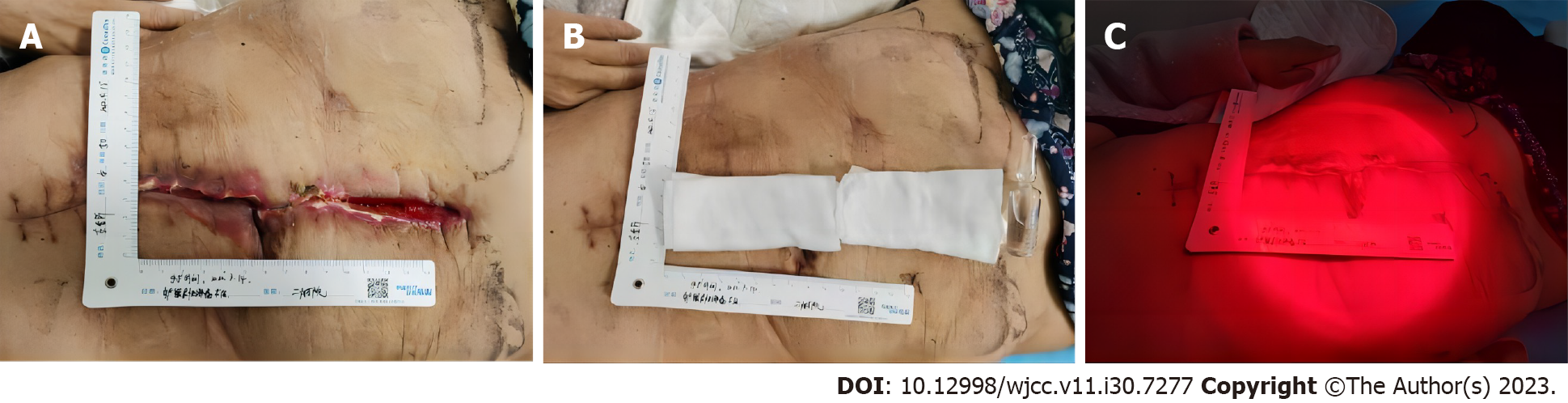
Dressing changes in the experimental group: The experimental group received the same dressing change (first change) at the outpatient clinic as the control group (Figure 1A). After the second dressing change, in addition to 2% LWC for 5 min and routine dressing changes, the experimental group underwent RLI on the wound for 10 min (Figure 1B). The distance between the red light and wound was < 15 cm[10], see the graph below (Figure 1C). Subsequent dressing change procedures were the same as those in the control group.
Degree of pain during dressing change: The degree of pain experienced during dressing changes was evaluated using a numerical scale. Patients had to self-evaluate their pain level during individual dressing changes and score their pain experience on a scale of 0-10. A score of 0 indicated no pain and a score of 10 indicated severe pain. The pain scores from the first to third dressing changes were compared between the two groups[11].
Wound healing evaluation: Wound healing was observed and recorded for both groups. Wound exudation, redness, swelling, wound bed temperature, and wound area were evaluated on day 28. If a wound healed within 28 d, the wound healing time was recorded and no dressing changes were performed. The evaluation criteria for different curative effects of the treatment were as follows: (1) cured: The wound completely healed with appropriate granulation tissue growth, and the wound area was completely covered by epithelial cells; (2) markedly effective: the ulcer and skin color were improved, and the wound area was reduced by > 70%; (3) improved: the wound area was reduced by 25%-75%; and (4) ineffective: The wound area was reduced by < 25%, and the skin color did not significantly improve[12]. The rate of effectiveness was calculated using the following formula: Total rate of effectiveness = (cured cases + markedly effective cases + improved cases)/total number of cases × 100. Rate of ineffectiveness = ineffective cases/total number of cases × 100.
Number of dressing changes: The first and second dressing changes were performed on days 1 and 2, respectively. The third dressing change was performed 3 d after the second change. The dressing change interval was determined based on wound exudation and pain. The patients had to visit for follow-up evaluations when the outermost wound dressing was soaked > 50% of the exudation, or if the wound pain was significantly aggravated[13]. The average number of dressing changes in the two groups within 28 days was recorded.
The SPSS19.0 software package (IBM, Armonk, NY, United States) was used for data entry and analysis. The data were tested for normality. Normally distributed numeric data were presented as the mean ± SD and compared using t-test between the two groups. Non-normally distributed numeric data were presented as median and interquartile range and then compared between the two groups using the rank-sum test. Count data were presented as frequency and percentage (%) and compared between the two groups using the chi-square test. Statistical significance was set at P < 0.05.
A total of 150 patients with chronic wounds were enrolled in this study. The patients were divided into control and experimental groups. The control group had 75 patients (36 men and 39 women; age range: 34-68 years; mean age: 46.2 ± 11.80 years; 22 cases of long-term unhealed incision, 21 cases of venous leg ulcers, 5 cases of pressure ulcers, 15 cases of mastitis, and 12 cases of other conditions). The experimental group had 75 patients (38 men and 37 women; age range: 35-65 years; mean age: 52.9 ± 11.5 years; 24 cases of long-term unhealed incision, 22 cases of venous leg ulcers, 6 cases of pressure ulcers, 13 cases of mastitis, and 10 cases of other conditions). No significant differences in sex, age, wound bed temperature, wound area, or pain score at the first dressing change were observed between the two groups (P > 0.05; Table 1).
| Group | Sex | Age (yr, mean ± SD) | Wound bed temperature (°C, mean ± SD) | Wound area (cm2, median ± IQR) | |
| Male | Female | ||||
| Control (n = 75) | 36 | 39 | 46.2 ± 11.80 | 32.80 ± 2.80 | 7.04 ± 4.17 |
| Experimental (n = 75) | 38 | 37 | 52.9 ± 11.5 | 32.74 ± 1.96 | 7.06 ± 3.29 |
| Statistic | 0.107 | 1.709 | -0.313 | -0.031 | |
| P value | 0.743 | 0.09 | 0.76 | 0.95 | |
No significant difference in the degree of pain was observed during the first dressing change between the two groups (P > 0.05), whereas at the second and third dressing changes, the degree of pain was significantly lower in the experimental group than in the control group (P < 0.05; Table 2).
| Group | Pain score at the first dressing change | Pain score at the second dressing change | Pain score at the third dressing change |
| Control | 7.80 ± 0.86 | 7.33 ± 0.98 | 6.47 ± 1.36 |
| Experimental | 7.67 ± 0.72 | 6.27 ± 0.70 | 5.27 ± 0.96 |
| t | -0.459 | -3.434 | -2.797 |
| P value | 0.650 | 0.002 | 0.009 |
The total effective rate of wound healing on day 28 was significantly higher in the experimental group (96%) than in the control group (86.7%). The rate of ineffectiveness of wound healing was significantly lower in the experimental group (4%) than in the control group (13.3%) (P < 0.05; Table 3).
| Groups | Cases | Cured | Markedly effective | Improved | Ineffective | Total rate of effectiveness | χ2 | P value |
| Control | 75 | 27 (36.0) | 25 (33.3) | 13 (17.3) | 10 (13.3) | 65 (86.7) | 4.127 | 0.042 |
| Experimental | 75 | 30 (40.0) | 27 (36.0) | 15 (20.0) | 3 (4) | 72 (96) |
From day 1 to 28, the number of dressing changes was significantly lower in the experimental group than in the control group. Specifically, the control group (n = 75) had an average of 9.47 ± 1.64 dressing changes, whereas the experimental group (n = 75) had an average of 6.60 ± 1.06 dressing changes. The difference between these two groups was statistically significant, with a t-value of –5.688 and a P-value of < 0.001 (P < 0.05).
For chronic wounds, the traditional debridement is to first clean and disinfect the wound with 3% hydrogen peroxide solution. The results highlighted the significant effectiveness of the combination of LWC and RLI in alleviating pain and promoting wound healing in patients with chronic wounds. From the second dressing change onward, the experimental group received the LWC+RLI treatment and reported significantly less pain than the control group (P < 0.05). Furthermore, on day 28, the overall rate of effective wound healing was significantly higher in the experimental group than that of in the control group (P < 0.05). Additionally, the experimental group required significantly fewer dressing changes within the 28-d period than the control group (P < 0.05). These findings suggest that the combination of LWC and RLI effectively reduced pain, enhanced wound healing, and decreased the frequency of dressing changes in patients with chronic wounds. These results were consistent with those of the previous studies[8,14-17].
For chronic wounds, traditional debridement initially involves cleaning and disinfecting the wound with a 3% hydrogen peroxide solution and then rinsing off the disinfectant with a sterile saline solution[18]. Hydrogen peroxide is a strong irritant that destroys epithelial cells and aggravates wound pain. According to a previous study, wet wrap therapy relieves pain and the economic burden, shortens healing time, and reduces the risk of wound infection. Lidocaine is a common clinically used local anesthetic. It can also be used on wounds in wet wrap therapy for 5 min to quickly alleviate pain. The combination of RLI and LWC compensates for the short duration of the effect of lidocaine, which reduces wound pain, but increases the workload of nurses and bacterial growth in the wound, which may not achieve the expected healing effect. The combination of RLI and LWC not only compensates for the shortcomings of lidocaine but also reduced the degree of wound pain. Red light is a cold light (low-temperature irradiation) that does not cause tissue burns. However, RLI improves the function of mononuclear macrophages in the wound and affects the local serotonin content, thereby achieving anti-inflammatory and analgesic effects[19]. Therefore, LWC+RLI effectively relieved pain in patients with chronic wounds.
Chronic wounds often remain in the inflammatory phase of wound healing[20]. For optimization of wound healing, the wound surface should be clean, have a healthy granulation tissue matrix, and be infection-free. LWC keeps the wound moist, and autolytic debridement of this moist wound allows endogenous enzymes (e.g., matrix metalloproteinases) to degrade nonviable substances from the wound bed and promote wound healing[21]. These results are consistent with previous reports[22]. Red light has a strong penetrability (up to a depth of 30 mm). Red light irradiation on the human body is absorbed by the mitochondria. Red light accelerates metabolism, enhances cell activity, and promotes epithelial cell regeneration, which is conducive to rapid wound healing[19]. Therefore, the combination of lidocaine and RLI effectively promoted the healing of chronic wounds.
This study has several limitations. For example, changes in liver and kidney functions, routine blood tests, and other indicators of the patients were not monitored and compared for financial reasons. Furthermore, unlike in this study, chronic wounds should be subdivided in future studies. With the increase in the number of patients with chronic wounds, the use of red light for wound dressing changes will have broad clinical applications and prospects. Further studies on dressing changes are warranted to provide evidence-based medicine for clinical application.
LWC+RLI effectively relieved pain in patients with chronic wounds. This combination can aid nursing staff in efficiently debriding wounds, thereby promoting wound healing, reducing the number of dressing changes and follow-up visits, and improving patients’ quality of life.
Chronic wounds, characterized by a failure to progress through normal healing phases, represent a substantial burden on healthcare systems and patients’ quality of life. Traditional wound care is often painful and may involve frequent dressing changes. Lidocaine wet compression (LWC) and red-light irradiation (RLI) are known for their potential to manage pain and promote healing in chronic wounds.
The need to enhance wound healing, reduce pain during treatment, and decrease the frequency of dressing changes motivated this research. The key problem addressed is the persistent pain and slow healing associated with chronic wounds, and solving this problem has significant implications for improving patient outcomes and reducing healthcare costs.
The main objective was to investigate the therapeutic efficacy of combining LWC with RLI in treating chronic wounds. Realizing this objective is pivotal for introducing new, effective treatment modalities in wound care management, potentially changing clinical practices.
The study involved 150 patients with chronic wounds, randomly divided into control and experimental groups. The experimental group received LWC+RLI treatment from the second dressing change onward. Pain levels, the number of dressing changes, and the degree of wound healing were systematically assessed and compared between the groups.
The experimental group reported significantly less pain during the second and third dressing changes compared to the control group. The overall rate of effective wound healing on day 28 was significantly higher in the experimental group. The experimental group required significantly fewer dressing changes within the 28-d period. These findings contribute to the understanding of the combined effects of LWC and RLI on chronic wounds, opening avenues for further research and clinical applications.
This study unveils new findings on the effectiveness of combining LWC with RLI in managing chronic wounds, highlighting its potential in reducing pain, promoting wound healing, and decreasing the frequency of dressing changes. It provides a holistic view of current knowledge in chronic wound management and offers original insights into the synergistic effects of LWC and RLI. The study implies that the integration of LWC+RLI could be a groundbreaking approach in clinical practices for treating chronic wounds in the future.
This study serves as a learning cornerstone, suggesting that a combined approach in chronic wound management can yield superior outcomes. Future research should focus on refining this combined approach, exploring its long-term effects, and validating its efficacy across diverse patient populations and wound types. Developing optimized protocols and exploring the economic aspects of implementing this combined approach in clinical settings should be the direction for upcoming research.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, general and internal
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Conway F, Italy; Lupu F, United States S-Editor: Yan JP L-Editor: A P-Editor: Yan JP
| 1. | van Koppen CJ, Hartmann RW. Advances in the treatment of chronic wounds: a patent review. Expert Opin Ther Pat. 2015;25:931-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 2. | Bickers DR, Lim HW, Margolis D, Weinstock MA, Goodman C, Faulkner E, Gould C, Gemmen E, Dall T; American Academy of Dermatology Association; Society for Investigative Dermatology. The burden of skin diseases: 2004 a joint project of the American Academy of Dermatology Association and the Society for Investigative Dermatology. J Am Acad Dermatol. 2006;55:490-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 460] [Cited by in RCA: 484] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 3. | Hopman WM, Harrison MB, Coo H, Friedberg E, Buchanan M, VanDenKerkhof EG. Associations between chronic disease, age and physical and mental health status. Chronic Dis Can. 2009;29:108-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 130] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 4. | Souza SC, Mendes CMC, Meneses JVL, Dias RM. Simplified vacuum dressing system: effectiveness and safety in wounds management. Acta Cir Bras. 2022;37:e370906. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 5. | Vowden K, Vowden P. Wound dressings: principles and practice. Surgery. 2017;35:489-494. [RCA] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 65] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 6. | Obilor HN, Adejumo PO, Ilesanmi RE. Assessment of patients' wound-related pain experiences in University College Hospital, Ibadan, Nigeria. Int Wound J. 2016;13:697-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Trelles MA, Allones I. Red light-emitting diode (LED) therapy accelerates wound healing post-blepharoplasty and periocular laser ablative resurfacing. J Cosmet Laser Ther. 2006;8:39-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 32] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | Adamskaya N, Dungel P, Mittermayr R, Hartinger J, Feichtinger G, Wassermann K, Redl H, van Griensven M. Light therapy by blue LED improves wound healing in an excision model in rats. Injury. 2011;42:917-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 96] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 9. | Wang C, Luan S, Panayi AC, Xin M, Mi B, Luan J. Effectiveness and Safety of Hyaluronic Acid Gel with Lidocaine for the Treatment of Nasolabial Folds: A Systematic Review and Meta-analysis. Aesthetic Plast Surg. 2018;42:1104-1110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 10. | Austin E, Huang A, Wang JY, Cohen M, Heilman E, Maverakis E, Michl J, Jagdeo J. Red Light Phototherapy Using Light-Emitting Diodes Inhibits Melanoma Proliferation and Alters Tumor Microenvironments. Front Oncol. 2022;12:928484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 11. | Williamson A, Hoggart B. Pain: a review of three commonly used pain rating scales. J Clin Nurs. 2005;14:798-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1542] [Cited by in RCA: 1938] [Article Influence: 96.9] [Reference Citation Analysis (0)] |
| 12. | Situm M, Kolić M, Redzepi G, Antolić S. [Chronic wounds as a public health problem]. Acta Med Croatica. 2014;68 Suppl 1:5-7. [PubMed] |
| 13. | Park H, Copeland C, Henry S, Barbul A. Complex wounds and their management. Surg Clin North Am. 2010;90:1181-1194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 14. | Andersen RM, Thyssen JP, Maibach HI. The role of wet wrap therapy in skin disorders – a literature review. Acta Derm Venereol. 2015;95:933-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 15. | Rohringer S, Holnthoner W, Chaudary S, Slezak P, Priglinger E, Strassl M, Pill K, Mühleder S, Redl H, Dungel P. The impact of wavelengths of LED light-therapy on endothelial cells. Sci Rep. 2017;7:10700. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 16. | Michanek P, Toth T, Bergström E, Treffenberg-Pettersson H, Bergh A. Effect of infrared and red monochromatic light on equine wound healing. Equine Vet J. 2021;53:143-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Shi HJ, Song H, Zhao QY, Tao CX, Liu M, Zhu QQ. Efficacy and safety of combined high-dose interferon and red light therapy for the treatment of human papillomavirus and associated vaginitis and cervicitis: A prospective and randomized clinical study. Medicine (Baltimore). 2018;97:e12398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 18. | Wang M, Huang X, Zheng H, Tang Y, Zeng K, Shao L, Li L. Nanomaterials applied in wound healing: Mechanisms, limitations and perspectives. J Control Release. 2021;337:236-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 76] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 19. | Avci P, Gupta A, Sadasivam M, Vecchio D, Pam Z, Pam N, Hamblin MR. Low-level laser (light) therapy (LLLT) in skin: stimulating, healing, restoring. Semin Cutan Med Surg. 2013;32:41-52. [PubMed] |
| 20. | Falanga V. Classifications for wound bed preparation and stimulation of chronic wounds. Wound Repair Regen. 2000;8:347-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 201] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 21. | Powers JG, Higham C, Broussard K, Phillips TJ. Wound healing and treating wounds: Chronic wound care and management. J Am Acad Dermatol. 2016;74:607-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 418] [Article Influence: 46.4] [Reference Citation Analysis (0)] |
| 22. | Purcell A, Buckley T, Fethney J, King J, Moyle W, Marshall AP. The Effectiveness of EMLA as a Primary Dressing on Painful Chronic Leg Ulcers: Effects on Wound Healing and Health-Related Quality of Life. Int J Low Extrem Wounds. 2017;16:163-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |