Published online Jan 26, 2023. doi: 10.12998/wjcc.v11.i3.709
Peer-review started: November 26, 2022
First decision: December 13, 2022
Revised: December 21, 2022
Accepted: January 5, 2023
Article in press: January 5, 2023
Published online: January 26, 2023
Processing time: 61 Days and 1.5 Hours
Soft tissue tuberculosis is rare and insidious, with most patients presenting with a localized enlarged mass or swelling, which may be factors associated with delayed diagnosis and treatment. In recent years, next-generation sequencing has rapidly evolved and has been successfully applied to numerous areas of basic and clinical research. A literature search revealed that the use of next-generation sequencing in the diagnosis of soft tissue tuberculosis has been rarely reported.
A 44-year-old man presented with recurrent swelling and ulcers on the left thigh. Magnetic resonance imaging suggested a soft tissue abscess. The lesion was surgically removed and tissue biopsy and culture were performed; however, no organism growth was detected. Finally, Mycobacterium tuberculosis was confirmed as the pathogen responsible for infection through next-generation sequencing analysis of the surgical specimen. The patient received a standardized anti-tuberculosis treatment and showed clinical improvement. We also performed a literature review on soft tissue tuberculosis using studies published in the past 10 years.
This case highlights the importance of next-generation sequencing for the early diagnosis of soft tissue tuberculosis, which can provide guidance for clinical treatment and improve prognosis.
Core Tip: The diagnosis of extrapulmonary tuberculosis can be challenging, especially for tuberculosis in rare sites such as soft tissues. Soft tissue tuberculosis is rare and easily misdiagnosed. A delay in soft tissue tuberculosis diagnosis may worsen the disease, increase tuberculosis transmission, and accelerate the evolution of drug resistance. This case report emphasizes the importance of next-generation sequencing for early diagnosis of soft tissue tuberculosis, which can provide guidance for clinical treatment and improve prognosis.
- Citation: He YG, Huang YH, Yi XL, Qian KL, Wang Y, Cheng H, Hu J, Liu Y. Soft tissue tuberculosis detected by next-generation sequencing: A case report and review of literature. World J Clin Cases 2023; 11(3): 709-718
- URL: https://www.wjgnet.com/2307-8960/full/v11/i3/709.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i3.709
Tuberculosis (TB) is a chronic infectious disease caused by mycobacterium TB (MTB). Extrapulmonary TB (EPTB) in China constitutes 15%-20% of all TB cases, it can involve any organ, with the most usual sites of infection being the pleura (49.8%), bronchi (14.8%), lymph nodes (8.56%), meninges (7.6%), thoracic vertebra (2.55%), and skeletal joints (0.56%)[1,2]. Isolated soft tissue TB is rare and accounts for only 1-2% of all pulmonary TB (PTB) and EPTB cases. Current knowledge of soft tissue TB is largely based on the analysis of a single patient and case series[3,4]. Therefore, the low incidence and lack of typical symptoms of soft tissue TB may lead to difficulties and delays in diagnosis.
The traditional gold standard for TB diagnosis is the MTB culture assay, which has a lower positivity rate of 23.03%. In EPTB, the positivity rate is approximately 18.45%[5]. The MTB culture assay demands laboratory biosafety requirements, and rapid diagnosis is challenging. Molecular biology techniques, such as real-time fluorescent polymerase chain reaction (PCR) and cross-primer amplification, have facilitated the rapid diagnosis. However, the diagnostic sensitivity of these methods for EPTB is limited and varies among specimen types[6]. For example, the sensitivity of Xpert MTB/ rifampin (RIF) assay for PTB and EPTB was found to be 95.5% and 76.5%, respectively[7].
Next-generation sequencing (NGS) is an emerging and promising technology that is used for disease diagnosis, drug resistance determination, and epidemiological investigations. It has the potential to significantly reduce response time for the identification of pathogens, such as bacteria, viruses, tuberculosis, fungi, and parasites[8-12]. For TB, next-generation sequencing can be used for early diagnosis, identification of drug resistance gene mutations associated with conventional anti-TB drugs, and detection of mixed infections[13]. However, there are only a few reports on the rapid diagnosis of MTB infection in soft tissue using NGS. In this report, we present a case of soft tissue TB in an immunocompetent patient with no history of TB. We used NGS technology to detect the surgically resected lesion tissue, and the patient was finally diagnosed with soft tissue TB. In addition, we reviewed the main features of soft tissue TB cases reported in the past decade.
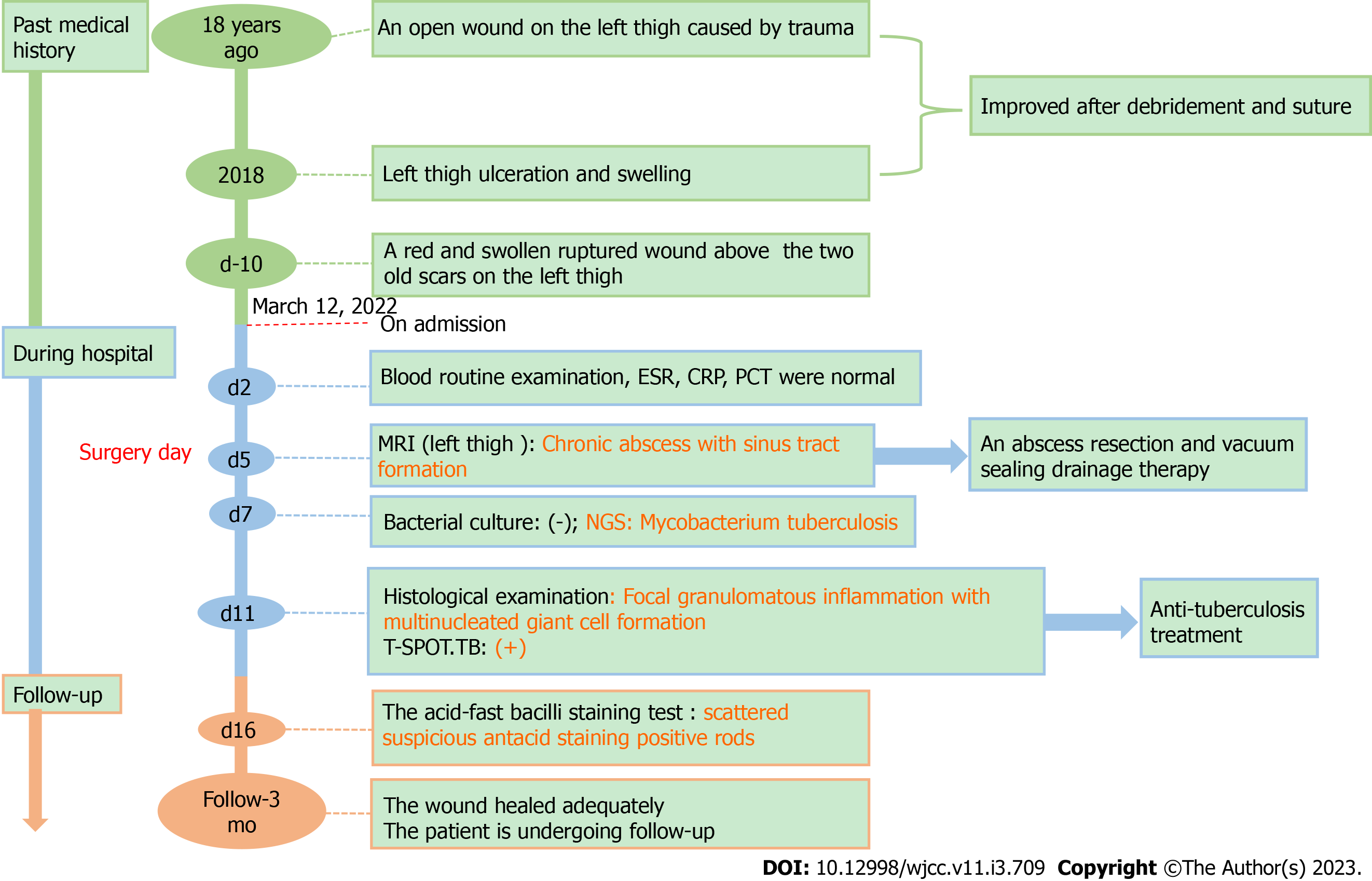
A 44-year-old man admitted to Department of Orthopaedics of the First Affiliated Hospital of Nanjing Medical University presented with a history of left thigh ulceration and swelling for 10 d.
The patient showed similar symptoms in 2018, some tests including bacterial and tuberculosis culture, acid-fast staining, and tuberculosis infection T-lymphocyte spot test (T-SPOT). TB tests, were negative at that time. And the patient recovered gradually after debridement. Through careful history investigation, we found that the patient had an open wound on the left thigh caused by trauma 18 years ago, which improved after debridement and suturing.
There is no relevant history of past illness.
The patient’s personal and family histories were unremarkable.
Two old scars were observed on the patient's left thigh, and there was a red and swollen ruptured wound above them. The skin temperature of the limb was normal, and no movement limitation was observed. A physical examination revealed no other positive signs. Breath sounds of both lungs were clear, no obvious dry or wet rales were heard, and there was no pleural friction rub.
On day 2 after admission, laboratory tests, such as routine blood examination, coagulation function tests, erythrocyte sedimentation rate, and serum biochemical indicators, did not show any significant abnormalities.
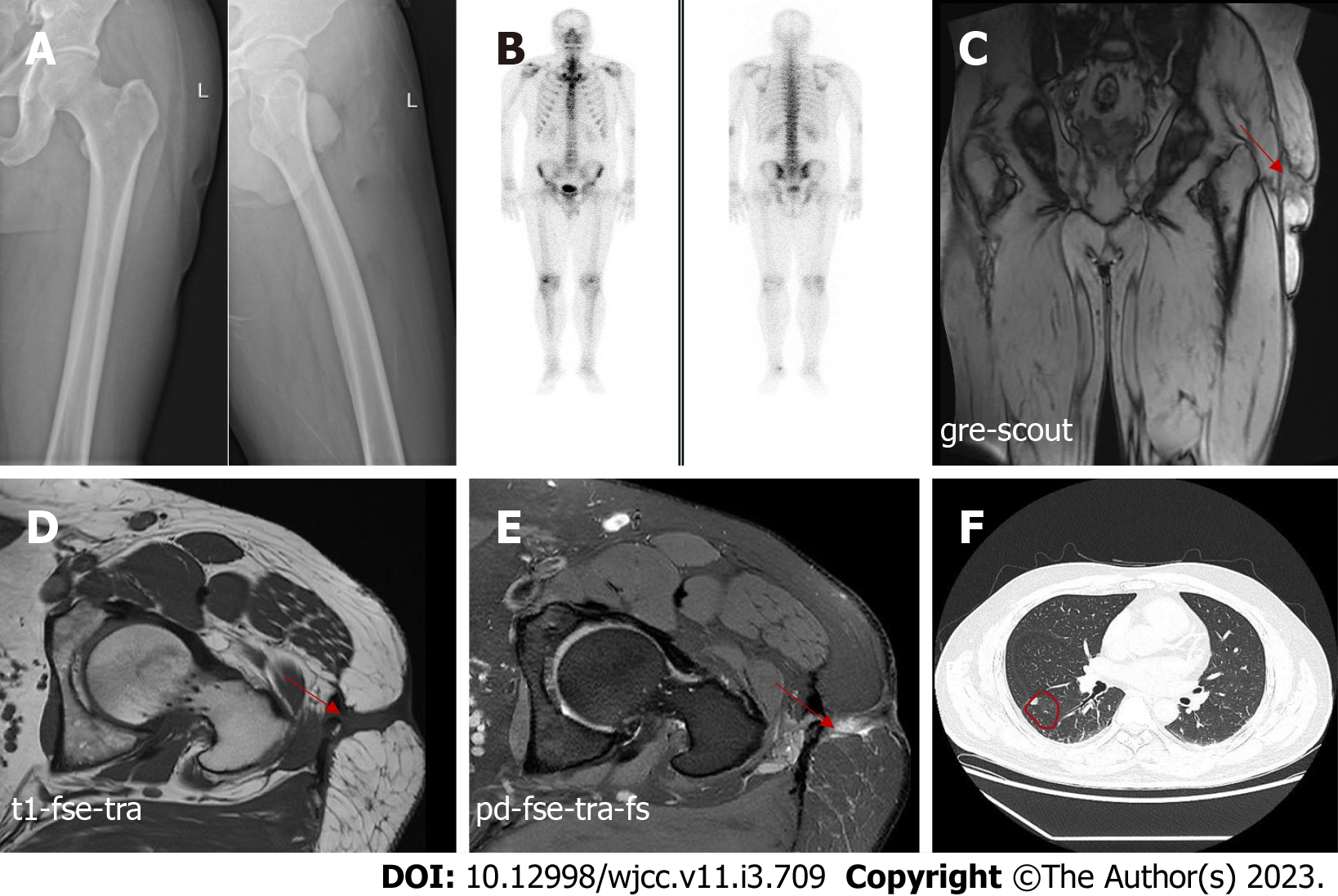
A radiograph of the left femur indicated no bone erosion (Figure 1A). Bone single-photon emission computed tomography did not reveal any abnormalities in bone metabolism (Figure 1B). On day five after admission, magnetic resonance imaging (MRI) of the left hip was performed, which revealed abnormal signals in the soft tissue of the left upper femur and oedema of the subcutaneous soft tissue. In addition, MRI revealed a chronic abscess with sinus tract formation (Figure 1C-E). Computed tomography of the chest showed scattered nodules in both lungs (Figure 1F). But the symptoms of tuberculosis are not obvious.
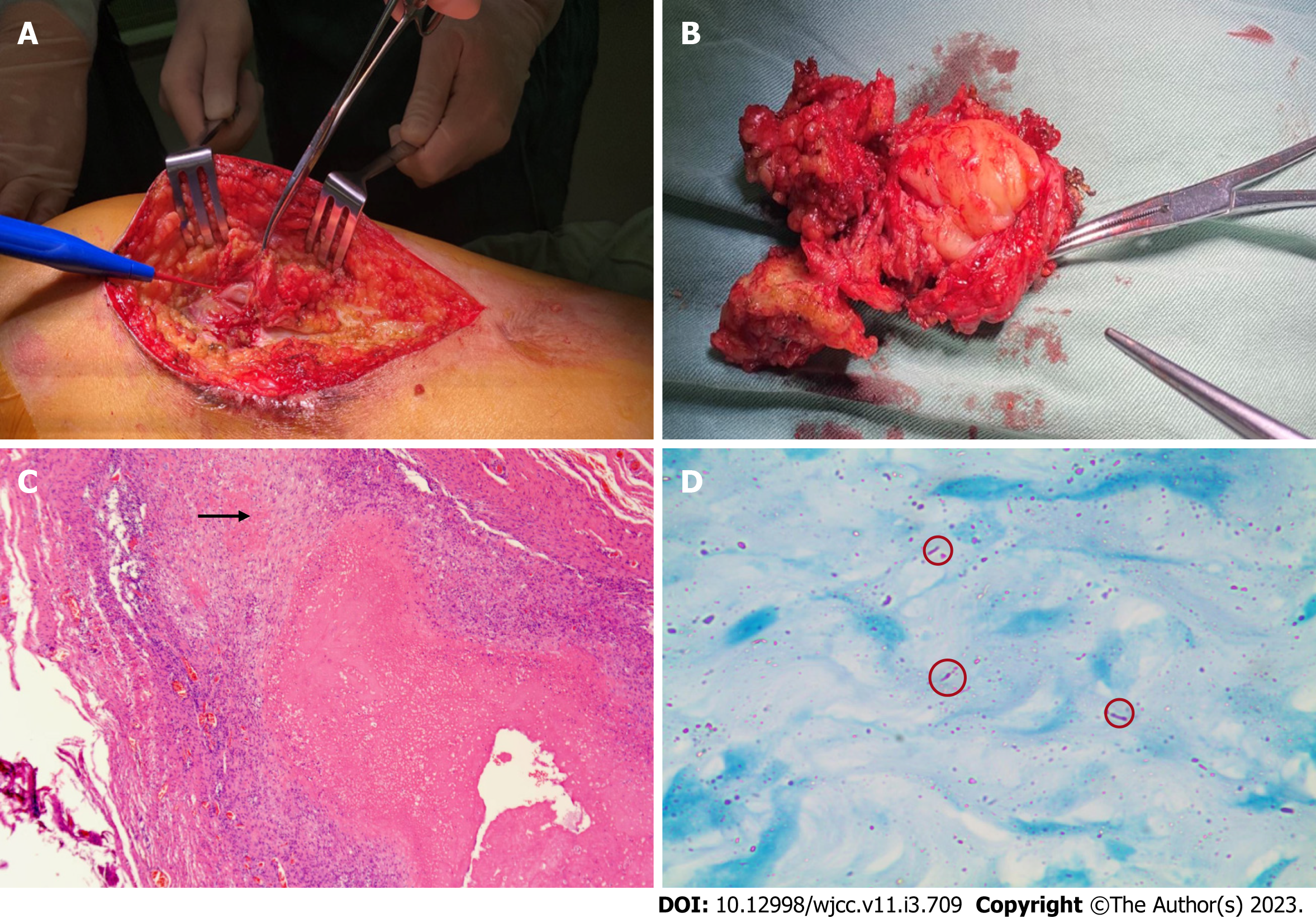
Considering that the abscess was large, we performed abscess resection of the left thigh and vacuum sealing drainage therapy. We observed inflammation and degeneration in subcutaneous tissue and cystic infected tissue wrapped in the deep layer, which had tough capsule walls and a size of approximately 8 cm × 6 cm (Figure 2A and B). After incision of the purulent cyst, gelatinous necrotic tissue which was grey and white, was observed. Furthermore, we performed histological examination, bacterial culture, and NGS testing of the specimen.
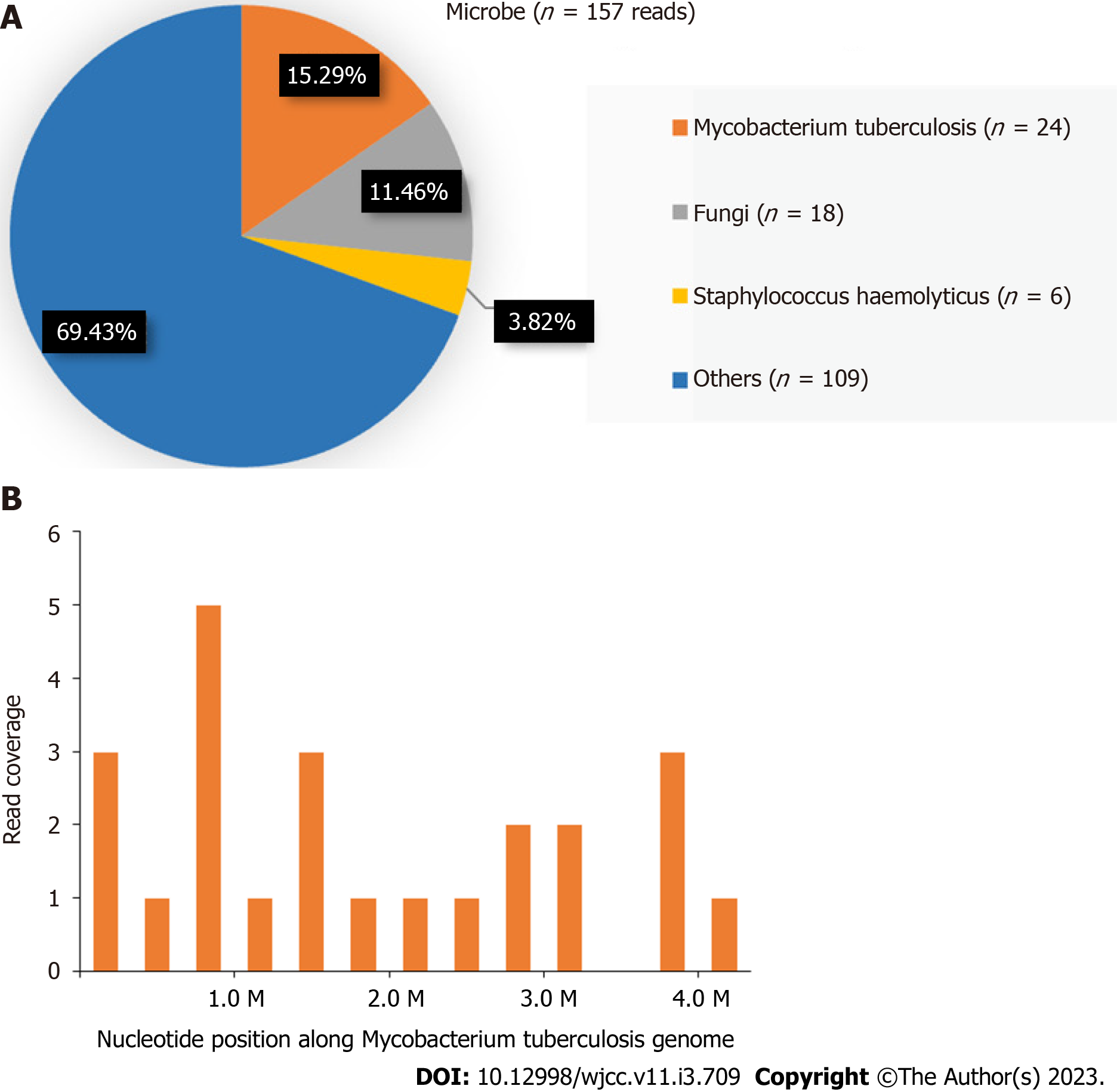
Histological examination revealed fibrous connective tissue hyperplasia with necrosis, acute and chronic inflammatory cell infiltration, and focal granulomatous inflammation with multinucleated giant cell formation (Figure 2C). Bacterial culture of the surgically excised specimen was negative on day seven after admission. However, MTB was detected by NGS simultaneously. The distribution of bacteria and fungi identified by NGS revealed that MTB was the main pathogen (Figure 3A), and 24 sequence reads were identified (Figure 3B).
The pathology department was then contacted for an additional acid-fast bacillus staining test of the intraoperative specimen, and scattered suspicious antacid staining positive rods were observed microscopically (Figure 2D). Further, the T-SPOT. TB test results were positive.
Combined with the patient’s medical history and outcome of NGS, the patient was finally diagnosed with soft tissue tuberculosis.
We planned a standardized anti-tuberculosis treatment with four drugs, isoniazid, rifampicin, ethambutol, and pyrazinamide, for 2 mo. Further isoniazid and rifampicin were given for four months. But the patient stopped the drug because of gastrointestinal discomfort during the 3rd month of treatment and suspended for 2 wk, then he continued to take the medicine again for 3 mo.
Currently, the wound has healed adequately and the patient is undergoing follow-up. A summary of the timeline is shown in Figure 4.
According to a global TB report published by WHO, TB deaths have increased because of reduced access to TB diagnosis and treatment in the face of the COVID-19 pandemic for the first time in over a decade[14]. China has the second-highest number of TB cases worldwide, accounting for approximately 9% of the global TB incidence. One study indicated that the prevalence of smear-positive TB in China decreased from 170/100 000 population in 1990 to 59 /100 000 population in 2010, a reduction of more than 50 percent[15]. Despite past success in controlling TB, the limited epidemiologic information available suggests that the incidence of EPTB may be increasing steadily worldwide, including in China[16,17].
The diagnosis of EPTB can be challenging, especially for TB in rare sites such as soft tissues. A delay in soft tissue TB diagnosis may worsen the disease, increase TB transmission, and accelerate the evolution of drug resistance. The conventional gold standards for TB diagnosis are MTB culture and drug sensitivity tests. However, it is tedious and can take 6-8 wk because of slow growth of MTB[18]. Molecular diagnostic techniques, such as Xpert MTB/RIF, loop-mediated isothermal amplification (LAMP), and line probe assay (LPA), can effectively reduce turnaround time and improve diagnostic performance. However, these techniques have limited diagnostic sensitivity for specimens with low bacterial content, such as EPTB[19,20]. For example, a meta-analysis found that the pooled sensitivity of Xpert MTB/RIF for diagnosing abdominal TB was only 23%[21]. Another meta-analysis reported that the pooled sensitivity of LAMP for detecting EPTB was 77%[22]. Only a few studies have evaluated the role of LPA in EPTB specimens. A study from India reported a sensitivity of 46.1% with a specificity of 91% in EPTB specimens, with liquid culture as the reference standard[23].
Soft tissue TB is rare and easily misdiagnosed. Therefore, rapid, efficient, and accurate diagnosis of soft tissue TB is an urgent clinical problem. NGS is a revolutionary development of first-generation sequencing methods that can sequence millions of DNA fragments simultaneously with high throughput and short detection cycles. A study found that the positivity rate of NGS is approximately 15% higher than that of traditional pathogen culture in a pairwise manner for infectious diseases[24]. Xpert MTB/RIF and NGS tests were performed on various samples of sputum, cerebrospinal fluid, and pus from patients with suspected active TB infection. Compared with Xpert MTB/RIF, NGS showed better sensitivity in all clinical (76.9% vs 61.5%), pulmonary (87.5% vs 75.0%), and extrapulmonary samples (60.0% vs 40.0%)[13]. In this case, NGS rapidly detected the sequence of MTB in the sample, which was important for us to confirm the diagnosis of soft tissue TB early and to treat it with anti-tuberculosis drugs in time. Below, we review the relevant literature on soft tissue TB.
Recent case reports on soft tissue TB published in the past 10 years were identified by searching PubMed (Table 1). We found 17 case reports including 10 males and 7 females. The ages of the patients ranged from 7 to 79 years. There were 12 cases distributed in Asian countries, four cases in African countries and one case in America. No underlying diseases were reported in 10 patients. In 13 patients, the lesions were located on the extremities, including the thigh, calf, forearm, and wrist. The other four patients had lesions in the gluteus, back, thorax, and iliopsoas.
| Ref. | Gender/age (yr) | Country | Underlying disease | Main clinical manifestations | Involved sites | Diagnostic methods |
| Arora et al[38], 2012 | Male/15 | India | None | Swelling, anorexia, weight loss | Left thigh | Mycobacterium tuberculosis culture |
| Lee et al[39], 2013 | Male/62 | Korea | Right total hip arthroplasty | Mass | Right thigh | Histological examination, and culture |
| Elshafie et al[40], 2013 | Male/25 | Oman | Exposed to suspected tuberculosis, diarrhea | Enlarging swelling | Right gluteus | Histological examination, and culture |
| Neogi et al[41], 2013 | Female/11 | India | None | Swelling | Right thigh, right calf, and left arm | Histological examination, and culture |
| Meena et al[42], 2015 | Male/25 | India | None | Swelling, fatigue, weight loss | Right triceps | Mycobacterium tuberculosis PCR |
| Dhakal et al[43], 2015 | Female/9 | Nepal | None | Swelling | Forearm, right calf | Histological examination |
| Sbai et al[44], 2016 | Male/45 | Tunisia | None | Pain, swelling | Right wrist | Tissue biopsy and culture |
| Al-khazraji et al[45], 2017 | Female/33 | America | Lupus nephritis, hormonal therapy | Pain, weakness, swelling, redness | Right calf | Fluid culture |
| Alaya et al[46], 2017 | Female/23 | Tunisia | None | Swelling, pain | Left thigh | Mycobacterium tuberculosis PCR |
| Manicketh et al[25], 2018 | Female/55 | India | Pulmonary tuberculosis | Swelling, fever | Left wrist and right calf | A Ziehl-Nielsen stain |
| Hashimoto et al[27], 2018 | Male/79 | Japan | None | Swelling, erythema | Left wrist | Histological examination |
| Zeng et al[4], 2019 | Male/49 | China | Pulmonary tuberculosis; steroid treatment | Pain, mass, swelling | Both thighs and calves | Mycobacterium tuberculosis PCR, Tissue biopsy and culture |
| Zitouna et al[47], 2019 | Female/42 | Tunisia | None | Mass, swelling | Right mid-back | Tissue biopsy and culture |
| Moyano et al[48], 2019 | Male/29 | Senegal | None | Pain, increase in size of hemithorax | Right hemithorax | Mycobacterium tuberculosis PCR and culture |
| Fahad et al[28], 2020 | Female/45 | Pakistan | None | Swelling, pain | Right forearm | Histological examination |
| Murugesh et al[49], 2020 | Male/31 | India | Renal transplant with immuno-suppressants | Fever, pain, swollen erythematous | Right foot and calf | Nucleic acid amplification test |
| Tone et al[26], 2021 | Male/29 | Japan | Right tuberculous pleurisy | Fever, pain | Right iliopsoas | Mycobacterium tuberculosis PCR |
Soft tissue TB occurs mostly in the extremities, and patients present with local masses, swelling, and weakness. Only a small percentage of patients present with constitutional symptoms such as fever and weight loss[25,26]. Most cases of soft tissue TB reported in the literature were diagnosed by histological examination and MTB culture, whereas some were confirmed by rapid PCR. Although the culture and gene Xpert were negative, two patients recovered after empirical anti-TB therapy[27,28]. Empirical treatment for TB was initiated without a confirmed bacterial diagnosis. Moreover, factors contributing to the probability of a patient developing TB and experiencing adverse outcomes were weighed against the threshold for initiating anti-TB therapy. This threshold is subjective and may vary among the clinicians. Factors considered in empirical anti-TB treatment include the background TB epidemiology in the geographic area, exposure to TB patients, clinical manifestations suggestive of TB disease, comorbidities such as HIV co-infection, and the results of other diagnostic methods such as imaging outcomes if available[29].
There are many traditional tests for TB, including smear, culture, pathological biopsy, imaging, purified protein derivative testing, interferon-gamma release assay, and TB antibody testing[30,31]. Confirmation of TB over the past few decades often requires a combination of these tests. Over the last decade, advances have been made in the field of TB diagnostics in the form of new molecular tests. Often referred to as nucleic acid amplification tests, these assays rely on amplification of a targeted genetic region of the MTB complex, typically by PCR[32]. GeneXpert MTB/RIF is a fully automated closed system that performs sample preparation and real-time PCR and produces results within 2 h. This system can detect RIF resistance (targeting the rpoB gene). In 2011, the WHO recommended GeneXpert MTB/RIF for the early diagnosis of drug-resistant TB, which was further expanded in 2013 to replace smear and culture for the rapid diagnosis of EPTB[33]. The new version of Xpert MTB Ultra improved overall sensitivity and was endorsed by the WHO in 2017[34]. In 2017, the UK used NGS for TB diagnosis, drug resistance detection, and MTB typing for the first time[35]. Several studies have shown its advantages in the diagnosis and treatment of EPTB[36,37]. However, the value of NGS in the rapid diagnosis of TB has not been verified in large samples, and there is a lack of unified standards and procedures. Guidelines for the clinical interpretation of NGS reports need to be improved.
We reported the case of a patient who was immunocompetent and had no history of TB and was diagnosed with soft tissue TB by NGS. The patient received timely anti-TB treatment, which improved. Clinicians should consider atypical pathogens such as MTB in the diagnosis of patients with local masses or swelling. NGS may be a useful method for identifying the pathogens responsible for soft tissue infections without typical clinical manifestations.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: Shen TC, Taiwan; Vyshka G, Albania S-Editor: Wang LL L-Editor: A P-Editor: Wang LL
| 1. | Baykan AH, Sayiner HS, Aydin E, Koc M, Inan I, Erturk SM. Extrapulmonary tuberculosıs: an old but resurgent problem. Insights Imaging. 2022;13:39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 60] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 2. | Kang W, Liu S, Du J, Tang P, Chen H, Liu J, Ma J, Li M, Qin J, Shu W, Zong P, Zhang Y, Dong Y, Yang Z, Mei Z, Deng Q, Wang P, Han W, Yan X, Chen L, Zhao X, Tan L, Li F, Zheng C, Liu H, Li X, A E, Du Y, Liu F, Cui W, Wang Q, Chen X, Han J, Xie Q, Feng Y, Liu W, Yang S, Zhang J, Zheng J, Chen D, Yao X, Ren T, Li Y, Wu L, Song Q, Shen X, Liu Y, Guo S, Yan K, Yang M, Lei D, Wu M, Li L, Tang S. Epidemiology of concurrent extrapulmonary tuberculosis in inpatients with extrapulmonary tuberculosis lesions in China: a large-scale observational multi-centre investigation. Int J Infect Dis. 2022;115:79-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 3. | Franco-Paredes C, Chastain DB, Allen L, Henao-Martínez AF. Overview of Cutaneous Mycobacterial Infections. Curr Trop Med Rep. 2018;5:228-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 4. | Zeng Y, Liu Y, Xie Y, Liang J, Kuang J, Lu Z, Zhou Y. Muscular Tuberculosis: A New Case and a Review of the Literature. Front Neurol. 2019;10:1031. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 5. | Sun WW, Gu J, Fan L. [Application value of metagenomic next-generation sequencing (mNGS) in the diagnosis of different types of tuberculosis]. Zhonghua Jie He He Hu Xi Za Zhi. 2021;44:96-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 6. | Tadesse M, Abebe G, Bekele A, Bezabih M, Yilma D, Apers L, de Jong BC, Rigouts L. Xpert MTB/RIF assay for the diagnosis of extrapulmonary tuberculosis: a diagnostic evaluation study. Clin Microbiol Infect. 2019;25:1000-1005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 7. | Allahyartorkaman M, Mirsaeidi M, Hamzehloo G, Amini S, Zakiloo M, Nasiri MJ. Low diagnostic accuracy of Xpert MTB/RIF assay for extrapulmonary tuberculosis: A multicenter surveillance. Sci Rep. 2019;9:18515. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 8. | Goelz H, Wetzel S, Mehrbarzin N, Utzolino S, Häcker G, Badr MT. Next- and Third-Generation Sequencing Outperforms Culture-Based Methods in the Diagnosis of Ascitic Fluid Bacterial Infections of ICU Patients. Cells. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 9. | Sahajpal NS, Mondal AK, Njau A, Petty Z, Chen J, Ananth S, Ahluwalia P, Williams C, Ross TM, Chaubey A, DeSantis G, Schroth GP, Bahl J, Kolhe R. High-Throughput Next-Generation Sequencing Respiratory Viral Panel: A Diagnostic and Epidemiologic Tool for SARS-CoV-2 and Other Viruses. Viruses. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Oreskovic A, Waalkes A, Holmes EA, Rosenthal CA, Wilson DPK, Shapiro AE, Drain PK, Lutz BR, Salipante SJ. Characterizing the molecular composition and diagnostic potential of Mycobacterium tuberculosis urinary cell-free DNA using next-generation sequencing. Int J Infect Dis. 2021;112:330-337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Tiew PY, Thng KX, Chotirmall SH. Clinical Aspergillus Signatures in COPD and Bronchiectasis. J Fungi (Basel). 2022;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Ryan U, Zahedi A, Feng Y, Xiao L. An Update on Zoonotic Cryptosporidium Species and Genotypes in Humans. Animals (Basel). 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 127] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 13. | Zhou X, Wu H, Ruan Q, Jiang N, Chen X, Shen Y, Zhu YM, Ying Y, Qian YY, Wang X, Ai JW, Zhang WH. Clinical Evaluation of Diagnosis Efficacy of Active Mycobacterium tuberculosis Complex Infection via Metagenomic Next-Generation Sequencing of Direct Clinical Samples. Front Cell Infect Microbiol. 2019;9:351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 99] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 14. | Daley CL. The Global Fight Against Tuberculosis. Thorac Surg Clin. 2019;29:19-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 15. | Wang L, Zhang H, Ruan Y, Chin DP, Xia Y, Cheng S, Chen M, Zhao Y, Jiang S, Du X, He G, Li J, Wang S, Chen W, Xu C, Huang F, Liu X, Wang Y. Tuberculosis prevalence in China, 1990-2010; a longitudinal analysis of national survey data. Lancet. 2014;383:2057-2064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 407] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 16. | Pang Y, An J, Shu W, Huo F, Chu N, Gao M, Qin S, Huang H, Chen X, Xu S. Epidemiology of Extrapulmonary Tuberculosis among Inpatients, China, 2008-2017. Emerg Infect Dis. 2019;25:457-464. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 180] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 17. | Chen L, Fu X, Tian P, Li Q, Lei D, Peng Z, Liu Q, Li N, Zhang J, Xu P, Zhang H. Upward trends in new, rifampicin-resistant and concurrent extrapulmonary tuberculosis cases in northern Guizhou Province of China. Sci Rep. 2021;11:18023. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Baker JJ, Johnson BK, Abramovitch RB. Slow growth of Mycobacterium tuberculosis at acidic pH is regulated by phoPR and host-associated carbon sources. Mol Microbiol. 2014;94:56-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 112] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 19. | Kohli M, Schiller I, Dendukuri N, Dheda K, Denkinger CM, Schumacher SG, Steingart KR. Xpert(®) MTB/RIF assay for extrapulmonary tuberculosis and rifampicin resistance. Cochrane Database Syst Rev. 2018;8:CD012768. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 137] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 20. | Mhimbira FA, Bholla M, Sasamalo M, Mukurasi W, Hella JJ, Jugheli L, Reither K. Detection of Mycobacterium tuberculosis by EasyNAT diagnostic kit in sputum samples from Tanzania. J Clin Microbiol. 2015;53:1342-1344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Sharma V, Soni H, Kumar-M P, Dawra S, Mishra S, Mandavdhare HS, Singh H, Dutta U. Diagnostic accuracy of the Xpert MTB/RIF assay for abdominal tuberculosis: a systematic review and meta-analysis. Expert Rev Anti Infect Ther. 2021;19:253-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (1)] |
| 22. | Yu G, Shen Y, Zhong F, Ye B, Yang J, Chen G. Diagnostic accuracy of the loop-mediated isothermal amplification assay for extrapulmonary tuberculosis: A meta-analysis. PLoS One. 2018;13:e0199290. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 23. | Sharma SK, Kohli M, Chaubey J, Yadav RN, Sharma R, Singh BK, Sreenivas V, Sharma A, Bhatia R, Jain D, Seenu V, Dhar A, Soneja M. Evaluation of Xpert MTB/RIF assay performance in diagnosing extrapulmonary tuberculosis among adults in a tertiary care centre in India. Eur Respir J. 2014;44:1090-1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 24. | Miao Q, Ma Y, Wang Q, Pan J, Zhang Y, Jin W, Yao Y, Su Y, Huang Y, Wang M, Li B, Li H, Zhou C, Li C, Ye M, Xu X, Li Y, Hu B. Microbiological Diagnostic Performance of Metagenomic Next-generation Sequencing When Applied to Clinical Practice. Clin Infect Dis. 2018;67:S231-S240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 591] [Article Influence: 98.5] [Reference Citation Analysis (0)] |
| 25. | Manicketh I, Panjwani P, Ravikumar G, Prince Mathan L. Soft tissue tuberculosis - An unusual presentation of a common disease. Indian J Tuberc. 2018;65:96-97. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (1)] |
| 26. | Tone K, Hirano Y, Kuwano K. Iliopsoas gravity abscess secondary to a tuberculous empyema. Int J Mycobacteriol. 2021;10:335-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 27. | Hashimoto K, Nishimura S, Oka N, Kakinoki R, Akagi M. Tuberculoma with phlegmon-like symptoms mimicking soft tissue sarcoma in the wrist: A case report. Mol Clin Oncol. 2018;9:207-210. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 28. | Fahad S, Baloch N, Din NU. Tuberculosis of the flexor carpi radialis muscle - a case report. J Pak Med Assoc. 2020;70:1645-1647. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 29. | Dartois VA, Rubin EJ. Anti-tuberculosis treatment strategies and drug development: challenges and priorities. Nat Rev Microbiol. 2022;20:685-701. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 205] [Cited by in RCA: 223] [Article Influence: 74.3] [Reference Citation Analysis (0)] |
| 30. | Nishimura T, Hasegawa N, Mori M, Takebayashi T, Harada N, Higuchi K, Tasaka S, Ishizaka A. Accuracy of an interferon-gamma release assay to detect active pulmonary and extra-pulmonary tuberculosis. Int J Tuberc Lung Dis. 2008;12:269-274. [PubMed] |
| 31. | Procop GW. Laboratory Diagnosis and Susceptibility Testing for Mycobacterium tuberculosis. Microbiol Spectr. 2016;4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 32. | Acharya B, Acharya A, Gautam S, Ghimire SP, Mishra G, Parajuli N, Sapkota B. Advances in diagnosis of Tuberculosis: an update into molecular diagnosis of Mycobacterium tuberculosis. Mol Biol Rep. 2020;47:4065-4075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 127] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 33. | WHO Guidelines Approved by the Guidelines Review Committee. Xpert MTB/RIF Implementation Manual: Technical and Operational ‘How-To’; Practical Considerations. Geneva: World Health Organization, 2014. |
| 34. | Chakravorty S, Simmons AM, Rowneki M, Parmar H, Cao Y, Ryan J, Banada PP, Deshpande S, Shenai S, Gall A, Glass J, Krieswirth B, Schumacher SG, Nabeta P, Tukvadze N, Rodrigues C, Skrahina A, Tagliani E, Cirillo DM, Davidow A, Denkinger CM, Persing D, Kwiatkowski R, Jones M, Alland D. The New Xpert MTB/RIF Ultra: Improving Detection of Mycobacterium tuberculosis and Resistance to Rifampin in an Assay Suitable for Point-of-Care Testing. mBio. 2017;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 286] [Cited by in RCA: 453] [Article Influence: 56.6] [Reference Citation Analysis (0)] |
| 35. | Satta G, Lipman M, Smith GP, Arnold C, Kon OM, McHugh TD. Mycobacterium tuberculosis and whole-genome sequencing: how close are we to unleashing its full potential? Clin Microbiol Infect. 2018;24:604-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 89] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 36. | Sun W, Lu Z, Yan L. Clinical efficacy of metagenomic next-generation sequencing for rapid detection of Mycobacterium tuberculosis in smear-negative extrapulmonary specimens in a high tuberculosis burden area. Int J Infect Dis. 2021;103:91-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 37. | Yu G, Wang X, Zhu P, Shen Y, Zhao W, Zhou L. Comparison of the efficacy of metagenomic next-generation sequencing and Xpert MTB/RIF in the diagnosis of tuberculous meningitis. J Microbiol Methods. 2021;180:106124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 38. | Arora S, Sabat D, Sural S, Dhal A. Isolated tuberculous pyomyositis of semimembranosus and adductor magnus: a case report. Orthop Surg. 2012;4:266-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 39. | Lee HJ, Kim KW, Kim KS, Ryu SH, Ha YC. Primary musculoskeletal mycobacterium infection with large cystic masses after total hip arthroplasty. J Arthroplasty. 2013;28:374.e1-374.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 40. | Elshafie KT, Al-Hinai MM, Al-Habsi HA, Al-Hattali MS, Hassan O, Al-Sukaiti R. A massive tuberculosis abscess at the erector spinae muscles and subcutaneous tissues in a young man. Sultan Qaboos Univ Med J. 2013;13:601-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 41. | Neogi DS, Bandekar SM, Chawla L. Skeletal muscle tuberculosis simultaneously involving multiple sites. J Pediatr Orthop B. 2013;22:167-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 42. | Meena M, Dixit R, Samaria JK, Vijayakandeepan Kumaresan SH. Tuberculosis of the triceps muscle. BMJ Case Rep. 2015;2015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 43. | Dhakal AK, Shah SC, Shrestha D, Banepali N, Geetika KC. Tuberculosis presenting as multiple intramuscular nodules in a child: a case report. J Med Case Rep. 2015;9:72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 44. | Sbai MA, Benzarti S, Msek H, Boussen M, Khorbi A. Pseudotumoral form of soft-tissue tuberculosis of the wrist. Int J Mycobacteriol. 2016;5:99-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 45. | Al-Khazraji A, Takher J, Alkhawam H, Fabbri M. Primary Tuberculous Pyomyositis of the Calf Muscles. Am J Med Sci. 2017;353:187-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 46. | Alaya Z, Osman W. Isolated muscular tuberculosis: unusual location of the Koch bacillus. Pan Afr Med J. 2017;26:158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 47. | Zitouna K, Riahi H, Goubantini A, Barsaoui M. Isolated tuberculous abscess in longissimus muscle. Int J Mycobacteriol. 2019;8:403-405. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 48. | Moyano-Bueno D, Blanco JF, López-Bernus A, Gutiérrez-Zubiaurre N, Gomez Ruiz V, Velasco-Tirado V, Belhassen-García M. Cold abscess of the chest wall: A diagnostic challenge. Int J Infect Dis. 2019;85:108-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 49. | Murugesh Anand S, Edwin Fernando M, Srinivasaprasad ND, Sujit S, Thirumalvalavan K. Tuberculous myositis and cellulitis in a renal transplant recipient. Indian J Tuberc. 2020;67:353-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |