Published online Jan 26, 2023. doi: 10.12998/wjcc.v11.i3.692
Peer-review started: November 24, 2022
First decision: December 13, 2022
Revised: December 22, 2022
Accepted: January 5, 2023
Article in press: January 5, 2023
Published online: January 26, 2023
Processing time: 63 Days and 6.9 Hours
Hemangioblastoma typically occurs in the cerebellum, spinal cord, and central nervous system. However, in rare cases, it could occur in the retina or optic nerve. The prevalence of retinal hemangioblastoma is 1 in 73080, and it occurs either alone or as the manifestation of von Hippel Lindau (VHL) disease. Here, we reported a rare case with the imaging features of retinal hemangioblastoma without VHL syndrome, along with the relevant literature review.
A 53-year-old man had progressive swelling, pain and blurred vision in the left eye without obvious inducement for 15 d. Ultrasonography revealed a possible optic nerve head melanoma. Computed tomography (CT) showed punctate calcification on the posterior wall of the left eye ring and small patchy soft tissue density in the posterior part of the eyeball. Magnetic resonance imaging showed slightly hyperintense signal on T1-weighted images and slightly hypointense-to-isointense signal on T2-weighted images at the medial and posterior edges of the left eyeball, a significant enhancement was observed in the contrast-enhanced scans. Positron emission tomography/CT fusion images showed that the glucose metabolism of the lesion was normal. Pathology was consistent with hemangioblastoma.
Early identification of retinal hemangioblastoma based on imaging features is of great value for its personalized treatment.
Core Tip: We reported a rare case of the imaging features of retinal hemangioblastoma without von Hippel Lindau syndrome, along with the relevant literature review. A 53-year-old man, who had progressive swelling, pain and blurred vision in the left eye without obvious inducement for 15 d. Ultrasonography revealed a possible optic nerve melanoma of the head. Computed tomography (CT) showed punctate calcification on the posterior wall of the left eye ring and small patchy soft tissue density in the posterior part of the eyeball. Magnetic resonance imaging showed slightly hyperintense on T1-weighted images and slightly hypointense-to-isointense on T2-weighted images at the medial and posterior edges of the left eyeball, a significant enhancement was observed after contrast-enhanced scans. positron emission tomography/CT fusion images showed that the glucose metabolism of the lesion was normal. Pathology was consistent with hemangioblastoma. Early identification of retinal hemangioblastoma by imaging features is of great value for its personalized treatment.
- Citation: Tang X, Ji HM, Li WW, Ding ZX, Ye SL. Imaging features of retinal hemangioblastoma: A case report. World J Clin Cases 2023; 11(3): 692-699
- URL: https://www.wjgnet.com/2307-8960/full/v11/i3/692.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i3.692
Retinal hemangioblastoma (RCH) is a rare benign tumor that was first reported by Von Hippel, a German ophthalmologist, in 1911. Since then, more than 900 families worldwide have been diagnosed with the disease[1]. RCH has also been shown to be the most common and earliest manifestation in 49%-85% of patients with von Hippel Lindau (VHL) disease, and only a very small number of cases are sporadic. Its diagnosis is mainly based on clinical suspicion and confirmation by molecular testing and imaging techniques[2-4]. Moreover, retinal hemangiomas can be usually observed directly and diagnosed by eye fundus examination, which may be the main reason why radiologists infrequently perform RCH diagnosis. We retrospectively analyzed the relevant literature and found that the imaging features of RCH are rarely reported[1-11]. Herein, we reported the computed tomography (CT), magnetic resonance (MR) imaging, and positron emission tomography (PET)/CT features of a sporadic RCH case.
On February 23, 2022, a 53-year-old male was admitted to our hospital because of 15 d history of progressive swelling, pain and blurred vision in the left eye, in the absence of obvious inducement.
Fifteen days ago, the patient developed swelling, pain and blurred vision in the left eye, and was admitted to the 9th Hospital of Hangzhou. He was diagnosed with "neovascular glaucoma” and was given tobramycin dexamethasone eye drops and pranoprofen eye drops for anti-inflammatory therapy, timolol eye drops and brinzolamide eye drops for ocular hypotensive therapy. However, the disease symptoms did not improve, and he visited the First People's Hospital of Hangzhou on February 23, 2022, for further diagnosis and treatment.
The patient had no past illness.
The patient had no special personal or family history of illness.
Ophthalmological examination showed that visio oculus dexter was 0.8 and Visus Oculi Sinistri was sensitive to light (mainly contains distorted light that is located above and below the nose). Noncontact tonometer showed that R/L = 16.3/Tn + 3 mmHg. There was no hyperemia of right bulbar conjunctiva. The cornea was clear and the depth of anterior chamber was satisfactory. Pupils were round in shape and reactive to light while light was mixed in the lens of right eye, optic disc boundary was clear and flat, while omentum was located in the fundus, mixed congestion in the conjunctiva of left eye and corneal edema were also noted. There was mild swelling in one-third of the anterior chamber, pupil was round in shape and not reactive to light and it was not extending to posterior chamber of eye, while the other details were unclear.
Relevant antibody tests and other laboratory tests were further performed, and the results were all negative.
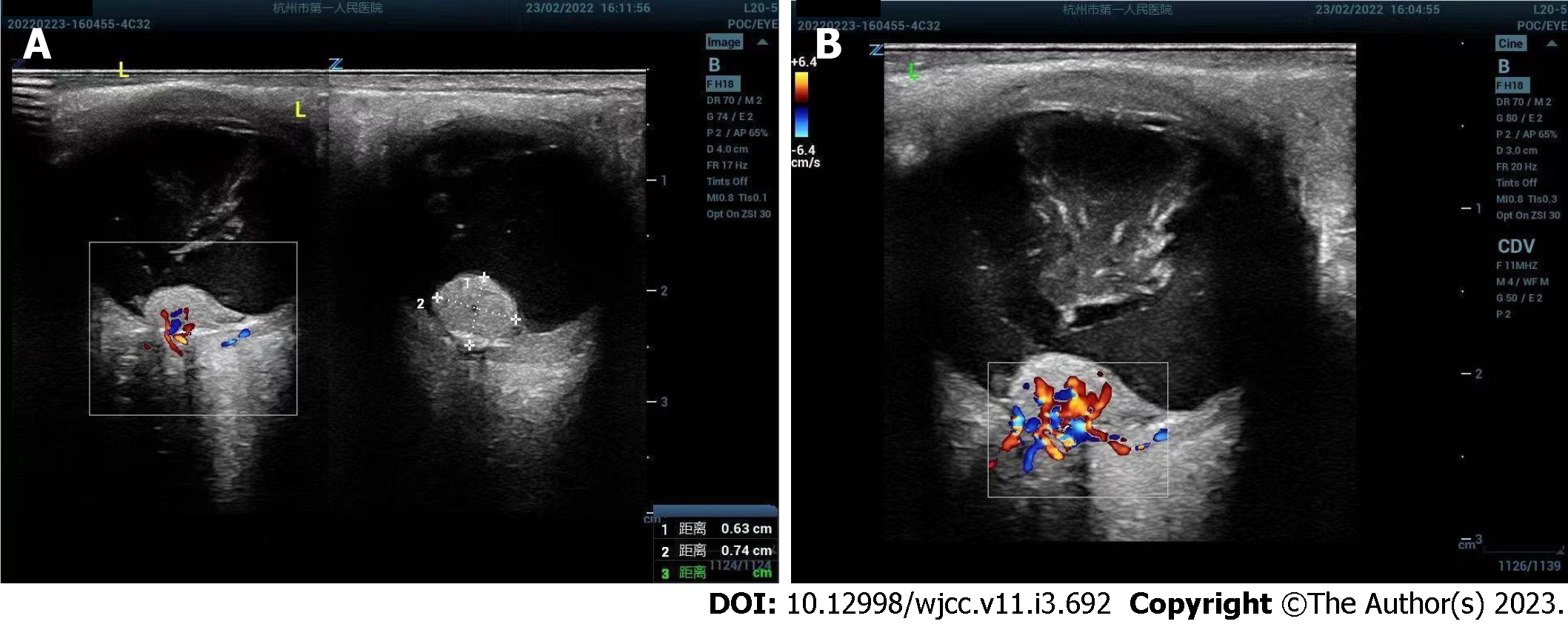
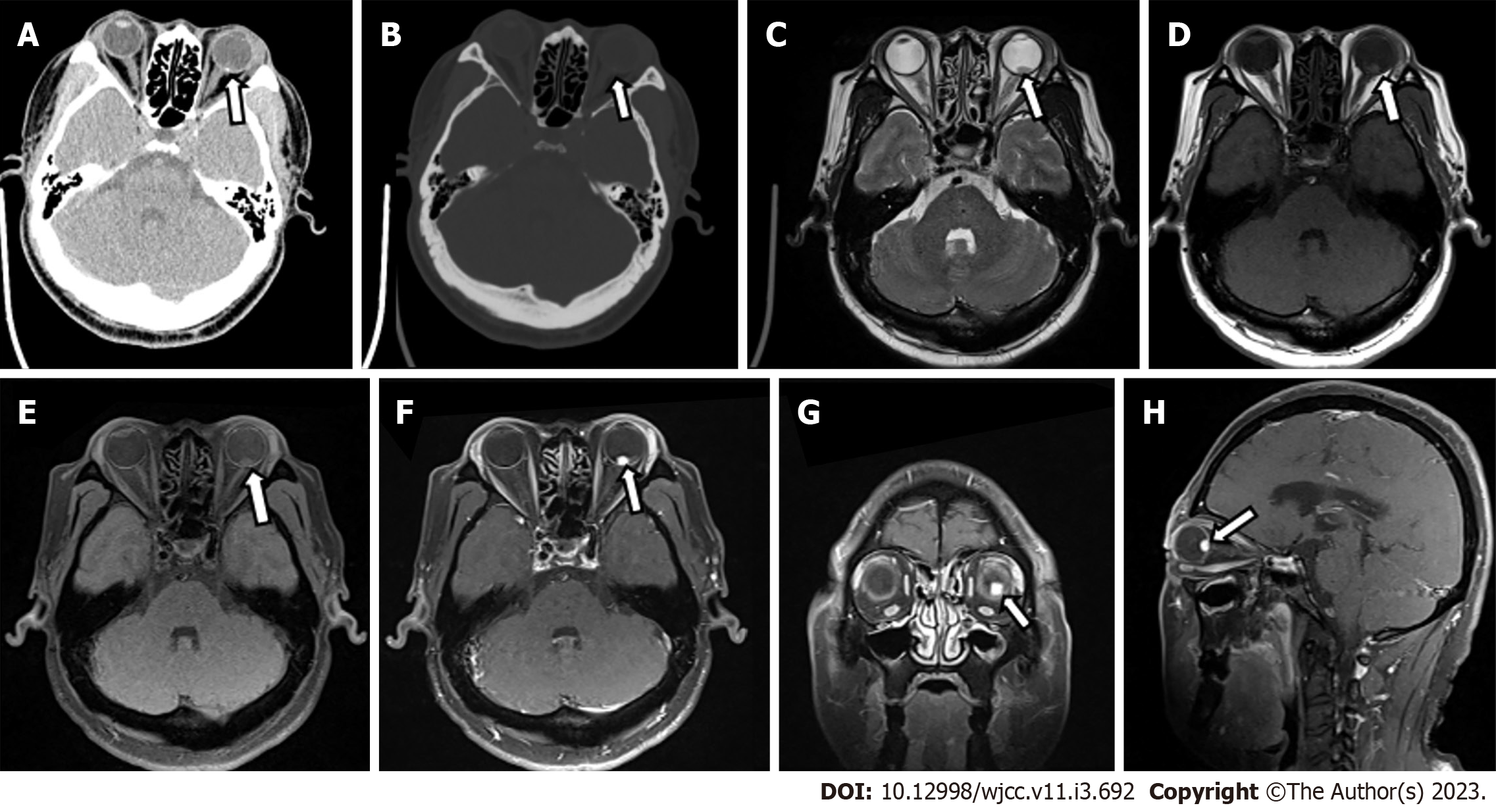
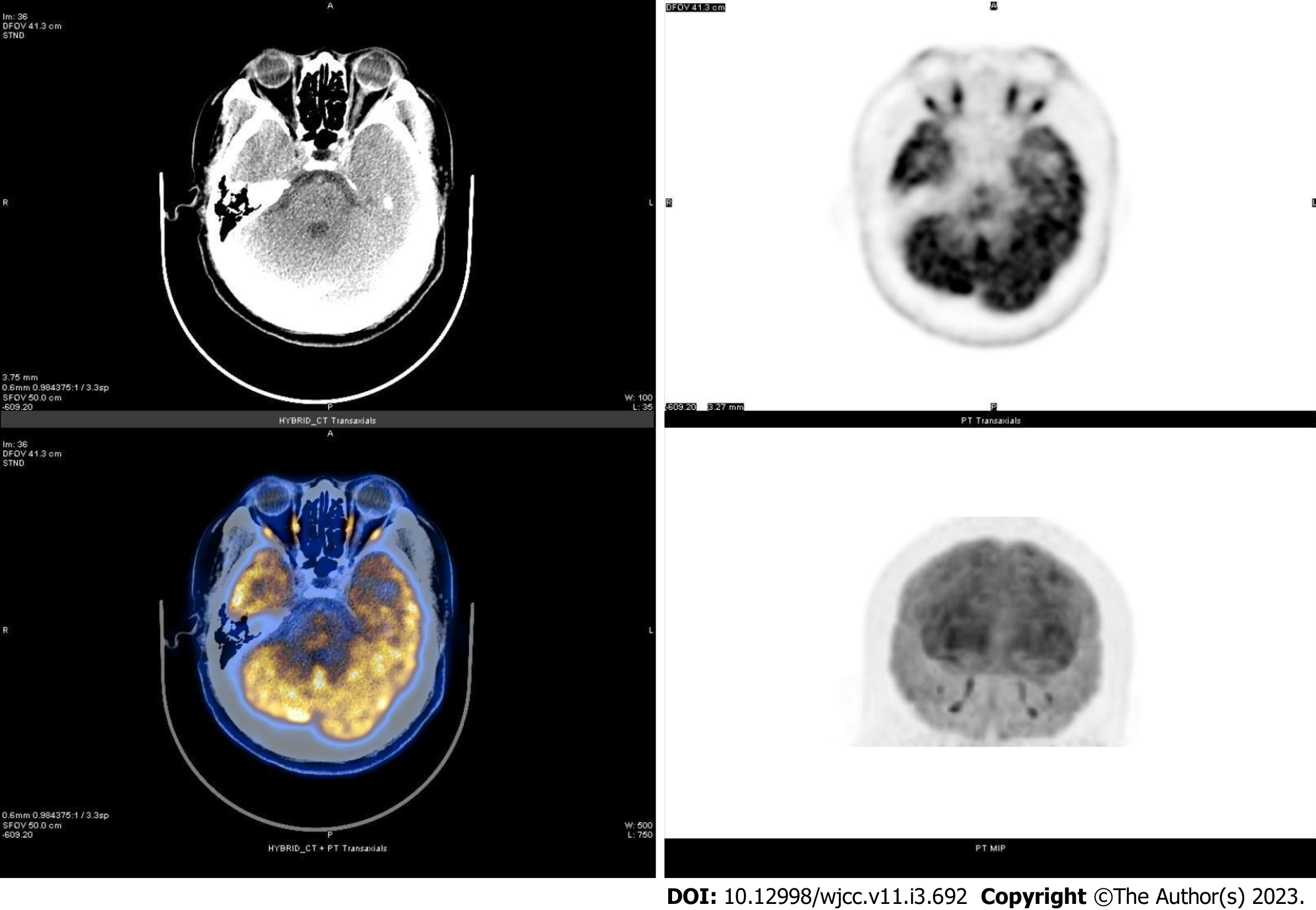
Ophthalmic ultrasound showed a solid lesion in the left eye, indicating possible optic nerve head melanoma (Figure 1). CT showed patchy slightly hyperdense shadows anterior to the posterior wall of the left eyeball, suggesting a mass (Figure 2A and B). MR imaging showed left eyeball mass with slightly short T1, equal short T2 abnormal signals, about 8 mm × 5 mm in size, which were significantly enhanced after contrast enhancement (Figure 2C-H). PET/CT fusion images showed that the posterior left eyeball was locally thickened. The glucose metabolism of the lesion was normal (Figure 3). No significant abnormality was observed in the pancreas, spinal cord, cerebellum, adrenal gland, or kidney. The patient signed a written informed consent form before the examination. This retrospective study involving human participants was reviewed and approved by the Medical Ethics Committee of Hangzhou First People’s Hospital, Zhejiang University School of Medicine (Approval No. 2022-007-01).
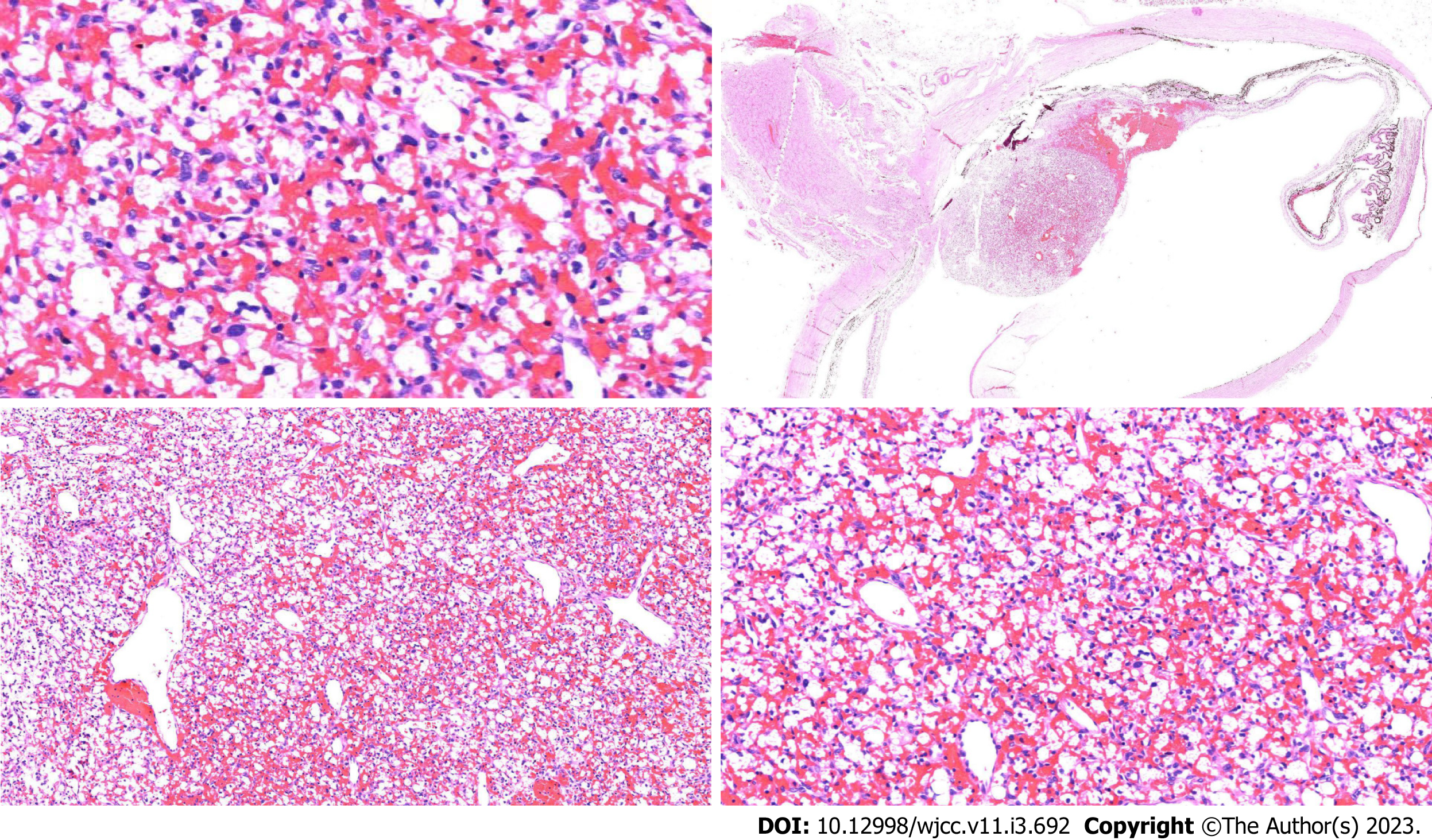
Postoperative histopathological findings showed that the "left eyeball" lesion was consistent with hemangioblastoma, with a maximum tumor diameter of 0.4 cm and no involvement of the optic nerve resection margin. Immunohistochemical results showed D2-40 focal [+], inhibin focal [+], S-100 few [+], CD34 vessels [+], CD56 [-], NSE [-], CD68 [-], CD163 [-], GFAP [-], CD10 [-], MelanA [-], EMA [-], CK [-], SOX10 [-], and Ki-67 [+] 1%-2% (Figure 4).
After excluding surgical contraindications, the patient underwent "left eye enucleation" under general anesthesia on March 4, 2022, and received postoperative anti-inflammatory treatment with levofloxacin eye drops and tobramycin dexamethasone eye ointment (TobraDex ointment).
Early identification of retinal hemangioblastoma based on imaging features is of great value for its personalized treatment.
The retinal hemangioblastoma in the present case was a solid mass, and ultrasound showed an isoechoic mass. CT showed a mildly hyperdense patchy lesion surrounded by spotty calcifications. MR imaging showed a slightly hyperintense signal on T1-weighted images and slightly hypointense-to-isointense signal on T2-weighted images, and the lesions were significantly enhanced after Gadolinium-DTPA injection. PET/CT showed no abnormally increased metabolism of the lesion. These imaging features mainly depended on its histological structure. Retinal hemangioblastoma is mainly composed of vacuolar interstitial cells and abundant capillary networks. Interstitial cells are the main component of the tumor, which are rarely associated with necrotic or specific infectious components, while PET metabolism is characterized by minor metabolic changes due to the competition between tumor cells and macrophages[12,13]. Moreover, retinal hemangioblastomas show low levels of 18F-FDG dose that may be associated with over-expression of somatostatin receptors on the surface of tumor cells, and the PET/CT findings are consistent with Papadakis et al[9]. Furthermore, retinal hemangioblastoma is essentially a vascular lesion, and these tumors release various angiogenic factors, including vascular endothelial growth factor (VEGR), erythropoietin, and platelet-derived growth factor[14,15]. Vascular endothelial growth factor was significantly increased, which can induce massive capillary proliferation and increase vascular permeability, which leads to significant vascular-like enhancement of the lesion. In addition, hemangioblastoma has different imaging features based on different sites of occurrence. Solid tumors are also the main form of optic nerve hemangioblastoma, but most of the lesions are surrounded by edema[16]. Large cysts with small nodules are characteristic of cerebellar and spinal hemangioblastomas, and are mainly associated with intratumoral and peritumoral flow void effects[17]. According to the location and imaging characteristics of the lesion, hemangioblastomas also need to be differentiated from the following diseases. Choroidal melanoma: It is the most common ocular malignancy in adults, wherein CT shows a localized well-defined mass isodense to the extraocular muscles, generally without calcification. MR imaging shows hyperintense signal on T1-weighted images and hypointense signal on T2-weighted images, which is the characteristic feature, because the tumor contains paramagnetic melanin material. It also shows mild to moderate enhancement after contrast enhancement. PET metabolism indicated that glucose uptake was often increased in choroidal melanoma, and its SUVmax was > 10[18,19]. Therefore, it is not difficult to differentiate from this case of retinal hemangioblastoma. Choroidal hemangioma: CT shows local thickening of eyeball wall. It shows progressive significant enhancement after contrast enhancement. MR imaging shows higher signal than vitreous on T1-weighted images and lower than vitreous on T2-weighted images, but isointense signal compared with optic nerve and extraocular muscles on T2-weighted images, 90% of patients have concomitant mild retinal detachment. It shows progressive significant enhancement after contrast enhancement. PET metabolism suggests that choroidal hemangioma usually has no change in glucose uptake[20,21]. Therefore, enhanced dynamic delayed scanning is of great significance in the diagnosis and differentiation of choroidal hemangioma. Retinoblastoma: It occurs in children within 5 years of age and presents with localized thickening or heterogeneous mass shadows of the eye ring on CT, more than 90% of which are mixed with dot-like calcifications. Typical MR imaging features of retinoblastoma include a slightly higher signal on T1-weighted images and low signal on T2-weighted images, with contrast enhancement and diffusion restriction. PET metabolism mostly shows a slight increase in glucose uptake in retinoblastoma[22,23]. Thus, it is not difficult to differentiate from this case. Retinal HB is usually the initial manifestation of VHL. A comprehensive clinical examination of the patient and systematic genetic review of his family revealed that the patient had no other features of VHL syndrome or a family history of genetic diseases. Thus, it was a case of sporadic retinal hemangioblastoma, which may be due to the loss of a tumor suppressor gene similar to retinoblastoma rather than somatic mutations in the VHL tumor suppressor gene[24]. Although RHB is inherently benign and slow-growing, it can lead to retinal exudation, detachment, or macular edema resulting in severe visual loss. Advanced cases can present with extensive retinal scarring leading to blind eye pain. Therefore, early detection of retinal hemangioblastoma is important for ocular preservation and long-term visual acuity[25]. Minimally invasive laser photocoagulation is the best early treatment with less side effects, and vitreoretinal surgery is the main treatment in the late stage[1].
Retinal hemangioblastoma, as a rare disease type, with or without VHL syndrome, should be used as a routine differential diagnosis when a solid retinal mass is found on ultrasound, CT, or MR imaging in patients with no change in PET/CT metabolic characteristics.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Aydin S, Turkey; Urbančič M, Slovenia S-Editor: Wang LL L-Editor: A P-Editor: Wang LL
| 1. | Mir Saeid Ghazi AA, Amouzegar A, Zadeh-Vakili A, Sheikh Rezaei A, Amirbaigloo A, Zarif Yeganeh M, Hashemi H, Azizi F. Clinical and Laboratory Characteristics of a Large Iranian Kindred Afflicted with Von Hippel Lindau Disease. Int J Endocrinol Metab. 2021;19:e105189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 2. | Golas L, Skondra D, Ittiara S, Bajic N, Jeng-Miller KW, Mukai S, Yonekawa Y, Blair MP. Efficacy of Retinal Lesion Screening in Von Hippel-Lindau Patients With Widefield Color Fundus Imaging Versus Widefield FA. Ophthalmic Surg Lasers Imaging Retina. 2019;50:e260-e265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 3. | Singh B, Singla M, Singh R, Rathore SS, Gupta A. Von Hippel-Lindau Syndrome: Multi-Organ Involvement Highlighting Its Diverse Clinical Spectrum in Two Adult Cases. Cureus. 2020;12:e9402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 4. | Maher ER, Yates JR, Harries R, Benjamin C, Harris R, Moore AT, Ferguson-Smith MA. Clinical features and natural history of von Hippel-Lindau disease. Q J Med. 1990;77:1151-1163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 576] [Cited by in RCA: 559] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 5. | Nabih O, Hamdani H, El Maaloum L, Allali B, El Kettani A. Retinal angioma of Von hippel-lindau disease: A case report. Ann Med Surg (Lond). 2022;74:103292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 6. | Russell JF, Villegas VM, Schwartz SG, Weng CY, Davis JL, Flynn HW Jr, Harbour JW. Multimodal Imaging in the Diagnosis of Exophytic Juxtapapillary Retinal Capillary Hemangioblastoma. Am J Ophthalmol. 2021;225:128-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Nguyen TH, Pham T, Strickland T, Brewer D, Belirgen M, Al-Rahawan MM. Von Hippel-Lindau with early onset of hemangioblastoma and multiple drop-metastases like spinal lesions: A case report. Medicine (Baltimore). 2018;97:e12477. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Nielsen SM, Rhodes L, Blanco I, Chung WK, Eng C, Maher ER, Richard S, Giles RH. Von Hippel-Lindau Disease: Genetics and Role of Genetic Counseling in a Multiple Neoplasia Syndrome. J Clin Oncol. 2016;34:2172-2181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 119] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 9. | Papadakis GZ, Millo C, Jassel IS, Bagci U, Sadowski SM, Karantanas AH, Patronas NJ. 18F-FDG and 68Ga-DOTATATE PET/CT in von Hippel-Lindau Disease-Associated Retinal Hemangioblastoma. Clin Nucl Med. 2017;42:189-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Reich M, Glatz A, Boehringer D, Evers C, Daniel M, Bucher F, Ludwig F, Nuessle S, Lagrèze WA, Maloca PM, Lange C, Reinhard T, Agostini H, Lang SJ. Comparison of Current Optical Coherence Tomography Angiography Methods in Imaging Retinal Hemangioblastomas. Transl Vis Sci Technol. 2020;9:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Ouederni M, Maamouri R, Sassi H, Nouri S, Cheour M. [Multimodal imaging in the diagnosis of retinal capillary hemangioblastoma]. J Fr Ophtalmol. 2021;44:912-914. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 12. | Hamza HS, Elhusseiny AM. Submacular sclerosing capillary hemangioblastoma. Am J Ophthalmol Case Rep. 2018;11:61-63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Reinfeld BI, Madden MZ, Wolf MM, Chytil A, Bader JE, Patterson AR, Sugiura A, Cohen AS, Ali A, Do BT, Muir A, Lewis CA, Hongo RA, Young KL, Brown RE, Todd VM, Huffstater T, Abraham A, O'Neil RT, Wilson MH, Xin F, Tantawy MN, Merryman WD, Johnson RW, Williams CS, Mason EF, Mason FM, Beckermann KE, Vander Heiden MG, Manning HC, Rathmell JC, Rathmell WK. Cell-programmed nutrient partitioning in the tumour microenvironment. Nature. 2021;593:282-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 631] [Cited by in RCA: 661] [Article Influence: 165.3] [Reference Citation Analysis (0)] |
| 14. | Custo Greig EP, Duker JS. Retinal hemangioblastoma vascular detail elucidated on swept source optical coherence tomography angiography. Am J Ophthalmol Case Rep. 2021;21:101005. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Wiley HE, Krivosic V, Gaudric A, Gorin MB, Shields C, Shields J, Aronow ME, Chew EY. Management of retinal hemangioblastoma in von hippel-lindau disease. Retina. 2019;39:2254-2263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 16. | Duan M, Yang L, Kang J, Wang R, You H, Feng M. Neuroimaging Features of Optic Nerve Hemangioblastoma Identified by Conventional and Advanced Magnetic Resonance Techniques: A Case Report and Literature Review. Front Oncol. 2021;11:763696. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 17. | Huntoon K, Shepard MJ, Lukas RV, McCutcheon IE, Daniels AB, Asthagiri AR. Hemangioblastoma diagnosis and surveillance in von Hippel-Lindau disease: a consensus statement. J Neurosurg. 2021;1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 18. | Jiblawi A, Chanbour H, Tayba A, Khayat H, Jiblawi K. Magnetic Resonance Imaging Diagnosis of Choroidal Melanoma. Cureus. 2021;13:e16628. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 19. | Marko M, Leško P, Jurenová D, Furda R, Greguš M. Importance of PET/CT examination in patients with malignant uveal melanoma. Cesk Slov Oftalmol. 2020;76:37-44. [PubMed] [DOI] [Full Text] |
| 20. | Sarrafpour S, Tsui E, Mehta N, Modi YS, Finger PT. Choroidal Hemangioma in a Black Patient With Sturge-Weber Syndrome: Challenges in Diagnosis. Ophthalmic Surg Lasers Imaging Retina. 2019;50:183-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 21. | Damento GM, Koeller KK, Salomão DR, Pulido JS. T2 Fluid-Attenuated Inversion Recovery Imaging of Uveal Melanomas and Other Ocular Pathology. Ocul Oncol Pathol. 2016;2:251-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Abramson DH, Dunkel IJ, Francis JH. Magnetic Resonance Imaging of Metastatic Retinoblastoma. J Pediatr Ophthalmol Strabismus. 2022;1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 23. | Orman G, Huisman TAGM. A descriptive neuroimaging study of retinoblastoma in children: magnetic resonance imaging features. Pol J Radiol. 2022;87:e363-e368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 24. | Chang JH, Spraul CW, Lynn ML, Drack A, Grossniklaus HE. The two-stage mutation model in retinal hemangioblastoma. Ophthalmic Genet. 1998;19:123-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 25. | Schoen MA, Shields CL, Say EAT, Douglass AM, Shields JA, Jampol LM. Clinically invisible retinal hemangioblastomas detected by spectral domain optical coherence tomography and fluorescein angiography in twins. Retin Cases Brief Rep. 2018;12:12-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |