Published online Jan 26, 2023. doi: 10.12998/wjcc.v11.i3.629
Peer-review started: July 18, 2022
First decision: October 17, 2022
Revised: October 21, 2022
Accepted: December 9, 2022
Article in press: December 9, 2022
Published online: January 26, 2023
Processing time: 192 Days and 4.8 Hours
Congenital biliary atresia (CBA) is a serious hepatobiliary disease in children with unknown etiology. Its outcome is often liver transplantation or death. Clarifying the etiology of CBA is of great significance for prognosis, treatment, and genetic counseling.
A male Chinese infant at an age of 6 mo and 24 d was hospitalized because of "yellow skin for more than 6 mo". Soon after birth, the patient developed jaundice, which then progressively intensified. A "laparoscopic exploration" indicated "biliary atresia". After coming to our hospital, genetic testing suggested a GPC1 mutation [loss 1 (exons 6-7)]. The patient recovered and was discharged after living donor liver transplantation. After discharge, the patient was followed up. The condition was controlled by oral drugs, and the patient’s condition was stable.
CBA is a complex disease with a complex etiology. Clarifying the etiology is of great clinical importance for treatment and prognosis. This case reports CBA caused by a GPC1 mutation, which enriches the genetic etiology of biliary atresia. However, its specific mechanism needs to be confirmed by further research.
Core tip: Congenital biliary atresia (CBA) often results in a poor prognosis. Most patients need liver transplantation as the final treatment. If the opportunity for liver transplantation is missed, death often occurs. However, the etiology of CBA is still unclear. Clarifying the etiology is of great significance for prognosis, treatment, and genetic counseling. Through the report of a patient with a GPC1 mutation, this paper enriches the genetic etiology of CBA and provides a new basis for clinical and scientific research.
- Citation: Kong YM, Yuan K, Wang CL. Congenital biliary atresia caused by GPC1 gene mutation in Chinese siblings: A case report. World J Clin Cases 2023; 11(3): 629-634
- URL: https://www.wjgnet.com/2307-8960/full/v11/i3/629.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i3.629
Congenital biliary atresia (CBA) is a congenital disease that occurs in infants and young children. Due to the atresia of intrahepatic and extrahepatic bile ducts, the discharge of conjugated bilirubin into the intestine is blocked. This causes inflammation of intrahepatic and extrahepatic bile ducts, resulting in cholestatic cirrhosis. This condition is usually called CBA. The incidence rate of this disease is different in different countries and regions. The incidence rate in Europe is 1/15000-1/19000, and the incidence rate in Japan is 1/9000-1/10000[1]. However, the cause of CBA is not clear. At present, there are many hypotheses, including viral infection (cytomegalovirus, reovirus, rotavirus, human papillomavirus, retrovirus, etc.), immune and/or autoimmune injury[2,3], gene-related factors (inversion and MDR3[2], EFEMP1[4]), abnormal bile duct development, microchimerism, cytokines, miRNA gene expression abnormalities, and biliary malformation[5,6]. In addition, its incidence rate also differs by race, geography, season, and other factors[7]. The main clinical manifestations include the following: (1) Jaundice, which is usually gradually exposed 2-3 wk after birth; and (2) the color of stool becomes lighter, and white pottery-like stool and, is very common in the clinical findings (also it can be just: acholia). The surgical management is the actual approach and it is a by hilar jejunal Roux-Y anastomosis (Kasai operation) within 3 mo after birth. However, the 5-year survival rate of this treatment is low, at approximately 30%-50%[8,9]. Liver transplantation is proposed when the Kassai operation fails to retain the hope of continued survival (as a suggestion you can give the survival rate with transplantation. Clarifying the etiology of CBA have great significance for its treatment and prognosis.
The patient came to our outpatient clinic because of "yellow skin and sclera staining for more than 6 mo".
Soon after birth, there was no obvious incentive for the child to have yellow skin and sclera staining, which gradually intensified. This yellowing was accompanied by a white stool color, but no other discomfort was reported. After many phototherapies at the local hospital, the conditions did not improve, and the yellow skin and sclera staining showed progressive aggravation. Then, the patient went to the local hospital and underwent "laparoscopic exploration suggestive of biliary atresia" (family members complained privately, and no report was found). Later, the family members did not allow further diagnoses and treatments. After discharge, oral symptomatic Chinese patent medicine was administered, but the above symptoms were not relieved. The patient was then admitted to the hospital with "bile duct atresia" for living donor liver transplantation.
Previous "history of laparoscopic surgery".
The patient's father and mother were in good health. The patient had a sister who had a history of "biliary atresia" and underwent "liver transplantation" at an age of more than 6 mo. This sister died at an age of more than 2 years.
Physical examination results were as follows: T 37°C, P 134 bpm, BP 95/52 mmHg, a clear mind, fair spirit, soft neck, yellow skin and sclera, thick respiratory sounds of both lungs, no dry and wet rales, uniform heart rhythm, no obvious pathological murmur in heart sound, abdominal distention, abdominal circumference of 44 cm, no exposure of abdominal wall vein, touch approximately 7 cm under liver rib, hard texture, blunt edge, 6 cm under spleen rib, negative neurological examination, and limb temperature.
The blood examination on December 18, 2019 results were as follows: Leukocyte count 20.0 × 109/L, neutrophils (%) 41.1%, lymphocytes (%) 49.6%, red blood cell 3.08 × 1012/L, hemoglobin 99 g/L, and platelet count 234 × 109/L. On December 18, 2019, the plasma D-dimer determination was 4597 μg/L FEU. Examination of coagulation function on December 18, 2019 was as follows: International standardized ratio 2.36, fibrinogen 0.50 g/L, activated partial thromboplastin time 90.5 seconds, thrombin time 26.6 s, and prothrombin time 27.4 s. On December 18, 2019, the liver examination and kidney lipid glucose electrolyte determination were as follows: total protein 78.4 g/L, albumin 59.3 g/L, globulin 19.1 g/L, white globulin ratio 3.1, alanine aminotransferase 16U/L, aspartate aminotransferase 53U/L, total bile acid 522.7 μmol/L, total bilirubin 347.6 μmol/L, direct bilirubin 222.5 μmol/L, indirect bilirubin 125.1 μmol/L, glomerular filtration rate (EPI-cr) 285.95 ml/min, creatinine 14 μmol/L, uric acid 88 μmol/L, total cholesterol 0.93 mmol/L, high-density lipoprotein-c 0.25 mmol/L, low-density lipoprotein-c 0.09 mmol/L, fasting blood glucose 3.54 mmol/L, and potassium 4.86 mmol/L.
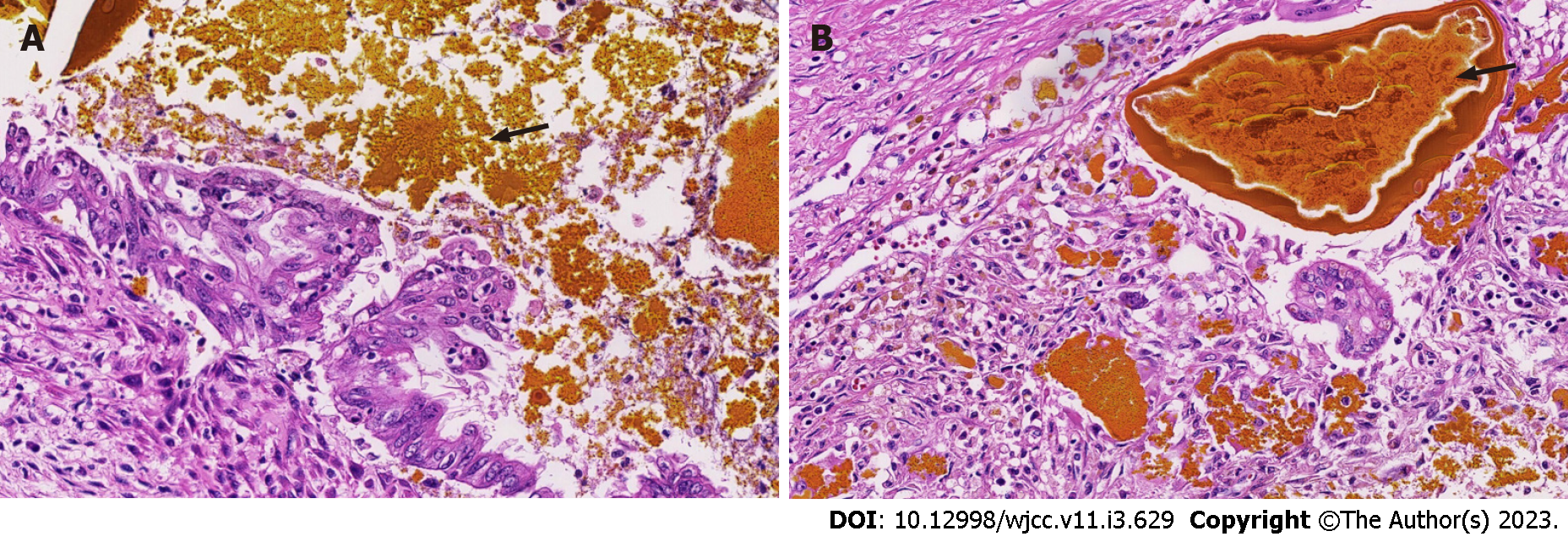
Full abdominal computed tomography showed that the intrahepatic bile duct and common bile duct were dilated. It also showed signs of exudation around the portal vein and an unclear display of the gallbladder. Thus, effusion of gallbladder fossa and peritoneal effusion were considered. Imaging also showed a patch shadow of the left lower lobe, bilateral pleural effusion, and bilateral hip soft tissue swelling. The biopsy results showed biliary stasis and hepatic cirrhosis (Figure 1).
The diagnosis was CBA and cholestatic hepatitis.
On January 7, 2020, "reduced volume liver transplantation" was performed under general anesthesia. After the operation, meropenem was administered to fight infection, ganciclovir was administered for virus infection prevention, liver protection, and phlegm removal, tacrolimus was administered to fight rejection, and albumin and human immunoglobulin were supplemented. The patient's condition improved. On January 31, 2020, the color Doppler ultrasound of the portal vein system of the transplanted liver (color Doppler ultrasound) revealed that the blood circulation of the transplanted liver was satisfactory, and there was a small amount of effusion near the cutting edge of the liver. On January 31, 2020, the serum drug concentration of FK506 was 8.1 ng/mL. Blood coagulation function was checked, and the international standardized ratio was 2.24. On January 21, 2020, the blood biochemical test results were as follows: Alanine aminotransferase 17 U/L, aspartate aminotransferase 19 U/L, total bilirubin 8.0 μmol/L, and glutamyl transphthalase 77 U/L. The patient recovered, the liver function was stable, and the antirejection drugs and coagulation function reached the standards. On January 10, 2020, whole liver + cholecystectomy specimens were submitted for examination. The liver surface was fine granular, the section was gray-red and gray-yellow, and diffuse fine granular. Microscopically, the structure of normal liver lobules was damaged, hepatocytes and small bile ducts were silted, small bile ducts in the portal area were proliferated, inflammatory cells were infiltrated, pseudolobules of liver tissue were formed, and cholestatic cirrhosis was changed. The first hilar lymph node was reactive hyperplasia. The bile duct indicated chronic cholecystitis. In conclusion, the findings in the total liver and cholecystectomy specimens are consistent with a diagnosis of cholestatic cirrhosis with CBA and chronic cholecystitis.
The patient was discharged after being in a stable condition. Follow-ups occurred regularly in the outpatient department of the liver transplantation department, and the patient took long-term oral drugs to control the condition after transplantation. At present, there is no obvious yellowing of the skin, the color of stool is normal, the abdominal circumference is significantly reduced, and the functional evaluation of the transplanted liver is basically within the normal range.
At present, there is no clear understanding of the etiology of CBA. Clinical scholars have put forward many independent and interrelated hypotheses that mainly focus on the following aspects.
The first aspect is congenital dysplasia. The developmental abnormality hypothesis is mainly aimed at CBA with other organ malformations. It is generally believed that biliary tract lesions begin at 5-6 wk of pregnancy, rather than during the development of the intrahepatic bile duct (7-10 wk of pregnancy). The differentiation and morphogenesis of embryonic cells are key factors of organ development[10]. The most important factor is gene mutations. For example, the inversion mutations in MDR3[2], EFEMP1[4], and GPC1 reported in this paper may lead to the production of CBA through corresponding signaling pathways. In addition, in animal experiments of miRNAs, zebrafish lacking miR-30a have abnormal bile duct development. This indicates that genetic factors contribute to the etiology of CBA.
The second aspect is the study of autoimmune-related microchimeras. Kasai hilar jejunostomy after birth in children with CBA can effectively alleviate the development of this disease. However, even if the bile drainage is unobstructed, the process of liver fibrosis does not stop, and histologically, the CBA intrahepatic bile duct is very similar to sclerosing cholangitis, graft-versus-host disease, and other immune diseases. Therefore, some scholars regard CBA as an autoimmune disease. There is also some theoretical and research support for the influence of maternal microchimeras and the second strike theory. In addition, studies have confirmed that there is a large amount of C4d deposition in portal vein blood endothelium[11], indicating that liver fibrosis and portal hypertension in the later stage of CBA are autoimmune reactions that participate in cellular and humoral immunity. Third, the immune imbalance caused by infection may also be an important cause of CBA. Since Benjamin first attributed CBA to viral infection in the liver and biliary system, the mechanism of viral infection immune imbalance has gradually become a hot spot in the etiology of CBA. From the initial discovery of a large amount of monocyte infiltration in the vascular and bile duct endothelium of the hilar region to the establishment of animal models and immune response research, an increasing number of results support this view. The most studied factor is virus infection. At present, research on external infection sources mainly focuses on respiratory enterovirus, cytomegalovirus, and rotavirus[12]. In addition, increasing attention has been given to specific immunity and natural immunity[13]. The presence of bile duct-specific autoantibodies was found in a mouse model infected with rotavirus[14], which also provides a new idea for the exploration of the etiology of CBA.
According to the current paper data, CBA is a serious disease with multiple etiologies and phenotypes, and the etiologies of different types are different. Multiple hypotheses are independent and interdependent and need to be carefully identified in further clinical practice.
CBA is a serious childhood disease with a difficult etiology and poor prognosis. GPC1 mutations are one of the important causes of CBA. Clarifying the etiology of CBA is of great importance for its early diagnosis and prognosis. It is also of great importance to popularize prenatal gene diagnoses in high-risk groups.
The authors would like to thank the patient’s family for agreeing to participate in this research.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Gupta MK, Germany; Obando A, Nicaragua S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Nakamura K, Tanoue A. Etiology of biliary atresia as a developmental anomaly: recent advances. J Hepatobiliary Pancreat Sci. 2013;20:459-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 2. | Danial E, Fleck-Derderian S, Rosenthal P. Has Rotavirus Vaccination Decreased the Prevalence of Biliary Atresia? J Clin Gastroenterol. 2019;53:e348-e351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | Bednarek J, Traxinger B, Brigham D, Roach J, Orlicky D, Wang D, Pelanda R, Mack CL. Cytokine-Producing B Cells Promote Immune-Mediated Bile Duct Injury in Murine Biliary Atresia. Hepatology. 2018;68:1890-1904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 4. | Chen Y, Gilbert MA, Grochowski CM, McEldrew D, Llewellyn J, Waisbourd-Zinman O, Hakonarson H, Bailey-Wilson JE, Russo P, Wells RG, Loomes KM, Spinner NB, Devoto M. A genome-wide association study identifies a susceptibility locus for biliary atresia on 2p16.1 within the gene EFEMP1. PLoS Genet. 2018;14:e1007532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 5. | Fabris L, Cadamuro M, Guido M, Spirli C, Fiorotto R, Colledan M, Torre G, Alberti D, Sonzogni A, Okolicsanyi L, Strazzabosco M. Analysis of liver repair mechanisms in Alagille syndrome and biliary atresia reveals a role for notch signaling. Am J Pathol. 2007;171:641-653. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 103] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 6. | Wang JY, Cheng H, Zhang HY, Ye YQ, Feng Q, Chen ZM, Zheng YL, Wu ZG, Wang B, Yao J. Suppressing microRNA-29c promotes biliary atresia-related fibrosis by targeting DNMT3A and DNMT3B. Cell Mol Biol Lett. 2019;24:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Muraji T, Tanaka H, Ieiri S. Ethnic variation in the incidence of biliary atresia correlates with the frequency of the most prevalent haplotype in its population. Hum Immunol. 2018;79:668-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Qisthi SA, Saragih DSP, Sutowo DW, Sirait DN, Imelda P, Kencana SMS, Makhmudi A, Gunadi. Prognostic Factors for Survival of Patients with Biliary Atresia Following Kasai Surgery. Kobe J Med Sci. 2020;66:E56-E60. [PubMed] |
| 9. | Shetty NS, Shah I. Incomplete Kawasaki Disease in an Infant with Cholangitis Post Kasai Surgery for Biliary Atresia. Ann Hepatol. 2018;17:332-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 10. | Keplinger KM, Bloomston M. Anatomy and embryology of the biliary tract. Surg Clin North Am. 2014;94:203-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 11. | Muraji T. Maternal microchimerism in biliary atresia: are maternal cells effector cells, targets, or just bystanders? Chimerism. 2014;5:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Oetzmann von Sochaczewski C, Pintelon I, Brouns I, Dreier A, Klemann C, Timmermans JP, Petersen C, Kuebler JF. Rotavirus particles in the extrahepatic bile duct in experimental biliary atresia. J Pediatr Surg. 2014;49:520-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Hadchouel M, Hugon RN, Odievre M. Immunoglobulin deposits in the biliary remnants of extrahepatic biliary atresia: a study by immunoperoxidase staining in 128 infants. Histopathology. 1981;5:217-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Mack CL, Tucker RM, Lu BR, Sokol RJ, Fontenot AP, Ueno Y, Gill RG. Cellular and humoral autoimmunity directed at bile duct epithelia in murine biliary atresia. Hepatology. 2006;44:1231-1239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 101] [Article Influence: 5.3] [Reference Citation Analysis (0)] |