Published online Oct 16, 2023. doi: 10.12998/wjcc.v11.i29.7227
Peer-review started: August 15, 2023
First decision: August 30, 2023
Revised: September 11, 2023
Accepted: September 19, 2023
Article in press: September 19, 2023
Published online: October 16, 2023
Processing time: 54 Days and 3.2 Hours
Acute leukemia in newborns is also known as neonatal or congenital leukemia (CL) and is a rare disease with an incidence rate of 1-5 per 1000000 live births. After birth, infants with CL exhibit infiltrative cutaneous nodules, hepatosplenomegaly, thrombocytopenia, and immature leukocytes in the peripheral blood. These symptoms are frequently accompanied by congenital abnormalities including trisomy 21, trisomy 9, trisomy 13, or Turner syndrome. Despite signifi
Here, we document a case of trisomy 21-related acute myeloid leukemia (AML) in a female neonate. The baby was sent to the neonatal intensive care unit because of anorexia, poor responsiveness, and respiratory distress. She was diagnosed with AML based on bone marrow aspiration and immunophenotyping. Genetic sequencing identified a mutation in the GATA1 gene. After receiving the diag
The newborn infant was diagnosed with trisomy 21-related AML. Genetic sequencing identified a mutation in the GATA1 gene. The parents abandoned medical treatment for their infant after receiving the diagnosis, and the infant died at home on the 9th day after birth.
Core Tip: We documented a case of trisomy 21-related acute myeloid leukemia (AML) in a newborn baby. She was sent to the newborn critical care unit due to anorexia, poor responsiveness, and respiratory distress. She was diagnosed with AML based on bone marrow aspiration and immunophenotyping. Genetic sequencing identified a mutation in GATA1. Parents abandoned medical care for their child after receiving the diagnosis. The baby died at home.
- Citation: Yang CX, Yang Y, Zhang FL, Wang DH, Bian QH, Zhou M, Zhou MX, Yang XY. Congenital leukemia: A case report and review of literature. World J Clin Cases 2023; 11(29): 7227-7233
- URL: https://www.wjgnet.com/2307-8960/full/v11/i29/7227.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i29.7227
Congenital leukemia (CL) accounts for < 1% of all the reported cases of pediatric leukemia and is often manifested during the first 28 d of life[1]. CL is the third-most prevalent malignancy in infants after teratoma and neuroblastoma[2]. The estimated frequency of newborn leukemia in the United Kingdom is 3 cases per million newborns with about 2 cases of newborn leukemia reported every year[3]. A retrospective investigation in Brazil confirmed 77 cases of newborn leukemia during a 25-year period between 1990 and 2013[4]. In 2002, Bresters et al[5] reported that 15 cases of CL were diagnosed over 25 years in the Netherlands. In China, the accurate incidence rates of CL are not available because of unreliable case recording and a lack of comprehensive investigations of leukemia. The spectrum of CL includes Down syndrome (DS)-related newborn leukemia, which often manifests as transitory abnormal myelodysplasia (TAM), and non-DS-related neonatal leukemia[6]. Non-DS-related neonatal leukemia is rarer than DS-related newborn leukemia.
Fetal exposure to a cancer-causing mutation is considered the starting point for childhood leukemia. However, for a newborn infant with the first mutation, a "second hit" must occur sometime after delivery to develop leukemia. The hematological and clinical hallmarks of neonatal leukemia are present in the newborn, as all the pathogenic cancer mutations already develop during pregnancy. Neonatal leukemia often presents as acute myeloid leukemia (AML) in 56%-64% of cases, but other patients may show one of many immunophenotypes, including blastic plasmacytoid dendritic cell neoplasm, mixed phenotype acute leukemia, or acute lymphoblastic leukemia (ALL)[1,3]. Infants with CL often exhibit aggressive characteristics including high white blood cell (WBC) counts, reduced platelet counts, hepatosplenomegaly, central nervous system (CNS) involvement, and skin-infiltrative cutaneous leukemia[7,8]. In extreme circumstances, multiple organs are affected. In contrast with childhood leukemia, lymphadenopathy is rare in newborn leukemia. The prognosis for CL is typically poor, with death rates of 68%-74% within 2 years of diagnosis in the neonates because of higher resistance to chemotherapy and increased toxicity from treatment[5,9]. However, spontaneous remission (SR) has been documented in at least 20 cases[10]. The following criteria are used to diagnose CL[11]: (1) Diagnosis within 28 d of birth; (2) specific number or proportion of primitive or immature cells in the bone marrow aspirate, and significantly high peripheral blood leukocyte counts (> 25 × 109/L); (3) spread of primitive cells to areas beyond the blood and bone marrow; and (4) no evidence of other disorders (e.g., newborn sepsis or neonatal hemolysis) that might cause infiltration of non-hematopoietic tissues.
Here, we describe a case of DS-related newborn leukemia caused by a GATA1 mutation. We also review the clinical, cytogenetic, and molecular characteristics of CL.
A 3-d-old female neonate was referred to our hospital with anorexia, poor reactivity for 2 d, and shortness of breath for 1 d.
The 3-d-old female infant was born at 38 wk and 3 d of gestation. At birth, she weighed 3300 grams, with a normal amniotic fluid, umbilical cord, and placenta, and achieved a perfect score of 10 on the Apgar scale. One day after birth, she was sent to the neonatal intensive care unit (NICU) because of anorexia and poor reaction. At first, she was suspected to have "neonatal pneumonia" and was provided with non-invasive ventilator support. She was also treated with an antibiotic combination of ceftazidime and penicillin. The patient was then sent to our facility because her symptoms did not improve and she continued to experience breathing difficulty.
No specific past medical history.
The patient was the family's third child. The first child had died because of an illness, within a month of delivery. Both parents and her elder sister (the family's second child) did not show any disease symptoms. The family did not have any significant medical history of a similar disease. The maternal age at delivery was 24 years. There was no history of the mother being exposed to quinolone antibiotics, pesticides, hormone medicines, or radiation, and she was a non-smoker and non-drinker. However, the mother had been exposed to mosquito repellents during pregnancy. A week before delivery, the mother suffered a respiratory illness and was treated with cefazolin.
The patient’s vitals at admission were as follows: Axillary temperature, 36.8 ℃; respiratory rate, 58 beats/min; pulse rate, 150 times/min; BP, 64/32 mmHg; and transcutaneous oxygen saturation, 90%.
Physical examination showed evidence of DS phenotype, namely simian crease, hypertelorism, and hypotonia. The trunk skin had developed red maculopapular rashes. Heart and lung auscultation did not reveal any murmurs or rales. Both the liver and spleen could be easily palpated at 3 and 5 cm below the costal border, respectively.
The results of complete blood analysis were as follows: Total leukocyte count, 79.37 × 109/L; neutrophils, 17.40%; lymphocytes, 8.40%; monocytes, 71.10%; hemoglobin, 16.2 g/dL; and platelet count, 514 × 109/L. Fifty-eight percent of the total cells in the peripheral blood smear were primitive and naive cells. The reticulocyte counts were 126.80 × 109/L (2.99%).
After testing the blood solution, the concentration of potassium ions was 7.44 mmol/L. All the body fluids of the patient, including the cerebrospinal fluid, blood culture, and urine, were within the normal range.
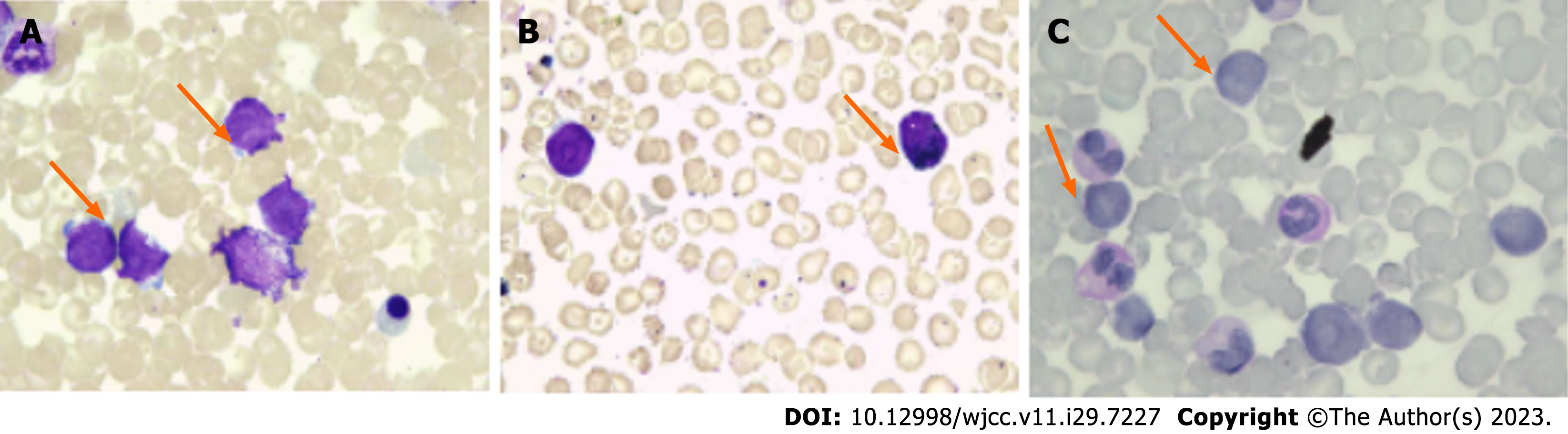
Details of the bone marrow smear are shown in Figure 1. Hyperplasia of the bone marrow was clearly observed in the bone marrow smear, and approximately 35% of the bone marrow cells were classified as primitive. These cells showed the following morphological characteristics: (1) Rounded nuclei, fine and loose nuclear chromatin, and the presence of 1-3 nucleoli; (2) dynamic proliferation of myeloid cells; (3) suppressed growth of new erythroblasts; (4) 8% of the total bone marrow cells were lymphocytes; and (5) megakaryocytes were rare and primordial cells.
The results of chromosome analysis showed DS. The karyotype was 47, xx, + 21. GATA1 mutation was identified based on gene sequencing analysis. The variation information was NM_002049: exon2: c.1A>G: p.M1. The chromosome position was chrX: 48649517A>G with a mutation frequency of 99.4%.
AML with trisomy 21.
The NICU staff of our hospital administered oxygen therapy; penicillin with cefoperazone sulbactam sodium to prevent infections; and hydration, alkalization, allopurinol, and hydroxyurea to aid in the decrease of WBCs after the baby was admitted. The whole treatment lasted for 3 d.
The parents abandoned medical care for the patient after the diagnosis. The baby passed away at home on day 9 d after birth.
Genetic factors play a significant role in the pathogenesis of CL. A high proportion of infant leukemias are cytogenetically characterized by balanced chromosomal translocations. The most frequent cytogenic abnormality in CL is t (4; 11) (q21.3; q23.3)/lysine methyltransferase 2A gene (KMT2A)-AFF1, followed by t (1; 22) (p13.3; q13.1)/RBM15-MKL1 and t (8; 16) (p11.2; p13.3)/KAT6A-CREBBP[3,12-14]. Among them, rearrangements in the histone KMT2A, [formerly known as the mixed lineage leukemia (MLL) gene] occur in 75% of ALL and 50% of AML in CL. KMT2A rearrangements results in the fusion of the N-terminus of the KMT2A gene with the C-terminus of a partner gene. In infant ALL, the most common KMT2A partner gene is AFF1, and in infant AML, the most common KMT2A partner gene is MLLT3[15]. Trisomy 21 is the most frequent chromosomal anomaly in the human genome. Leukemia or lymphoma was the cause of death in one-fifth of DS patients in the < 1-year-old group group in Japan[16]. Children with DS are associated with a 150-fold higher risk of AML and a 20-fold higher risk of ALL than children without DS[17]. CRLF2 gene rearrangement (either inter- or intra-chromosomal) is the most prevalent genetic abnormality that causes DS-ALL followed by ETV6: RUNX1 and C/EBP-altered[18]. Moreover, GATA1 mutations are reported in 30% of children with DS. GATA1s mutations induce uncontrolled proliferation of the fetal megakaryocytic cells and aberrant terminal erythroid differentiation[19]. GATA1 mutations cause TAM in 50% infants diagnosed with DS[20]. According to the literatures, we summarized the clinical features, laboratory tests, treatment and prognosis of TAM and acute megakaryoblastic leukemia associated with mutations in the GATA1 gene in children with DS, as shown in Table 1[21-25]. Transient aplasia of the megakaryoblast and erythroblast cells in the fetal liver, peripheral blood, and bone marrow is a characteristic feature of DS and has not been reported for any other disorder[26,27]. About 5 and 10% patients with TAM develop AML within 4 years because of the accumulation of additional oncogenic mutations[28]. Our patient was diagnosed with DS-related AML accompanied by a GATA1 mutation. She had signs of organ invasion soon after delivery. Her parents decided to stop treatment after diagnosis. Additional risk factors for CL include prenatal exposure to adrenocortical hormones, antihistamines, psychiatric therapeutics, and exposure to radiation and magnetic fields[29]. Viral infections have also been linked with CL[30,31]. The patient’s mother was exposed to chemicals during pregnancy.
| Case number | Age (yr) | Sex | Clinical symptoms | Signs | Physical examination | Treatment | Outcome | Ref. | ||
| Bone marrow/peripheral blood, Blasts (%) | Cytogenetics | GATA1 mutation | ||||||||
| 1 | 1 d | Male | Limp and cyanotic | Dysmorphic features, hepatomegaly | 33/45 | 47, XY, þ21[21] | c.49C>T | Cytarabine | Died of respiratory failure 8 d after birth | Yin et al[21] |
| 2 | 1 d | Male | Weak with respiratory distress | Dysmorphic features, hepatomegaly | 42/95 | 47, XY, þ21c[19] | c.37G>T | Cytarabine | Died of cardiovascular failure at the age of 8 mo | Yin et al[21] |
| 3 | 1 d | Male | Purpura and petechiae all over the body | No abnormal signs | 12/57 | 48, XY, + 21, + mar[20] | c.129_148dup20 | Intensive chemotherapy | Complete remission for 6 yr | Ono et al[22] |
| 4 | 18 mo | Male | Pale complexion | Hepatosplenomegaly (liver 3 cm and spleen 8 cm below costal margin) | 43/64 | 47, XY, + 21[14]/46, XY[6] | c.128_220 + 144del237 | Intensive chemotherapy and unrelated cord blood transplantation | Died of disease 10 mo after diagnosis | Ono et al[22] |
| 5 | 2 d | Male | Decreased tone | Dysmorphic features | -/10-15 | None | Yes | Untreated | Spontaneous remission | Bombery et al[23] |
| 6 | 1 d | Female | Mild respiratory distress | Hepatosplenomegaly, phenotypic features of DS | -/8 | None | Yes | Cytarabine | CR for more than 23 mo | Moritake et al[24] |
| 7 | 1 d | Female | Respiratory distress | Hepatosplenomegaly and jaundice | -/86 | 47, XX, + 21 | Yes | None | Died of overwhelming sepsis and hepatic failure at the age of 3 mo | Tsai et al[25] |
| 8 | 1 d | Male | None | Physical characteristics of Down syndrome | 13/22 | 47, XY, + 21[13]/46, XY[10] | Yes | None | Leukemia free survival (15 mo +) | Tsai et al[25] |
| 9 | 1 d | Male | Generalized edema | Massive hepatosplenomegaly | -/71 | 47, XY, + 21 | Yes | Symptomatic treatment | Died 1 wk after cardiac surgery at the age of 82 d | Tsai et al[25] |
The most prevalent clinical manifestations of non-DS newborn leukemia include pleural effusions, ascites, jaundice, skin lesions, splenomegaly, hepatomegaly, renal and hepatic failure, acidosis, hypoxia, and respiratory distress[32]. CNS infiltration has been reported in newborn leukemia[3]. Nodules of varying firmness and color (blue, red, brown, or purple) are a common symptom of cutaneous infiltrates and are referred to as "blueberry muffin" rash. Newborns with AML are more likely to exhibit cutaneous infiltrates than those with ALL[5,33]. Cutaneous infiltrates are also observed in patients with blast plasmacytoid dendritic cell neoplasms[34]. Symptoms of DS-related newborn leukemia range from the absence of any symptoms to those caused by multiple organ failure[35]. Peripheral blood smear is recommended for all the infants with DS because abnormal blood counts and circulating megakaryoblasts are highly prevalent in hematologic malignancies[36]. The first symptom reported in this case was dyspnea. Peripheral blood tests indicated significantly high WBC counts. In our patient, dyspnea was the most prominent symptom. Hepatosplenomegaly was also detected, but skin infiltration was not observed.
Leukocytosis, anemia, thrombocytopenia, and coagulation disorders are the most common hematological manifestations of newborn leukemia. The counts of WBCs and blast cells were higher than 50 × 109/L in newborn leukemias, in contrast to other infections that may cause neonatal leukocytosis. Neonatal leukemia must be differentiated from congenital infections, hemolytic illnesses, and leukemoid responses. Clinically, newborns with severe infections show high counts of myelocytes, metamyelocytes, and neutrophils; whereas, circulating blast cells seldom surpass 8% of the total peripheral blood cells. Moreover, these variations disappear within the third or fourth day after delivery.
A “watch and wait” approach with careful monitoring (including frequent physical examinations and blood counts) is necessary for CL with stable illness, because there is a possibility of SR without the use of chemotherapeutic medications that are associated with adverse effects[10,37]. According to the newly released guidelines, a low-dose cytarabine therapy is recommended for cases with multiorgan failure, high WBC counts (> 100 × 109/L), hepatopathy, and other life-threatening diseases. Besides, hematopoietic stem cell transplantation is recommended for patients with juvenile myelomonocytic leukemia[38].
CL is associated with poor survival outcomes. The main factors for poor prognosis are as follows: (1) Presence of leukemia detected in the CNS at the time of diagnosis; (2) the initial WBC counts are > 50 × 109/L; (3) leukocyte differentiation antigen CD10 is negative in the bone marrow; (4) myeloid leukemia cell surface antigens are present on the leukemic cells; and (5) AML genes have been rearranged at the 11q23 locus[39].
Our patient was a female neonate diagnosed with DS-related AML. Gene sequencing identified a mutation in the GATA1 gene. Because the prognosis was poor, the parents decided to abandon therapy and rejected further investigation. The child died at home on day 9 after birth.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Watanabe T, Japan S-Editor: Qu XL L-Editor: A P-Editor: Cai YX
| 1. | Tsujimoto H, Kounami S, Mitani Y, Watanabe T, Takifuji K. Neonatal Acute Megakaryoblastic Leukemia Presenting with Leukemia Cutis and Multiple Intracranial Lesions Successfully Treated with Unrelated Cord Blood Transplantation. Case Rep Hematol. 2015;2015:610581. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 2. | Orbach D, Sarnacki S, Brisse HJ, Gauthier-Villars M, Jarreau PH, Tsatsaris V, Baruchel A, Zerah M, Seigneur E, Peuchmaur M, Doz F. Neonatal cancer. Lancet Oncol. 2013;14:e609-e620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 3. | Roberts I, Fordham NJ, Rao A, Bain BJ. Neonatal leukaemia. Br J Haematol. 2018;182:170-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (1)] |
| 4. | Moura SV, Andrade F, Magalhães IQ, Costa I, Silva DB, D'Andrea ML, Pinheiro VP, Lee ML, Werneck F, Emerenciano M, Pombo-de-Oliveira MS. Clinical and molecular epidemiology of neonatal leukemia in Brazil. Leuk Lymphoma. 2015;56:903-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Bresters D, Reus AC, Veerman AJ, van Wering ER, van der Does-van den Berg A, Kaspers GJ. Congenital leukaemia: the Dutch experience and review of the literature. Br J Haematol. 2002;117:513-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 94] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 6. | Wagenblast E, Araújo J, Gan OI, Cutting SK, Murison A, Krivdova G, Azkanaz M, McLeod JL, Smith SA, Gratton BA, Marhon SA, Gabra M, Medeiros JJF, Manteghi S, Chen J, Chan-Seng-Yue M, Garcia-Prat L, Salmena L, De Carvalho DD, Abelson S, Abdelhaleem M, Chong K, Roifman M, Shannon P, Wang JCY, Hitzler JK, Chitayat D, Dick JE, Lechman ER. Mapping the cellular origin and early evolution of leukemia in Down syndrome. Science. 2021;373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 52] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 7. | Hilden JM, Dinndorf PA, Meerbaum SO, Sather H, Villaluna D, Heerema NA, McGlennen R, Smith FO, Woods WG, Salzer WL, Johnstone HS, Dreyer Z, Reaman GH; Children's Oncology Group. Analysis of prognostic factors of acute lymphoblastic leukemia in infants: report on CCG 1953 from the Children's Oncology Group. Blood. 2006;108:441-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 215] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 8. | Creutzig U, Zimmermann M, Bourquin JP, Dworzak MN, Kremens B, Lehrnbecher T, von Neuhoff C, Sander A, von Stackelberg A, Schmid I, Starý J, Steinbach D, Vormoor J, Reinhardt D. Favorable outcome in infants with AML after intensive first- and second-line treatment: an AML-BFM study group report. Leukemia. 2012;26:654-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 9. | Isaacs H Jr. Fetal and neonatal leukemia. J Pediatr Hematol Oncol. 2003;25:348-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 88] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 10. | Rossoff J, Akpan I, Platanias LC. Spontaneous remission in congenital leukemia. Leuk Lymphoma. 2018;59:2271-2272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (1)] |
| 11. | Green K, Tandon S, Ahmed M, Toscano W, O'Connor D, Ancliff P, Vora A, Bartram J, Samarasinghe S, Ghorashian S, Pavasovic V, Rao A. Congenital acute myeloid leukemia: challenges and lessons. A 15-year experience from the UK. Leuk Lymphoma. 2021;62:688-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Wan Ariffin E, Jones H, Bhatnagar N. Congenital acute myeloid leukaemia with KMT2A rearrangement. Br J Haematol. 2018;182:169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Pieters R, De Lorenzo P, Ancliffe P, Aversa LA, Brethon B, Biondi A, Campbell M, Escherich G, Ferster A, Gardner RA, Kotecha RS, Lausen B, Li CK, Locatelli F, Attarbaschi A, Peters C, Rubnitz JE, Silverman LB, Stary J, Szczepanski T, Vora A, Schrappe M, Valsecchi MG. Outcome of Infants Younger Than 1 Year With Acute Lymphoblastic Leukemia Treated With the Interfant-06 Protocol: Results From an International Phase III Randomized Study. J Clin Oncol. 2019;37:2246-2256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 183] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 14. | Brown PA. Neonatal Leukemia. Clin Perinatol. 2021;48:15-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 15. | Forgione MO, McClure BJ, Eadie LN, Yeung DT, White DL. KMT2A rearranged acute lymphoblastic leukaemia: Unravelling the genomic complexity and heterogeneity of this high-risk disease. Cancer Lett. 2020;469:410-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 16. | Motegi N, Yamaoka Y, Moriichi A, Morisaki N. Causes of death in patients with Down syndrome in 2014-2016: A population study in Japan. Am J Med Genet A. 2022;188:224-236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 17. | Murphy BR, Roth M, Kolb EA, Alonzo T, Gerbing R, Wells RJ. Development of acute lymphoblastic leukemia following treatment for acute myeloid leukemia in children with Down syndrome: A case report and retrospective review of Children's Oncology Group acute myeloid leukemia trials. Pediatr Blood Cancer. 2019;66:e27700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Li Z, Chang TC, Junco JJ, Devidas M, Li Y, Yang W, Huang X, Hedges DJ, Cheng Z, Shago M, Carroll AJ, Heerema NA, Gastier-Foster J, Wood BL, Borowitz MJ, Sanclemente L, Raetz EA, Hunger SP, Feingold E, Rosser TC, Sherman SL, Loh ML, Mullighan CG, Yu J, Wu G, Lupo PJ, Rabin KR, Yang JJ. Genomic landscape of Down syndrome-associated acute lymphoblastic leukemia. Blood. 2023;142:172-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 19. | Gialesaki S, Mahnken AK, Schmid L, Labuhn M, Bhayadia R, Heckl D, Klusmann JH. GATA1s exerts developmental stage-specific effects in human hematopoiesis. Haematologica. 2018;103:e336-e340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Gupte A, Al-Antary ET, Edwards H, Ravindranath Y, Ge Y, Taub JW. The paradox of Myeloid Leukemia associated with Down syndrome. Biochem Pharmacol. 2022;201:115046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Yin L, Lovell MA, Wilson ML, Wei Q, Liang X. Distinct GATA1 Point Mutations in Monozygotic Twins With Down Syndrome and Transient Abnormal Myelopoiesis From a Triplet Pregnancy: A Case Report and Review of Literature. Am J Clin Pathol. 2016;146:753-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 22. | Ono R, Hasegawa D, Hirabayashi S, Kamiya T, Yoshida K, Yonekawa S, Ogawa C, Hosoya R, Toki T, Terui K, Ito E, Manabe A. Acute megakaryoblastic leukemia with acquired trisomy 21 and GATA1 mutations in phenotypically normal children. Eur J Pediatr. 2015;174:525-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Bombery M, Vergilio JA. Transient abnormal myelopoiesis in neonates: GATA get the diagnosis. Arch Pathol Lab Med. 2014;138:1302-1306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 24. | Moritake H, Yamada A, Kimoto Y, Sawa D, Shimonodan H, Nunoi H. Acute megakaryoblastic leukemia and severe pulmonary fibrosis in a child with Down syndrome: successful treatment with ultra low-dose cytarabine using GATA1 mutation to monitor minimal residual disease. Am J Hematol. 2012;87:447-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 25. | Tsai MH, Hou JW, Yang CP, Yang PH, Chu SM, Hsu JF, Chiang MC, Huang HR. Transient myeloproliferative disorder and GATA1 mutation in neonates with and without Down syndrome. Indian J Pediatr. 2011;78:826-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 26. | Hasle H, Kline RM, Kjeldsen E, Nik-Abdul-Rashid NF, Bhojwani D, Verboon JM, DiTroia SP, Chao KR, Raaschou-Jensen K, Palle J, Zwaan CM, Nyvold CG, Sankaran VG, Cantor AB. Germline GATA1s-generating mutations predispose to leukemia with acquired trisomy 21 and Down syndrome-like phenotype. Blood. 2022;139:3159-3165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 27. | Yamato G, Park MJ, Sotomatsu M, Kaburagi T, Maruyama K, Kobayashi T, Nishi A, Sameshima K, Ohki K, Hayashi Y. Clinical features of 35 Down syndrome patients with transient abnormal myelopoiesis at a single institution. Int J Hematol. 2021;113:662-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 28. | Nikolaev SI, Santoni F, Vannier A, Falconnet E, Giarin E, Basso G, Hoischen A, Veltman JA, Groet J, Nizetic D, Antonarakis SE. Exome sequencing identifies putative drivers of progression of transient myeloproliferative disorder to AMKL in infants with Down syndrome. Blood. 2013;122:554-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 29. | Whitehead TP, Metayer C, Wiemels JL, Singer AW, Miller MD. Childhood Leukemia and Primary Prevention. Curr Probl Pediatr Adolesc Health Care. 2016;46:317-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 79] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 30. | Francis SS, Wallace AD, Wendt GA, Li L, Liu F, Riley LW, Kogan S, Walsh KM, de Smith AJ, Dahl GV, Ma X, Delwart E, Metayer C, Wiemels JL. In utero cytomegalovirus infection and development of childhood acute lymphoblastic leukemia. Blood. 2017;129:1680-1684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 31. | Bagri DR, Yadav KS, Sharma R, Gulati S. Congenital B-cell Acute Lymphoblastic Leukemia with Congenital Rubella Infection. Indian Pediatr. 2019;56:67-68. [PubMed] |
| 32. | Qin B, Dong X, Ding J. Neonatal congenital leukemia caused by several missense mutations and AFF1-KMT2A fusion: A case report. Oncol Lett. 2022;24:283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 33. | Lee EG, Kim TH, Yoon MS, Lee HJ. Congenital leukemia cutis preceding acute myeloid leukemia with t(9;11)(p22;q23), MLL-MLLT3. J Dermatol. 2013;40:570-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 34. | Tokuda K, Eguchi-Ishimae M, Yagi C, Kawabe M, Moritani K, Niiya T, Tauchi H, Ishii E, Eguchi M. CLTC-ALK fusion as a primary event in congenital blastic plasmacytoid dendritic cell neoplasm. Genes Chromosomes Cancer. 2014;53:78-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 35. | Tunstall O, Bhatnagar N, James B, Norton A, O'Marcaigh AS, Watts T, Greenough A, Vyas P, Roberts I, Wright M; British Society for Haematology. Guidelines for the investigation and management of Transient Leukaemia of Down Syndrome. Br J Haematol. 2018;182:200-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 36. | Gamis AS, Alonzo TA, Gerbing RB, Hilden JM, Sorrell AD, Sharma M, Loew TW, Arceci RJ, Barnard D, Doyle J, Massey G, Perentesis J, Ravindranath Y, Taub J, Smith FO. Natural history of transient myeloproliferative disorder clinically diagnosed in Down syndrome neonates: a report from the Children's Oncology Group Study A2971. Blood. 2011;118:6752-9; quiz 6996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 138] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 37. | Salvatori G, Foligno S, Sirleto P, Genovese S, Russo S, Coletti V, Dotta A, Luciani M. Sometimes it is better to wait: First Italian case of a newborn with transient abnormal myelopoiesis and a favorable prognosis. Oncol Lett. 2017;13:191-195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 38. | Bertrums EJM, Zwaan CM, Hasegawa D, De Haas V, Reinhardt DN, Locatelli F, De Moerloose B, Dworzak M, Buijs A, Smisek P, Kolenova A, Pronk CJ, Klusmann JH, Carboné A, Ferster A, Antoniou E, Meshinchi S, Raimondi SC, Niemeyer CM, Hasle H, Van den Heuvel-Eibrink MM, Goemans BF. Guideline for management of non-Down syndrome neonates with a myeloproliferative disease on behalf of the I-BFM AML Study Group and EWOG-MDS. Haematologica. 2022;107:759-764. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 39. | He Y, Wang D, Li X, Hu Y, Wang W, Huang R. Expression of partial tandem duplication of mixed lineage leukaemia in patients with acute leukaemia and their relatives. Chin Med J (Engl). 2014;127:284-289. [PubMed] |