Published online Oct 16, 2023. doi: 10.12998/wjcc.v11.i29.7207
Peer-review started: July 18, 2023
First decision: August 9, 2023
Revised: August 22, 2023
Accepted: September 19, 2023
Article in press: September 19, 2023
Published online: October 16, 2023
Processing time: 87 Days and 5.9 Hours
Central venous catheters (CVCs) often cause life-threatening complications, especially CVC-related bloodstream infection (CVC-BSI) and catheter-related thrombosis (CRT). Here, we report an unusual case of misplaced CVC-induced emphysematous thrombophlebitis, a rare but potentially lethal form of CRT and CVC-BSI characterized by both thrombosis and gas formation.
A 48-year-old male presented to the emergency room of a local hospital with sudden-onset headache and coma for 4 h. Computed tomography (CT) revealed right basal ganglia hemorrhage, so emergency decompressive craniotomy was performed and a CVC was inserted through the right subclavian vein for fluid resuscitation during anesthesia. Two days later, the patient was transferred to the intensive care unit of our hospital for further critical care. On day 9 after CVC insertion, the patient suddenly developed fever and hypotension. Point-of-care ultrasound (POCUS) demonstrated thrombosis and dilatation of the right internal jugular vein (IJV) filled with thrombosis. Ultrasonography also revealed that the CVC tip had been misplaced into the IJV and was surrounded by gas bubbles, which manifested as hyperechoic lines with dirty shadowing and comet-tail artifacts. Further CT scan confirmed air bubbles surrounding the CVC in the right neck. The final diagnosis was septic emphysematous thrombophlebitis induced by a misplaced CVC and ensuing septic shock. The responsible CVC was removed immediately. The patient received fluid resuscitation, intravenous noradrenaline, and a 10-d ultra-broad spectrum antibiotic treatment to combat septic shock. Both CVC and peripheral venous blood cultures yielded methicillin-resistant Staphylococcus cohnii. The patient was gradually weaned off vasopressors and the symptoms of redness and swelling in the right neck subsided within 7 d.
Emphysematous thrombophlebitis is a fulminant and life-threatening CVC-BSI associated with thrombosis and gas formation in the vein. A misplaced CVC may facilitate the development of emphysematous thrombophlebitis. POCUS can easily identify the artifacts produced by gas and thrombosis, facilitating rapid diagnosis at the bedside.
Core Tip: Central venous catheters (CVCs) often cause life-threatening complications, especially CVC-related bloodstream infection and catheter-related thrombosis. Emphysematous thrombophlebitis is a rare but life-threatening CVC-related bloodstream infection associated with thrombosis and gas formation in the vein. However, timely diagnosis remains a challenge for clinicians. Here, we report an unusual case of misplaced CVC-induced emphysematous thrombophlebitis rapidly diagnosed by point-of-care ultrasound (POCUS). POCUS revealed thrombosis surrounding the misplaced CVC in the right internal jugular vein and gas bubbles manifesting as hyperechoic lines with dirty shadowing and comet-tail artifacts. Knowledge of air-related artifacts may help physicians rapidly diagnose emphysematous thrombophlebitis.
- Citation: Chen N, Chen HJ, Chen T, Zhang W, Fu XY, Xing ZX. Emphysematous thrombophlebitis caused by a misplaced central venous catheter: A case report. World J Clin Cases 2023; 11(29): 7207-7213
- URL: https://www.wjgnet.com/2307-8960/full/v11/i29/7207.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i29.7207
Central venous catheters (CVCs) are commonly used in anesthesiology, emergency and critical care medicine, nephrology, hematology, and oncology for delivering medications and nutrition, fluid resuscitation, hemodialysis, and measuring blood pressure in central veins[1]. However, CVCs often cause complications, especially CVC-related bloodstream infection (CVC-BSI) and catheter-related thrombosis (CRT)[2]. In fact, CVC-BSI is the most frequent hospital-acquired blood stream infection and is documented to increase the length of intensive care unit (ICU) stay, in-hospital morbidity and mortality, and therapy-related costs[3], while CRT occurs in approximately one-third of patients with indwelling CVCs and accounts for up to 50% of all deep venous thromboses in children and 10% in adults[4]. Similarly, CRT can lead to catheter stenosis and dysfunction, higher infection risk, increased care costs, and substantial morbidity and mortality[4].
Emphysematous thrombophlebitis is a very rare condition defined as septic thrombophlebitis in a vein complicated by gas formation[5]. Like emphysematous infection in other organs such as emphysematous pyelonephritis, the air bubbles in emphysematous thrombophlebitis are usually produced by the pathogenic organism via fermentation of glucose and lactate in infected tissues[6]. While emphysematous thrombophlebitis is rare, it is frequently fulminant and lethal. Here, we reported a rare case of misplaced CVC-related emphysematous thrombophlebitis, a unique subtype of CRT and CVC-BSI. In addition, we report for the first time the imaging features of emphysematous thrombophlebitis on point-of-care ultrasound (POCUS) to facilitate early diagnosis at the bedside.
A 48-year-old Asian male suddenly developed fever, tachycardia, and hypotension 9 d after CVC insertion. In addition, the right neck was swollen, with redness, tenderness, and warmth.
The patient presented to the emergency room of a local hospital with sudden onset headache and coma for 4 h. Emergency decompressive craniotomy was performed at the local hospital, and a CVC was inserted through the right subclavian vein without ultrasound assistance for fluid resuscitation during anesthesia. Further, CVC catheter tip position was not assessed by chest X-ray after the operation. Two days later, the patient was transferred to the ICU of our tertiary teaching hospital for further critical care. Seven days after admission to ICU (9 d after CVC insertion), his right neck was severely swollen, red, warm, and sensitive to touch.
The patient had a 7-year history of poorly controlled hypertension and did not have diabetes.
The patient was a farmer with no history of smoking, alcohol, or drug abuse.
On physical examination, the patient presented with acute fever (39 °C), hypotension (80/60 mmHg), tachycardia (140 bpm), and slightly elevated respiratory rate (22 breaths/min). These findings combined with other laboratory and imaging results indicated septic shock. In addition, CVC-BSI was suspected given the severe neck swelling and hypotension.
Laboratory studies prior to and after the onset of the right neck swelling and hypotension were obtained and are summarized in Table 1. After the onset of neck symptoms, blood analysis revealed leukocytosis (13.69 × 109/L) and elevated inflammatory markers including C-reactive protein (75.9 mg/L) and procalcitonin (5.8 ng/mL). In addition, alanine aminotransferase (82 U/L) and aspartate aminotransferase (179 U/L) were elevated. Alternatively, renal function parameters were normal. Arterial blood gas measures were as follows: pH, 7.30; PaCO2, 33 mmHg; PaO2, 108 mmHg; lactate, 3.8 mmol/L; HCO3-, 16.8 mmol/L.
| Variables | Before | After | Normal range |
| White blood cells | 10.1 × 109/L | 13.69 × 109/L | 3.5-9.5 × 109/L |
| Platelets | 184 × 109/L | 244 × 109/L | 100-300 × 109/L |
| Alanine aminotransferase | 23 U/L | 82 U/L | 9-50 U/L |
| Aspartate aminotransferase | 27 U/L | 179 U/L | 15-40 U/L |
| C-reactive protein | 33.3 mg/L | 75.9 mg/L | 0.068-8.2 mg/L |
| Procalcitonin | 0.07 ng/mL | 5.8 ng/mL | < 0.05 ng/mL |
| Lactate | 0.6 mmol/L | 3.8 mmol/L | 0.5-1.7 mmol/L |
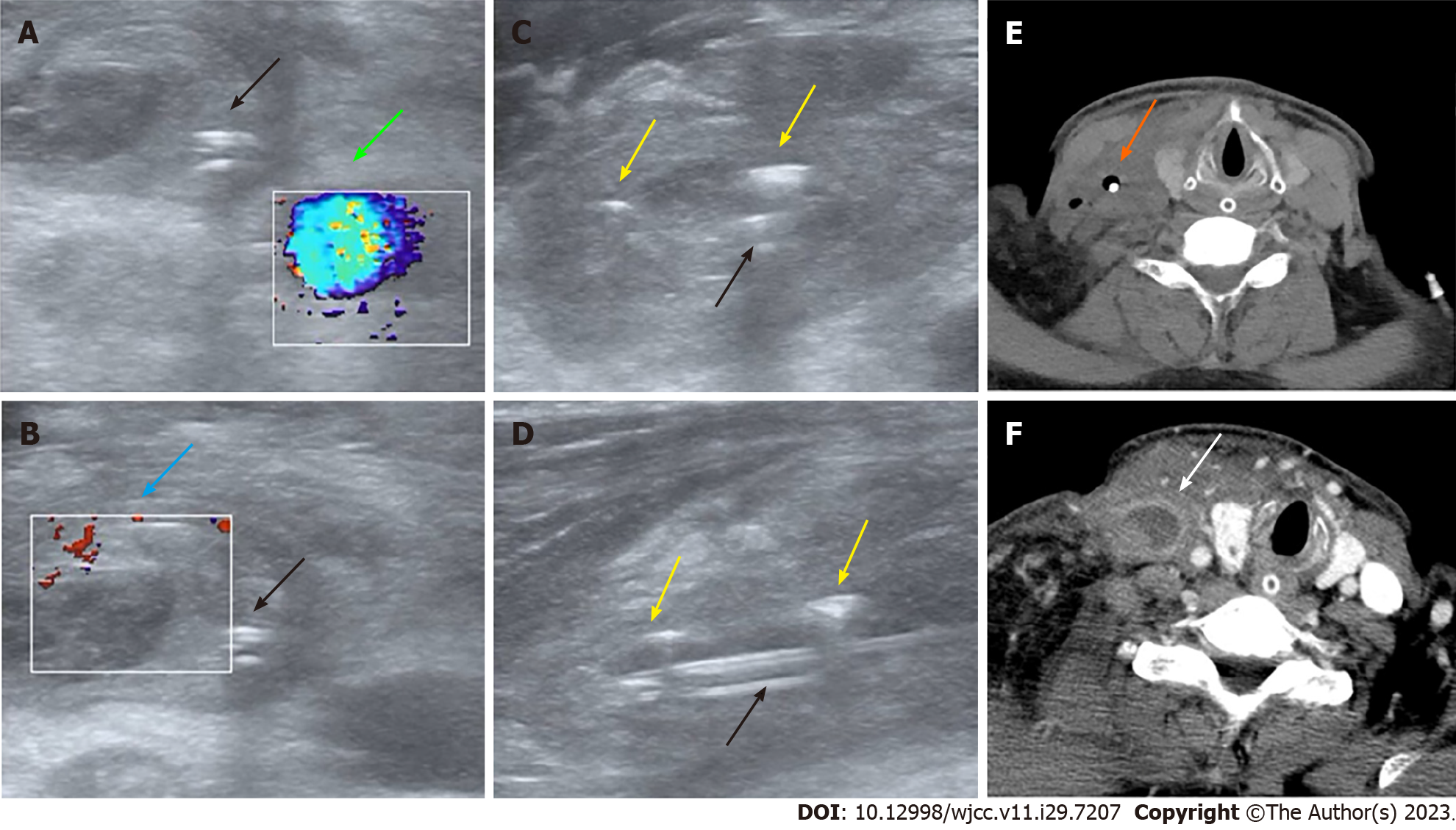
Computed tomography scans were not obtained immediately at presentation due to the critical status of the patient. Rather, we conducted urgent POCUS using a portable machine (Mindray M9, Shenzhen, China) to assess the clinical status at the bedside, which demonstrated normal blood flow in the right internal carotid artery (Figure 1A) but thrombosis and dilatation of the right internal jugular vein (IJV) without detectable blood flow (Figure 1B). Also, ultrasonography revealed that the misplaced CVC had been placed into the IJV rather than into the ideal position of superior vena cava (SVC) through the right subclavian vein approach and was surrounded by gas bubbles, manifesting as hyperechoic lines with dirty shadowing and comet-tail artifacts (Figure 1C and D; Video 1). The patient’s hypotension was improved by fluid resuscitation. A subsequent CT scan was performed afterwards on the same day and also confirmed air bubbles surrounding the CVC in the right neck (Figure 1E), and septic emphysematous thrombophlebitis induced by a misplaced CVC and septic shock was definitively diagnosed. The responsible CVC was removed immediately after paired peripheral venous blood and CVC blood sampling for aerobic and anaerobic culture to identify potential causative pathogens.
Septic emphysematous thrombophlebitis induced by a misplaced CVC and septic shock was definitively diagnosed based on POCUS findings, clinical symptoms, and physical signs.
Figure 2 illustrates the clinical course of the patient. The responsible CVC was removed immediately after paired peripheral venous blood and CVC blood sampling for aerobic and anaerobic culture to identify potential causative pathogens. The patient received fluid resuscitation, intravenous noradrenaline, and a 10-d ultra-broad spectrum antibiotic treatment including intravenous vancomycin (1 g every 12 h per day) and imipenem-cilastatin (1 g every 8 h per day) to combat the CVC-BSI and septic shock. In addition, an urgent vascular surgery consultation was obtained and low-molecular-weight heparin was administered to treat CRT. On day 2 after CVC removal, contrast-enhanced CT scan again revealed a swollen right IJV filled by a thrombus but no gas bubbles (Figure 1F). Both CVC and peripheral venous blood cultures on day 4 after CVC removal yielded methicillin-resistant Staphylococcus cohnii (S. cohnii) with differential time to positivity (DTP) of 5 h. The S. cohnii strain isolated by blood culture demonstrated moderate sensitivity to vancomycin (minimum inhibitory concentration of 1 mg/L). Thus, CVC-BSI caused by S. cohnii was finally diagnosed.
The patient was gradually weaned off vasopressors and the symptoms of redness and swelling in the right neck subsided within 7 d. At this time, the patient was fully conscious but exhibited left limb weakness due to stroke and emphysematous thrombophlebitis, and so was transferred from the ICU to a general ward for neurological rehabilitation. The patient was discharged in 8 days with full recovery. At one-month followed-up, the right IJV thrombus appeared organized and blood flow was stable. The patient and family expressed satisfaction with the medical care received from our team.
A CVC provides convenient venous access in the ICU, saving the patient from repeated needle punctures, but long-term CVC indwelling can result in infection, including serious CVC-BSI and thrombosis (CRT). Either condition may increase the risk or severity of the other[7]. Major risk factors for CVC-BSI include CRT, longer duration of catheterization, emergency surgery, use of steroids, coexistence of infection, and mechanical ventilation[8-10]. In addition, jugular-vein or femoral-vein catheterization may carry greater risk than subclavian-vein catheterization[11]. Risk factors associated with CRT include catheter-related infection, malposition of the CVC tip, older age, longer duration of catheterization, catheter-to-vein ratio > 0.45, a greater number of insertion attempts, absence of ultrasound assistance for insertion, and no use of anticoagulants[4]. In the current case, we suggest that emphysematous thrombophlebitis may be attributed to emergency surgery, absence of anticoagulant use, insertion without ultrasound assistance, and misplacement of the CVC tip from the right subcalvian vein into the IJV[4,12]. The term ‘misplacement’ refers to migration of the CVC tip to an improper position[13].It is recommended that the CVC tip be positioned at the junction of the SVC and right atrium[14], a location with high blood flow to reduce the risk of thrombosis but still lying outside the atrium, thereby preventing arrhythmia[15]. In the current case, the tip of the CVC was implanted via subclavian vein access towards the head rather than caudally toward the junction of the SVC and right atrium. Improper positioning of the catheter tip has been reported to greatly increase the risk of CRT due to reflux of blood[4]. We speculate that the relatively slow blood flow in the IJV and reflux of blood across the distal tip of the misplaced CVC resulted in CRT.
Emphysematous thrombophlebitis may manifest with clinical symptoms and physical signs similar to other CRT and CVC-BSI types. In this case, the patient presented with common symptoms of CVC-BSI including fever, septic shock, bacteremia, and leukocytosis[16]. While most patients with CRT are asymptomatic, the reported case exhibited localized swelling in the neck which may be attributed to IJV stenosis[4]. In addition, emphysematous thrombophlebitis with gas formation produced by pathogenic bacteria may lead to more severe sepsis-associated symptoms than common CVC-BSI without gas, such as circulatory shock as reported in this case. The most common causative pathogen for CVC-BSI is Staphylococcus[3], and indeed this Gram-positive bacteria was isolated from blood samples of the reported case. Other pathogens such as Klebsiella pneumoniae and E. coli may also cause similar clinical manifestations due to gas production.
Treatment of emphysematous thrombophlebitis is presently empirical and largely focused on managing CVC-BSI or CRT as there are few published reports on this specific condition. As shown in this case, CVC-BSI is diagnosed based on quantitative blood cultures from a catheter hub and another peripheral vein and by a DTP ≥ 2 h[17-19]. Duplex ultrasound plays a key role in the initial diagnosis of CRT by highlighting the absence of blood flow in the thrombotic vein[20,21]. POCUS has revolutionized the diagnosis and management of critical illnesses, especially lesions associated with gas bubbles[22]. Due to an inherent impedance mismatch with other human tissues, air has characteristic manifestations on ultrasound, including irregular hyperechoic structures, “dirty shadowing”, and A-lines that impede visualization of deeper structures[22]. These gas bubbles are easily detected by POCUS[23]. In the reported case, the comet-tail artifacts and dirty shadowing on POCUS facilitated rapid diagnosis of emphysematous thrombophlebitis and so may have contributed to favorable clinical outcome after antibiotic treatment, fluid resuscitation, rapid removal of the catheter, and anticoagulant administration.
Emphysematous thrombophlebitis is a fulminant and life-threatening CVC-BSI with hallmark signs of thrombosis and gas formation in the vein. POCUS can easily identify the artifacts produced by gas and thrombosis, facilitating rapid diagnosis at the bedside. Emergency and critical care professionals should consider this CVC-BSI type in differential diagnosis, especially in cases with a misplaced CVC, and recognize the value of POCUS for early diagnosis of emphysematous thrombophlebitis. Last, this case highlights the value of radiographic guidance for ensuring proper CVC tip location and thus preventing complications such as thrombosis.
We would like to acknowledge the patient and his family.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Critical care medicine
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Carlan SJ, United States; Ferraioli G, Italy; Juneja D, India S-Editor: Yan JP L-Editor: A P-Editor: Yan JP
| 1. | Geerts W. Central venous catheter-related thrombosis. Hematology Am Soc Hematol Educ Program. 2014;2014:306-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 138] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 2. | Fahy B, Sockrider M. Central Venous Catheter. Am J Respir Crit Care Med. 2019;199:P21-P22. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 3. | Hebeisen UP, Atkinson A, Marschall J, Buetti N. Catheter-related bloodstream infections with coagulase-negative staphylococci: are antibiotics necessary if the catheter is removed? Antimicrob Resist Infect Control. 2019;8:21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 4. | Citla Sridhar D, Abou-Ismail MY, Ahuja SP. Central venous catheter-related thrombosis in children and adults. Thromb Res. 2020;187:103-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 98] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 5. | Khor T, Anderson J, McRae P. Central venous thrombophlebitis diagnosed by computerized tomography scanning. Aust N Z J Surg. 1992;62:820-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 6. | Ubee SS, McGlynn L, Fordham M. Emphysematous pyelonephritis. BJU Int. 2011;107:1474-1478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 158] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 7. | Buetti N, Timsit JF. Management and Prevention of Central Venous Catheter-Related Infections in the ICU. Semin Respir Crit Care Med. 2019;40:508-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 8. | Pawar M, Mehta Y, Kapoor P, Sharma J, Gupta A, Trehan N. Central venous catheter-related blood stream infections: incidence, risk factors, outcome, and associated pathogens. J Cardiothorac Vasc Anesth. 2004;18:304-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Dedunska K, Dyk D. Prevention of central venous catheter-associated bloodstream infections: A questionnaire evaluating the knowledge of the selected 11 evidence-based guidelines by Polish nurses. Am J Infect Control. 2015;43:1368-1371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Septimus EJ, Moody J. Prevention of Device-Related Healthcare-Associated Infections. F1000Res. 2016;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 11. | Parienti JJ, Mongardon N, Mégarbane B, Mira JP, Kalfon P, Gros A, Marqué S, Thuong M, Pottier V, Ramakers M, Savary B, Seguin A, Valette X, Terzi N, Sauneuf B, Cattoir V, Mermel LA, du Cheyron D; 3SITES Study Group. Intravascular Complications of Central Venous Catheterization by Insertion Site. N Engl J Med. 2015;373:1220-1229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 401] [Cited by in RCA: 455] [Article Influence: 45.5] [Reference Citation Analysis (0)] |
| 12. | Saugel B, Scheeren TWL, Teboul JL. Ultrasound-guided central venous catheter placement: a structured review and recommendations for clinical practice. Crit Care. 2017;21:225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 182] [Cited by in RCA: 255] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 13. | Gibson F, Bodenham A. Misplaced central venous catheters: applied anatomy and practical management. Br J Anaesth. 2013;110:333-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 161] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 14. | Şahinkaya HH, Parlak M, Tekgul ZT. Assessment of the Tip Position of Central Venous Catheters Inserted Using Peres' Height Formula. Cureus. 2022;14:e31988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 15. | Bannon MP, Heller SF, Rivera M. Anatomic considerations for central venous cannulation. Risk Manag Healthc Policy. 2011;4:27-39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 83] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 16. | Hanna H, Afif C, Alakech B, Boktour M, Tarrand J, Hachem R, Raad I. Central venous catheter-related bacteremia due to gram-negative bacilli: significance of catheter removal in preventing relapse. Infect Control Hosp Epidemiol. 2004;25:646-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 78] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 17. | Mermel LA, Allon M, Bouza E, Craven DE, Flynn P, O'Grady NP, Raad II, Rijnders BJ, Sherertz RJ, Warren DK. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;49:1-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2225] [Cited by in RCA: 2399] [Article Influence: 149.9] [Reference Citation Analysis (0)] |
| 18. | Orihuela-Martín J, Rodríguez-Núñez O, Morata L, Cardozo C, Puerta-Alcalde P, Hernández-Meneses M, Ambrosioni J, Linares L, Bodro M, de Los Angeles Guerrero-León M, Del Río A, Garcia-Vidal C, Almela M, Pitart C, Marco F, Soriano A, Martínez JA. Performance of differential time to positivity as a routine diagnostic test for catheter-related bloodstream infections: a single-centre experience. Clin Microbiol Infect. 2020;26:383.e1-383.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Javeri Y, Jagathkar G, Dixit S, Chaudhary D, Zirpe KG, Mehta Y, Govil D, Mishra RC, Samavedam S, Pandit RA, Savio RD, Clerk AM, Srinivasan S, Juneja D, Ray S, Sahoo TK, Jakkinaboina S, Jampala N, Jain R. Indian Society of Critical Care Medicine Position Statement for Central Venous Catheterization and Management 2020. Indian J Crit Care Med. 2020;24:S6-S30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Bhagra A, Tierney DM, Sekiguchi H, Soni NJ. Point-of-Care Ultrasonography for Primary Care Physicians and General Internists. Mayo Clin Proc. 2016;91:1811-1827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 155] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 21. | Huang Z, Chen H, Liu Z. The 100 top-cited systematic reviews/meta-analyses in central venous catheter research: A PRISMA-compliant systematic literature review and bibliometric analysis. Intensive Crit Care Nurs. 2020;57:102803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 22. | Buttar S, Cooper D Jr, Olivieri P, Barca M, Drake AB, Ku M, Rose G, Siadecki SD, Saul T. Air and its Sonographic Appearance: Understanding the Artifacts. J Emerg Med. 2017;53:241-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 23. | Shabana W, Bude RO, Rubin JM. Comparison between color Doppler twinkling artifact and acoustic shadowing for renal calculus detection: an in vitro study. Ultrasound Med Biol. 2009;35:339-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |