Published online Oct 16, 2023. doi: 10.12998/wjcc.v11.i29.7200
Peer-review started: August 6, 2023
First decision: August 30, 2023
Revised: September 9, 2023
Accepted: September 26, 2023
Article in press: September 26, 2023
Published online: October 16, 2023
Processing time: 68 Days and 0.5 Hours
Immunosuppressive therapy and matched sibling donor hematopoietic stem cell transplantation (MSD-HSCT) are the preferred treatments for aplastic anemia (AA).
In this report, we describe a 43-year-old male patient with severe AA who carried BRIP1 (also known as FANCJ), TINF2, and TCIRG1 mutations. Screening of the family pedigree revealed the same TINF2 mutation in his mother and older brother, with his older brother also carrying the BRIP1 variant and demonstrating normal telomere length and hematopoietic function. The patient was successfully treated with oral cyclosporine A, eltrombopag, and acetylcysteine, achieving remission 4 years after receiving MSD-HSCT from his older brother.
This case provides a valuable clinical reference for individuals with suspected pathogenic gene mutations, normal telomere length, and hematopoietic function, highlighting them as potential donors for patients with AA.
Core Tip: Aplastic anemia (AA) is a bone marrow failure syndrome. In this report, we present a case of an adult patient with severe AA who was successfully treated with matched sibling donor hematopoietic stem cell transplantation from his older brother. Despite his brother carrying BRIP1 and TINF2 mutations, his telomere length and hematopoietic function remained normal. The patient achieved and maintained remission for more than four years after transplantation. This case provides a clinical reference for individuals with suspected pathogenic gene mutations and normal telomere length and hematopoietic function, as potential donors for patients with AA.
- Citation: Yan J, Jin T, Wang L. Hematopoietic stem cell transplantation of aplastic anemia by relative with mutations and normal telomere length: A case report. World J Clin Cases 2023; 11(29): 7200-7206
- URL: https://www.wjgnet.com/2307-8960/full/v11/i29/7200.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i29.7200
Aplastic anemia (AA) is a syndrome characterized by bone marrow failure, marked by reduced bone marrow cell proliferation and peripheral pancytopenia. AA can be classified as either inherited or acquired. Inherited AA is attributed to germline mutations, while acquired AA is suspected to result from cytotoxic T cell-mediated immune attacks on hematopoietic stem and progenitor cells[1]. The most prevalent inherited bone marrow failure syndromes (IBMFSs) encompass Fanconi anemia (FA), dyskeratosis congenita (DC), Shwachman–Diamond syndrome, congenital amegakaryocytic thrombocytopenia, Blackfan–Diamond anemia, and reticular dysgenesis. Treatment options for AA encompass immunosuppressive therapy (IST) and hematopoietic stem cell transplantation (HSCT). Comprehensive medical history, clinical manifestations and signs, and special laboratory tests such as chromosome karyotype analysis, genetic testing related to congenital bone marrow failure diseases, and telomere length measurements should be part of the diagnostic evaluation of all patients with AA to tailor therapeutic regimens. Carriers of a pathogenic variant of gene-related IBMFS can consider HSCT regimens for acquired severe AA (SAA)[2].
In this report, we present the case of an adult patient with SAA who underwent successful treatment with a matched sibling donor-HSCT from an older brother. The older brother carried both BRIP1 and TINF2 mutations and had normal telomere length. The patient achieved and maintained remission for more than four years following transplantation.
A 43-year-old male patient experienced sudden fainting for 6 h and presented with prolonged paleness.
In April 2018, a 43-year-old male patient was admitted to our hospital after experiencing sudden fainting for 6 h and presenting with prolonged paleness.
The patient was treated for skin ecchymosis at the age of nine. At that point, he was diagnosed with AA, and tests revealed pancytopenia. At that time, he received treatment with stanozolol and traditional Chinese medicine. In May 2012, a bone biopsy revealed a few megakaryocytes and a maturation deficiency without obvious abnormalities on flow cytometric immunophenotyping. Chromosomal analysis of bone marrow cells indicated a conventional karyotype (46, XY). Bone marrow CD34+ cells accounted for 0.02%; paroxysmal nocturnal hemoglobinuria (PNH) examination was negative; and the patient was positive for a TET2 gene mutation and negative for FANCA gene. The bone marrow biopsy did not show myelodysplastic syndrome, fluorescence in situ hybridization was negative, and the cytogenetic panel was normal. Six years later, the patient progressed to SAA. IBMFS high-throughput sequencing revealed that the patient carried mutations in three genes: BRIP1, TINF2, and TCIRG1.
The patient’s family had no history of hematological diseases. The patient’s older brother, aged 45, carried BRIP1 and TINF2 mutations, and shared the same TINF2 variant as their mother (Table 1, Figure 1). The blood count, bone marrow cytology, bone marrow biopsy, and small megakaryocyte enzyme labels were all normal. Bone marrow CD34+ cells accounted for 0.74% of all cells. Average telomere length was quantified using a telomere restriction fragment assay. The patient’s telomere was significantly shorter than that of his brother. In contrast, the telomere lengths of his mother and brother were normal compared to age-matched healthy controls.
| Site | Gene | Mutation | Type | Proportion (%) |
| 17:59793364 | c.2440C>T; p.Arg814Cys | Missense mutation | 43.78 | |
| 11:67812500 | c.1096C>T; p.Arg366Cys | Missense mutation | 51.53 | |
| 14:24709074 | c.1285C>G; p.Leu429Val | Missense mutation | 49.85 |
Physical examination revealed severe anemia without other abnormalities.
Initial laboratory evaluation of peripheral blood revealed the following: White blood cell count: 2.22 × 109/L; red blood cell count: 1.56 × 1012/L; hemoglobin level, 59 g/L; and platelet (PLT) count, 8 × 109/L.
In conjunction with the patient’s medical history, it is evident that he has progressed to SAA.
The patient, with had a human leukocyte antigen (HLA) 6/6 match compatible with that of his older brother, underwent bone marrow combined and peripheral blood stem cell transplantation. The pre-transplant conditioning regimen included fludarabine 30 mg/m2/d, cyclophosphamide 300 mg/m2/d, and anti-thymocyte globulin 2.5 mg/kg/d from days 5 to 2. Mononuclear (5.35 × 108/kg) and CD34+ (2.39 × 106/kg) were injected within two days. Subsequently, cyclosporine A (CsA) was administered (12 mo following transplantation, with a reduced dose after nine months), along with short-term methotrexate were used for graft-versus-host disease prophylaxis. Neutrophil and PLT engraftment commenced on day + 13. After the transplantation, the patient received oral CsA, eltrombopag, and acetylcysteine.
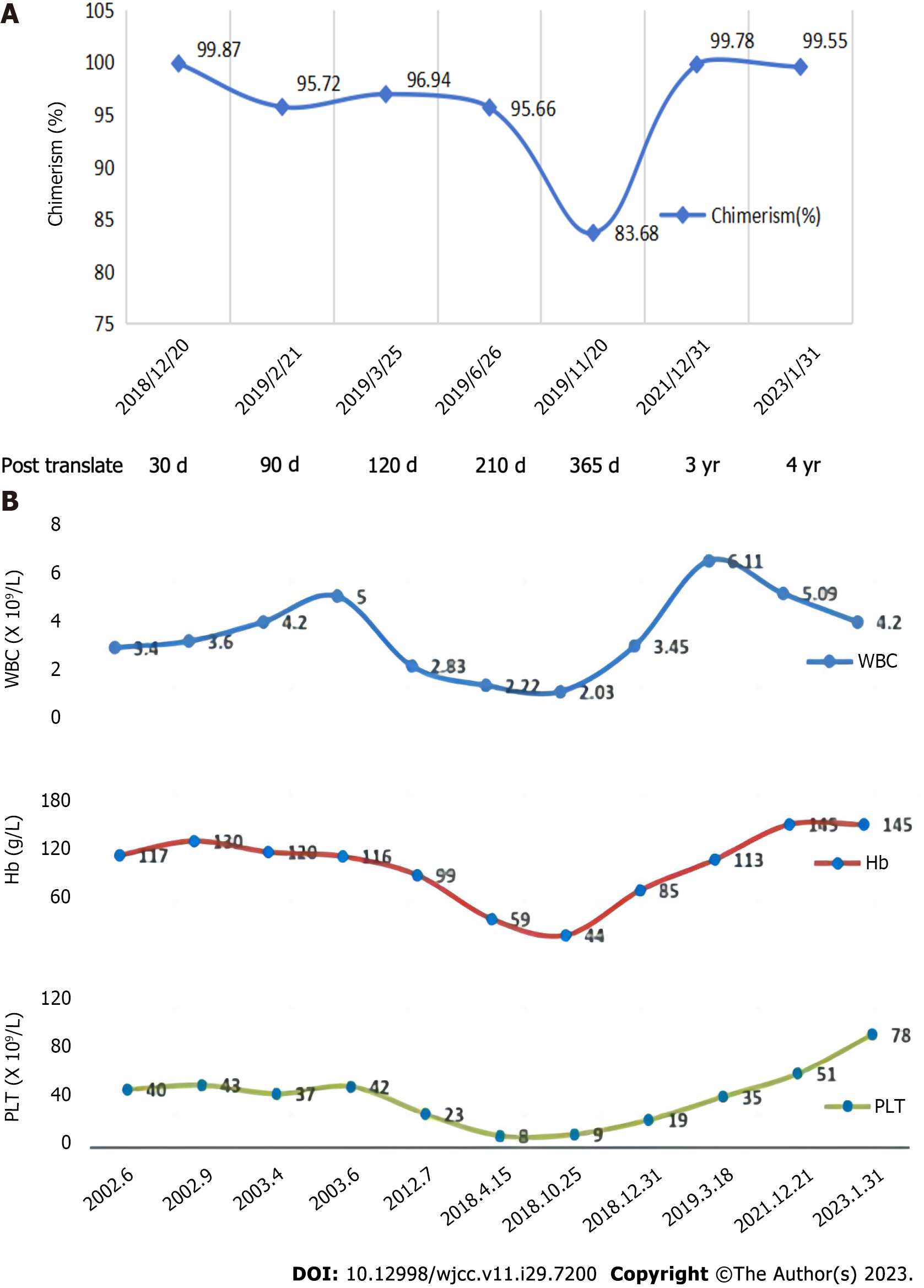
Figures 2 and 3 display the results of the bone marrow cytology and biopsy, chimerism, and blood counts. Telomere length measurements were repeatedly performed for both the patient and his brother three years after transplantation. The patient’s telomere length remained short, while his brother’s was within the normal range for individuals of the same age (Figure 4). The patient achieved and sustained remission for four years throughout the follow-up period. His blood cell counts returned to be relatively stable, and he continued to exhibit complete chimerism.
BRIP1 mutations have been detected in 3% of FA cases, and TINF2 mutations have been reported as the cause in approximately 15% of DC cases, often occurring de novo[3]. TINF2 protects chromosomes and modulates telomerase activity. Patients with DC typically exhibit short telomeres, leading to premature stem cell exhaustion and tissue failure[4-6]. In contrast, most TINF2 mutations in patients with DC are located in exon 6. However, TINF2 mutations in our patient and his brother were located in exon 9, a novel finding[7,8]. Heterozygous TINF2 mutations have been identified in 1%-5% of acquired AA patients with AA[9]. Despite carrying heterozygous BRIP1 and TINF2 mutations, the patient was diagnosed with acquired AA because he did not exhibit any signs or symptoms involving other organs or tissues typically associated with FA or DC. Although his brother was asymptomatic and had a normal hematopoietic function, he harbored the two mutations.
The decision to select HSCT and IST as the initial therapy for acquired AA depends on the patient's age and the availability of an HLA-matched donor. Several predictive biomarkers for IST response have been identified, including age, sex, pretreatment blood cell count, cytokines, gene mutations, PNH, and telomere length[10,11]. Significant telomere shortening in lymphocytes in patients with AA is presumed to occur secondary to hematopoietic stress. Telomere erosion reduces the replication of hematopoietic stem cells and progenitor cells. However, the value of telomere length in predicting response to IST is debatable. A study from the National Institute of Health has reported that baseline telomere length was associated with the risk of relapse, clonal transformation, and overall survival with hematologic response in adult patients with AA[12]. A study in Japan indicated that two patients with acquired AA who had TINF2 mutations did not respond clinically to IST[13]. Therefore, HSCT, which offers a potential cure, may be preferable to IST[14].
Miano et al[15] described the case of a patient with AA who experienced graft failure eight years after matched sibling donor HSCT and successfully received a secondary transplantation from the same donor. The Ser245Tyr mutation in TINF2 was discovered seven years after the secondary transplantation, and the patient survived for another 16 years. Although his father carried the mutation, he remained asymptomatic. This suggests that the Ser245Tyr TINF2 variant results in milder phenotypes compared to those in other significant TINF2 mutations, which are often associated with bone marrow involvement and interfere with stem cell implantation after transplantation.
In this case, the TINF2 mutation was located in exon 9, suggesting that this TINF2 mutation could be a clinically important variant related to either AA or asymptomatic phenotypes. This variant can be observed in a wide group of telomeropathies; generation anticipation and telomere length may partly explain the broad phenotype. Gadalla et al[16] assessed the association between leukocyte telomere length and outcomes in matched unrelated HSCT donors with SAA[17]. The patients did not have alternative donors, such as unrelated or umbilical cord blood donors. Although our patient’s brother carried mutations in BRIP1 and TINF2, his telomere length and hematopoietic function were normal. Therefore, the brother should not be excluded as a potential donor. The donor carried the same gene mutation; however, the transplantation succeeded, and the patient maintained remission.
Patients diagnosed with AA in childhood, especially those with BRIP1 or TINF2 mutations, should be carefully distinguished from congenital AA conditions like FA and DC. The only possible therapeutic strategy for these patients is allo-HSCT. However, selecting family members as potential donors should undergo a thorough evaluation. After transplantation, a series of bone marrow and peripheral blood tests should be carried out continuously, including donor chimerism status. Timely intervention is crucial to preventing both primary and secondary graft failures. However, whether a TINF2 mutation should be considered clinically important depends on the specific variant and its impact on protein function. Certain TINF2 mutations can disrupt telomere maintenance, leading to severe clinical phenotypes like DC. Other variants may result in milder effects associated with less severe or asymptomatic presentations. If further basic experiments are conducted to confirm the differential effects of TINF2 mutations at various sites on protein expression and function and on the clinical characteristics of patients, it will be helpful to more effectively define the pathogenesis and guide treatment.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Jaing TH, Taiwan S-Editor: Qu XL L-Editor: A P-Editor: Zhang XD
| 1. | Durrani J, Groarke EM. Clonality in immune aplastic anemia: Mechanisms of immune escape or malignant transformation. Semin Hematol. 2022;59:137-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 2. | McReynolds LJ, Rafati M, Wang Y, Ballew BJ, Kim J, Williams VV, Zhou W, Hendricks RM, Dagnall C, Freedman ND, Carter B, Strollo S, Hicks B, Zhu B, Jones K, Paczesny S, Marsh SGE, Spellman SR, He M, Wang T, Lee SJ, Savage SA, Gadalla SM. Genetic testing in severe aplastic anemia is required for optimal hematopoietic cell transplant outcomes. Blood. 2022;140:909-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 3. | Yoshizato T, Dumitriu B, Hosokawa K, Makishima H, Yoshida K, Townsley D, Sato-Otsubo A, Sato Y, Liu D, Suzuki H, Wu CO, Shiraishi Y, Clemente MJ, Kataoka K, Shiozawa Y, Okuno Y, Chiba K, Tanaka H, Nagata Y, Katagiri T, Kon A, Sanada M, Scheinberg P, Miyano S, Maciejewski JP, Nakao S, Young NS, Ogawa S. Somatic Mutations and Clonal Hematopoiesis in Aplastic Anemia. N Engl J Med. 2015;373:35-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 408] [Cited by in RCA: 457] [Article Influence: 45.7] [Reference Citation Analysis (0)] |
| 4. | Savage SA, Giri N, Baerlocher GM, Orr N, Lansdorp PM, Alter BP. TINF2, a component of the shelterin telomere protection complex, is mutated in dyskeratosis congenita. Am J Hum Genet. 2008;82:501-509. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 344] [Cited by in RCA: 310] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 5. | Gramatges MM, Bertuch AA. Short telomeres: from dyskeratosis congenita to sporadic aplastic anemia and malignancy. Transl Res. 2013;162:353-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 6. | Kallen ME, Dulau-Florea A, Wang W, Calvo KR. Acquired and germline predisposition to bone marrow failure: Diagnostic features and clinical implications. Semin Hematol. 2019;56:69-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 7. | Walne AJ, Vulliamy T, Beswick R, Kirwan M, Dokal I. TINF2 mutations result in very short telomeres: analysis of a large cohort of patients with dyskeratosis congenita and related bone marrow failure syndromes. Blood. 2008;112:3594-3600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 234] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 8. | Mason PJ, Bessler M. The genetics of dyskeratosis congenita. Cancer Genet. 2011;204:635-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 109] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 9. | Du HY, Mason PJ, Bessler M, Wilson DB. TINF2 mutations in children with severe aplastic anemia. Pediatr Blood Cancer. 2009;52:687. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | Scheinberg P. Prognostic value of telomere attrition in patients with aplastic anemia. Int J Hematol. 2013;97:553-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Narita A, Kojima S. Biomarkers for predicting clinical response to immunosuppressive therapy in aplastic anemia. Int J Hematol. 2016;104:153-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Scheinberg P, Cooper JN, Sloand EM, Wu CO, Calado RT, Young NS. Association of telomere length of peripheral blood leukocytes with hematopoietic relapse, malignant transformation, and survival in severe aplastic anemia. JAMA. 2010;304:1358-1364. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 161] [Cited by in RCA: 161] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 13. | Yamaguchi H, Inokuchi K, Takeuchi J, Tamai H, Mitamura Y, Kosaka F, Ly H, Dan K. Identification of TINF2 gene mutations in adult Japanese patients with acquired bone marrow failure syndromes. Br J Haematol. 2010;150:725-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Iftikhar R, Chaudhry QUN, Anwer F, Neupane K, Rafae A, Mahmood SK, Ghafoor T, Shahbaz N, Khan MA, Khattak TA, Shamshad GU, Rehman J, Farhan M, Khan M, Ansar I, Ashraf R, Marsh J, Satti TM, Ahmed P. Allogeneic hematopoietic stem cell transplantation in aplastic anemia: current indications and transplant strategies. Blood Rev. 2021;47:100772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 15. | Miano M, Lanciotti M, Giardino S, Dufour C. Ser245Tyr TINF2 mutation in a long-term survivor after a second myeloablative SCT following late graft failure for Aplastic Anaemia. Blood Cells Mol Dis. 2015;55:187-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 16. | Gadalla SM, Wang T, Haagenson M, Spellman SR, Lee SJ, Williams KM, Wong JY, De Vivo I, Savage SA. Association between donor leukocyte telomere length and survival after unrelated allogeneic hematopoietic cell transplantation for severe aplastic anemia. JAMA. 2015;313:594-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 75] [Article Influence: 7.5] [Reference Citation Analysis (0)] |