Published online Oct 16, 2023. doi: 10.12998/wjcc.v11.i29.7144
Peer-review started: June 19, 2023
First decision: August 4, 2023
Revised: August 15, 2023
Accepted: September 25, 2023
Article in press: September 25, 2023
Published online: October 16, 2023
Processing time: 116 Days and 3.4 Hours
The co-infection of Chlamydia psittaci (C. psittaci) and Tropheryma whipplei (T. whipplei) is unusual, and the detection of pathogenic microorganisms is particularly important for patients with severe diseases or poor experience in treatment. Early identification of pathogens can significantly improve the prognosis of the patients. Targeted next-generation sequencing (tNGS) is currently widely used in clinical practice for various infectious diseases, including respiratory infections, to achieve early, accurate, and rapid microbial diagnosis.
We report a case of a 40-year-old female patient with a history of contact with parrots who was diagnosed with C. psittaci and T. whipplei infection through bronchial lavage fluid targeted next generation sequencing. After moxifloxacin treatment, the patient's symptoms improved significantly, and the imaging changes were obviously resolved.
Coinfection with C. psittaci and T. whipplei is not common. In this case, timely and accurate identification of both pathogens was achieved using tNGS. Moreover, the efficacy of monotherapy with moxifloxacin was confirmed.
Core Tip: The co-infection of Chlamydia psittaci and Tropheryma whipplei is not common. Due to its ability to cause severe infections, timely and reliable diagnosis is crucial for improving prognosis. In recent years, the development of targeted next-generation sequencing has made the diagnosis of pathogenic microorganisms more economical and efficient.
- Citation: Du ZM, Chen P. Co-infection of Chlamydia psittaci and Tropheryma whipplei: A case report. World J Clin Cases 2023; 11(29): 7144-7149
- URL: https://www.wjgnet.com/2307-8960/full/v11/i29/7144.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i29.7144
Psittacosis is a zoonotic infectious disease caused by Chlamydia psittaci (C. psittaci), which is a gram-negative bacterium belonging to the Chlamydia spp. This pathogen mainly parasitizes parrots, pigeons, poultry, etc. Humans are mainly affected by direct contact with birds and poultry excrement and respiratory secretions. Handling the feathers, tissues, or corpses of infected birds also carries a risk of infection[1]. The first description of psittacosis in the medical literature was in 1879[2], and according to subsequent reports, psittacosis may be the cause of one percent of cases among community-acquired pneumonia[3]. Clinical manifestations of psittacosis vary from asymptomatic infection to severe systemic symptoms and may even lead to fatal systemic illness. Symptoms of infected individuals may include headache, chills, fever (even high fever), myalgia, and dry cough during the course of the disease, which may be accompanied by dyspnoea and chest tightness[4]. Tropheryma whipplei (T. whipplei) is a gram-positive bacterium first described by American pathologist George Hoyt Whipple in 1907 and belongs to the trophozoite genus[5]. The most obvious symptoms include arthralgia, diarrhoea, abdominal pain, and weight loss, and reports of endocarditis, nervous system infection, uveitis, arthritis and osteoarthritis infection can also be seen[6]. However, there are few reports about pneumonia caused by T. whipplei. A previous study found that the respiratory infection rate was only 13%-14%[7], of which only approximately 30% of patients had respiratory symptoms[8], but the symptoms were not typical and included dyspnoea, cough, chest pain, and vital capacity reduction[9]. Reports of coinfection of C. psittaci and T. whipplei are extremely rare, but due to their ability to cause severe infections, rapid progression, and life-threatening conditions, timely and reliable diagnosis is crucial for treatment.
In recent years, high-throughput sequencing methods have developed rapidly, mainly including whole genome sequencing (WGS), metagenomic next-generation sequencing (mNGS), and targeted next-generation sequencing (tNGS). Among them, WGS is less directly applied in clinical practice and is generally used in epidemiological investigations and research on the evolution of drug-resistant strains. MNGS can detect all pathogens in the sample without discrimination, while tNGS mainly focuses on common infectious pathogens in clinical practice. TNGS designs specific primers, uses a super multiplex polymerase chain reaction (PCR) library system to target and amplify the target sequence, and then uses high-throughput sequencing for synchronous detection of amplification products, achieving a new method of broad-spectrum accurate detection of pathogens. In this report, tNGS was used to confirm the clinical rarity of pneumonia caused by C. psittaci and T. whipplei.
A 40-year-old female patient was admitted to the hospital for fever on November 6, 2022.
The patient had a fever for 10 d and a maximum body temperature of 39.6 °C, accompanied by dizziness and headache, but she had no other symptoms, such as cough, abdominal pain, or diarrhea.
The patient had previously been physically fit, but she had bought three parrots one week before the onset of symptoms, one of which was sick.
The patient had no special history of personal history or family history.
On examination, body temperature was 36.0 °C; heart rate was 94 beats/min; respiratory rate was 21 breaths/min; blood pressure was 100/72 mmHg; and pulse oxygen saturation was 96%. The patient was well built and cooperative, had no pharyngeal hyperaemia, and had no enlargement or purulent discharge of the tonsils. Breath sounds in both lungs were rough, and moist rales could be heard in the lower lobe of the right lung. Heart and abdominal examinations showed no significant findings.
A preliminary hematological examination (Table 1) showed that the hypersensitive C-reactive protein was significantly increased, transaminase and direct bilirubin were slightly increased, and electrolyte metabolism was deranged. The white blood cells and their respective classification ratios were normal, and procalcitonin was normal. In addition, during coronavirus disease 2019 (COVID-19), we tested the patient for COVID-19 nucleic acid, and the results were negative.
| Blood tests | Results | Normal range |
| White blood cells (/L) | 5.78 × 109 | 3.5 × 109-9.5 × 109 |
| Neutrophils (%) | 70.5 | 40.0-75.0 |
| Lymphocytes (%) | 22.2 | 20.0-50.0 |
| Red blood cells (/L) | 4.2 × 1012 | 3.8 × 1012-5.1 × 1012 |
| Haemoglobin (g/L) | 119 | 115-150 |
| Platelets (/L) | 186 × 109 | 125 × 109-350 × 109 |
| Total protein (g/L) | 68.9 | 65.0-85.0 |
| Albumin (g/L) | 42.0 | 40.0-55.0 |
| Total bilirubin (umol/L) | 16.3 | < 23.0 |
| Direct bilirubin (umol/L) | 9.4 | < 6.8 |
| Aspartate aminotransferase (IU/L) | 53 | 13.0-35.0 |
| Alanine aminotransferase (IU/L) | 61 | 7.0-40.0 |
| Lactate dehydrogenase (IU/L) | 431.2 | 120.0-250.0 |
| Creatinine (umol/L) | 48.1 | 41.0-73.0 |
| Sodium (mmol/L) | 128 | 137.0-147.0 |
| Potassium (mmol/L) | 3.08 | 3.5-5.5 |
| Chloride (mmol/L) | 92.4 | 99-110 |
| Hypersensitive C-reactive (mg/L) | 165.75 | 0.5-10 |
| Procalcitonin (ng/mL) | < 0.05 | < 0.5 |
| Prothrombin time (s) | 13.5 | 9-14 |
| Activated partial thromboplastin time (s) | 28.9 | 20-40 |
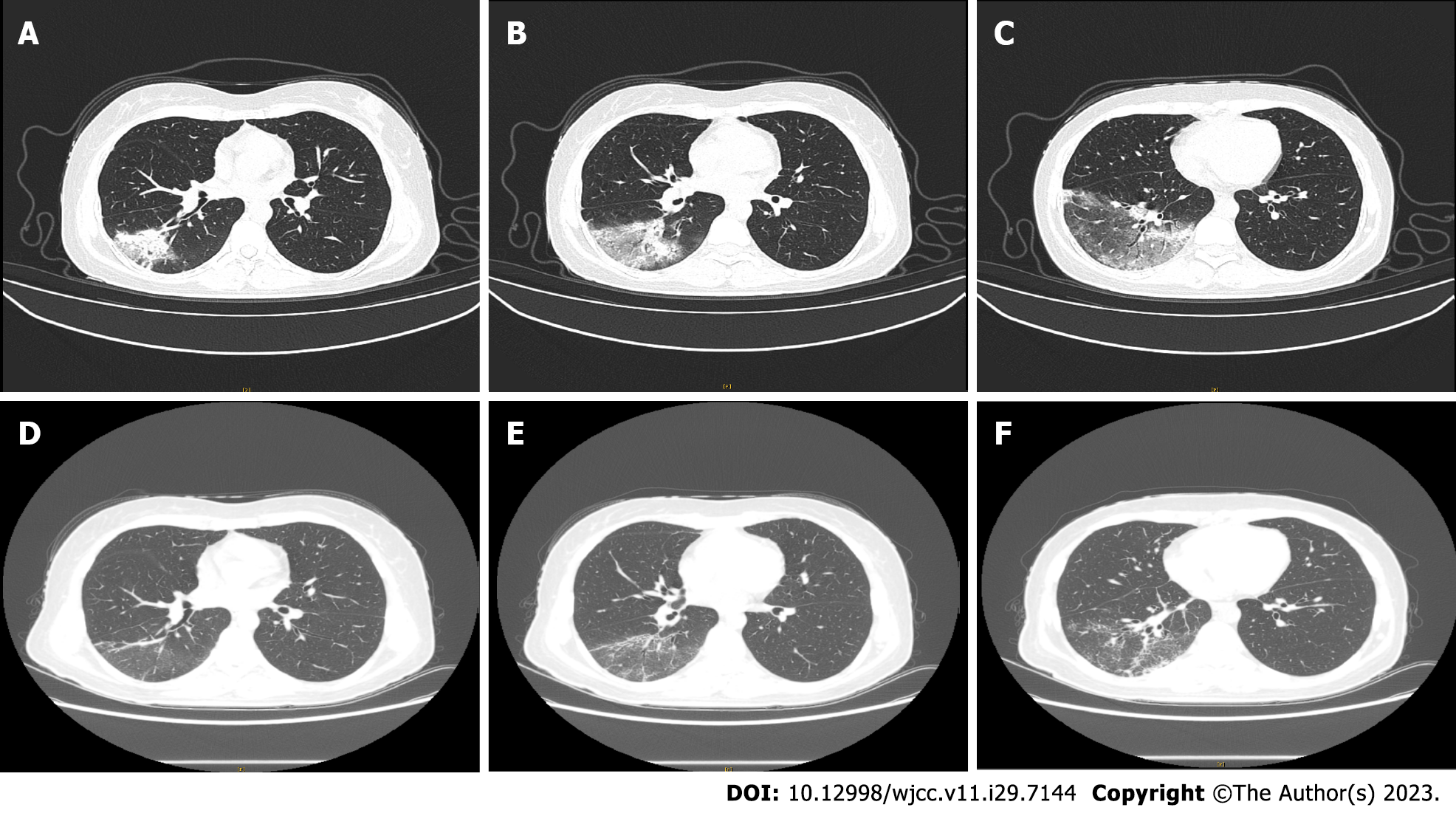
A computed tomography (CT) scan on November 6, 2022 (Figure 1A-C) revealed multiple large uneven ground glass shadows in the lower lobe of the right lung, with grid shadows visible inside, close to the pleura, and special pathogen infections could not be ruled out.
The patient received fiber bronchoscopy examination, and fiber bronchoscopy reached the lower lobe bronchus of the right lung. The lower lobe bronchus of the right lung was repeatedly irrigated. The collected samples were then sent to the laboratory for tNGS testing and analysis, which revealed C. psittaci and T. whipplei infection.
The patient was treated with moxifloxacin 0.4 g once daily for 10 d.
During hospitalization, the patient experienced coughing, phlegm, and diarrhea. Following treatment, the body temperature returned to normal, and the above symptoms disappeared completely. Hypersensitive C-reactive protein, transaminase and direct bilirubin returned to normal values. After 10 d of anti-infection treatment with moxifloxacin, a CT scan on November 16, 2022 (Figure 1D-F) showed that patch shadows and ground glass shadows in the right lower lobe lesions were obviously resolved. After discharge, the patient insisted on taking moxifloxacin orally for one week, but she was unwilling to undergo a recheck chest CT again for a review as her physical condition was good.
This article reports a rare case of co-infection caused by C. psittaci and T. whipplei. There are no specific clinical manifestations of psittacosis, and routine detection methods lack sensitivity and specificity, making it difficult to determine the epidemiology of psittacosis. Before 2013, some literature mentioned the occurrence of psittacosis cases, but with the increasing number of poultry raised in households and farms, large-scale outbreaks of psittacosis have frequently occurred, especially since 2020. The majority of transmission modes of psittacosis are limited to human exposure to contaminated birds and their excreta. However, some literature has reported the possibility of human-to-human transmission, and there is no definitive conclusion on whether there is human-to-human transmission in the second and third generations[4]. The diagnosis and treatment of Whipple's disease in the digestive system is relatively mature, but reports of infection in the respiratory system are rare. In addition, strong evidence shows that T. whipplei is a pathogen of acute pneumonia. Previous studies have found that T. whipplei is detected in the alveolar lavage fluid of patients with immune deficiency and patients with pneumonia who were admitted to intensive care units[10,11]. This evidence strongly supports a link between T. whipplei and pulmonary infection. It is noteworthy that T. whipplei can also exist in the saliva of asymptomatic patients[12], who inhale pathogenic bacteria from the mouth, leading to pneumonia[13]. In this case, the patient had a clear history of contact with sick parrots, with fever symptoms initially appearing and headache and respiratory symptoms gradually developing during the course of the disease. Hematological examination showed that the hypersensitive C-reactive protein was significantly increased, and transaminase and direct bilirubin were slightly increased. These symptoms and examinations are consistent with psittacosis and T. whipplei pneumonia, and tNGS testing confirmed the presence of C. psittaci and T. whipplei in the alveolar lavage fluid.
In clinical practice, traditional microbial culture methods and empirical diagnosis are still the mainstream, and the efficiency of traditional pathogen diagnosis methods is not high. Bacterial cultivation takes a long time, and in clinical practice, it is usually not possible to wait until the pathogenic microorganisms have been reported before taking medication. In addition, the positive rate of microbial culture is relatively low, resulting in many pathogenic infections that cannot obtain microorganisms. The emergence of high-throughput sequencing technology has improved this situation to some extent. At present, a variety of high-throughput sequencing technology have been used for the identification of pathogenic microorganisms in clinical infectious diseases, especially when there is a lack of diagnostic clues, it has significant advantages in identifying pathogenic microorganisms. According to different detection strategies, high-throughput sequencing methods are mainly divided into tNGS, mNGS and WGS. MNGS technology is to directly conduct unbiased detection and sequence analysis of all nucleic acids in the specimen, including but not limited to the detection of pathogenic bacteria nucleic acids, and also includes the nucleic acid detection of other pathogens and a large number of human host cells[14]. Due to the complex and time-consuming data processing of mNGS, it has the characteristics of high cost. In particular, mNGS is greatly influenced by human genes making the results easily misunderstood and interfered with. TNGS is a combination of multiplex PCR amplification and sequencing technology, which can detect dozens to hundreds of common pathogenic microorganisms and their virulence and drug resistance genes in samples. Through targeted amplification, it can improve the reliability of pathogen detection. At the same time, it can also provide personalized medication tips for suspected pathogenic microorganisms according to different infection sites. Because only specific target regions need to be sequenced, data processing and analysis are usually easier, making it faster and more economical. However, as mentioned earlier, due to the high cost and time-consuming nature of mNGS, the clinical application of pathogen metagene sequencing is still limited to a small range, leaving a collaborative space for tNGS. But rather than replacing the application space of mNGS in clinical practice with tNGS, the two complement each other and jointly improve the efficiency of hospital pathogen diagnosis. An article published in 2022 pointed out that there is no significant difference in the detection efficiency of bacteria, fungi, and viruses between mNGS and tNGS, but the removal of human genetic background by tNGS may affect the detection rate of G-negative bacteria, viruses, and intracellular bacteria, leading to higher false-negatives[15].
The guidelines are consistent with the literature recommendations for psittacosis. The first-line medication is tetracyclines, with a duration of at least 7-14 d, and the second choice of antibiotics include azithromycin, clarithromycin, erythromycin, and chloramphenicol[1]. There is not much evidence for the clinical use of quinolones alone. However, there are also studies suggesting that moxifloxacin has an anti-chlamydia effect, and moxifloxacin in this case has shown a good therapeutic effect, so first-line treatment is not used. This case also provides new evidence for the use of quinolones for psittacosis. The drugs for the treatment of T. whipplei mainly include penicillin, streptomycin, tetracycline, ceftriaxone, meropenem, compound trimethoprim, doxycycline and hydroxychloroquine. In the past, tetracycline was once listed as a first-line treatment drug, but a high recurrence rate was observed after treatment[16]. However, most of the treatments target digestive disorders caused by T. whipplei, and there are few reports on the treatment experience of pneumonia related to Whipple's disease. Therefore, there is still no broad consensus on the treatment of Whipple's disease-related pneumonia, and there is a lack of clinical treatment experience for a large number of cases. The anti-infection treatment plans are all empirical treatments, lacking sufficient evidence support.
Collectively, we believe that tNGS is promising in the diagnosis of unexplained pneumonia, and quinolones are effective in the treatment of psittacosis and Whipple's disease, which deserves more clinical attention.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Emran TB, Bangladesh; Yeoh SW, Australia S-Editor: Lin C L-Editor: Ma JY P-Editor: Lin C
| 1. | Balsamo G, Maxted AM, Midla JW, Murphy JM, Wohrle R, Edling TM, Fish PH, Flammer K, Hyde D, Kutty PK, Kobayashi M, Helm B, Oiulfstad B, Ritchie BW, Stobierski MG, Ehnert K, Tully TN Jr. Compendium of Measures to Control Chlamydia psittaci Infection Among Humans (Psittacosis) and Pet Birds (Avian Chlamydiosis), 2017. J Avian Med Surg. 2017;31:262-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 2. | Khadka S, Timilsina B, Pangeni RP, Regmi PR, Thapa AS. Importance of clinical history in the diagnosis of psittacosis: A case report. Ann Med Surg (Lond). 2022;82:104695. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 3. | Hogerwerf L, DE Gier B, Baan B, VAN DER Hoek W. Chlamydia psittaci (psittacosis) as a cause of community-acquired pneumonia: a systematic review and meta-analysis. Epidemiol Infect. 2017;145:3096-3105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 145] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 4. | Cui Z, Meng L. Psittacosis Pneumonia: Diagnosis, Treatment and Interhuman Transmission. Int J Gen Med. 2023;16:1-6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 5. | Li W, Zhang Q, Xu Y, Zhang X, Huang Q, Su Z. Severe pneumonia in adults caused by Tropheryma whipplei and Candida sp. infection: a 2019 case series. BMC Pulm Med. 2021;21:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 6. | Wang S, Xia D, Wu J, Jia D, Li L, Xu S. Severe Pneumonia Caused by Infection With Tropheryma whipplei Complicated With Acinetobacter baumannii Infection: A Case Report Involving a Young Woman. Front Public Health. 2021;9:729595. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Dolmans RA, Boel CH, Lacle MM, Kusters JG. Clinical Manifestations, Treatment, and Diagnosis of Tropheryma whipplei Infections. Clin Microbiol Rev. 2017;30:529-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 105] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 8. | Kutlu O, Erhan SŞ, Gökden Y, Kandemir Ö, Tükek T. Whipple's Disease: A Case Report. Med Princ Pract. 2020;29:90-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Dutly F, Altwegg M. Whipple's disease and "Tropheryma whippelii". Clin Microbiol Rev. 2001;14:561-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 157] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 10. | Bousbia S, Papazian L, Auffray JP, Fenollar F, Martin C, Li W, Chiche L, La Scola B, Raoult D. Tropheryma whipplei in patients with pneumonia. Emerg Infect Dis. 2010;16:258-263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 78] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 11. | Lozupone C, Cota-Gomez A, Palmer BE, Linderman DJ, Charlson ES, Sodergren E, Mitreva M, Abubucker S, Martin J, Yao G, Campbell TB, Flores SC, Ackerman G, Stombaugh J, Ursell L, Beck JM, Curtis JL, Young VB, Lynch SV, Huang L, Weinstock GM, Knox KS, Twigg H, Morris A, Ghedin E, Bushman FD, Collman RG, Knight R, Fontenot AP; Lung HIV Microbiome Project. Widespread colonization of the lung by Tropheryma whipplei in HIV infection. Am J Respir Crit Care Med. 2013;187:1110-1117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 154] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 12. | Keita AK, Raoult D, Fenollar F. Tropheryma whipplei as a commensal bacterium. Future Microbiol. 2013;8:57-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | García-Álvarez L, Pérez-Matute P, Blanco JR, Ibarra V, Oteo JA. High prevalence of asymptomatic carriers of Tropheryma whipplei in different populations from the North of Spain. Enferm Infecc Microbiol Clin. 2016;34:340-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Wilson MR, Naccache SN, Samayoa E, Biagtan M, Bashir H, Yu G, Salamat SM, Somasekar S, Federman S, Miller S, Sokolic R, Garabedian E, Candotti F, Buckley RH, Reed KD, Meyer TL, Seroogy CM, Galloway R, Henderson SL, Gern JE, DeRisi JL, Chiu CY. Actionable diagnosis of neuroleptospirosis by next-generation sequencing. N Engl J Med. 2014;370:2408-2417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 609] [Cited by in RCA: 678] [Article Influence: 61.6] [Reference Citation Analysis (0)] |
| 15. | Gaston DC, Miller HB, Fissel JA, Jacobs E, Gough E, Wu J, Klein EY, Carroll KC, Simner PJ. Evaluation of Metagenomic and Targeted Next-Generation Sequencing Workflows for Detection of Respiratory Pathogens from Bronchoalveolar Lavage Fluid Specimens. J Clin Microbiol. 2022;60:e0052622. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 98] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 16. | Fenollar F, Ponge T, La Scola B, Lagier JC, Lefebvre M, Raoult D. First isolation of Tropheryma whipplei from bronchoalveolar fluid and clinical implications. J Infect. 2012;65:275-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |