Published online Oct 16, 2023. doi: 10.12998/wjcc.v11.i29.7136
Peer-review started: June 12, 2023
First decision: August 16, 2023
Revised: August 27, 2023
Accepted: September 18, 2023
Article in press: September 18, 2023
Published online: October 16, 2023
Processing time: 123 Days and 0.6 Hours
Mucoepidermoid carcinoma of the lung is a rare malignant tumor, accounting for 0.1%–0.2% of all lung malignancies. It is a primary salivary gland tumor of the lung. Surgical resection is the primary treatment for pulmonary mucoepidermoid carcinoma, for which there has been no standardized treatment strategy. This article reports a case of a young woman with pulmonary mucoepidermoid carcinoma with hemoptysis as the first symptom.
A 24-year-old female patient presented with "4 d of hemoptysis" as the chief complaint. She had no special history and denied any smoking or drinking history. Physical examination revealed that the vital signs were stable and scattered small wet rales were heard in the left lung. After admission, the lung tumor markers were checked, and no abnormalities were found. After completing the bronchoscopy, a spherical lesion was observed at the main bronchus 1.5 cm away from the protubercle, with obvious pulsation and little blood seepage on the surface, and histopathological biopsy results showed acute and chronic inflammation. She was transferred to the Department of Thoracic Surgery for surgical treatment on the 16th day after admission. After exclusion of surgical conjunctures, the patient underwent resection of the tumor in the left main bronchus with single-pore video-assisted thoracic surgery on the 19th day after admission. The postoperative histopathological biopsy results showed mucoepidermoid carcinoma of the lung. The patient and her family refused to complete genetic testing and she was discharged from the hospital on the 8th day after surgery. During the follow-up period, the patient experienced shortness of breath after feeling active and had no special discomfort.
We have documented a case of moderately differentiated mucoepidermoid lung cancer with hemoptysis as the first symptom to improve clinicians' understanding of the disease and provide a new dimension of thinking for its future diagnosis and treatment.
Core Tip: Mucoepidermoid carcinoma of the lung is a rare tumor. This paper reports a young female patient who was diagnosed with mucoepidermoid carcinoma of the lung with hemoptysis as the first symptom to improve the clinician's understanding of the disease and to help its clinical diagnosis and treatment.
- Citation: Xie WX, Liu R, Li Z, Zhou PL, Duan LN, Fu DD. Mucoepidermoid carcinoma of the lung with hemoptysis as initial symptom: A case report. World J Clin Cases 2023; 11(29): 7136-7143
- URL: https://www.wjgnet.com/2307-8960/full/v11/i29/7136.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i29.7136
Pulmonary mucoepidermoid carcinoma (PMEC) was first reported by Smetana in 1952 as a rare malignant tumor of the lung[1]. It is the most common primary salivary gland carcinoma of the lung, originating from the small salivary gland in the submucosa of the atmospheric tract[2]. At present, there are few reports on PMEC, and most are case reports. Its clinical symptoms and epidemiological characteristics are not specific or typical. In addition, there is no specific treatment, and surgical excision remains the main treatment. Therefore, it is necessary to increase the attention of clinicians to the disease. This paper reports the case data of a young woman with hemoptysis as the first symptom who was clearly diagnosed with the disease after two lung histopathological biopsies and was discharged from the hospital after surgical treatment, aiming to provide some help for the diagnosis and treatment of the disease and improve the awareness of clinicians about it.
A 24-year-old female patient presented with "4 d of hemoptysis" as the chief complaint.
Bright red blood appeared 4 d prior, the amount was approximately 20 mL/d, accompanied by cough and sputum, and the sputum was a small amount of frothy sputum.
The patient had a good health previously and had no history of specific diseases.
The patient’s personal history and family history were unremarkable.
The patient’s vital signs were stable, and she had a clear mind and cooperated with the physical examination. The skin mucosa color was normal, and the superficial lymph nodes of the whole body were not enlarged. Bilateral respiratory movement was normal, palpation of both lungs was normal, percussive sound of both lungs was clear, small wet rales were heard in the left lung, and pleural fricative sounds were not heard on either side. No abnormal positive signs were found in the remaining physical examination.
D-dimer was 1.17 µg/mL, and hypersensitivity C-reactive protein was 32.88 mg/L. There were no obvious abnormalities in lung tumor markers, routine blood tests, coagulation function, liver function, renal function, or Mycobacterium tuberculosis culture.
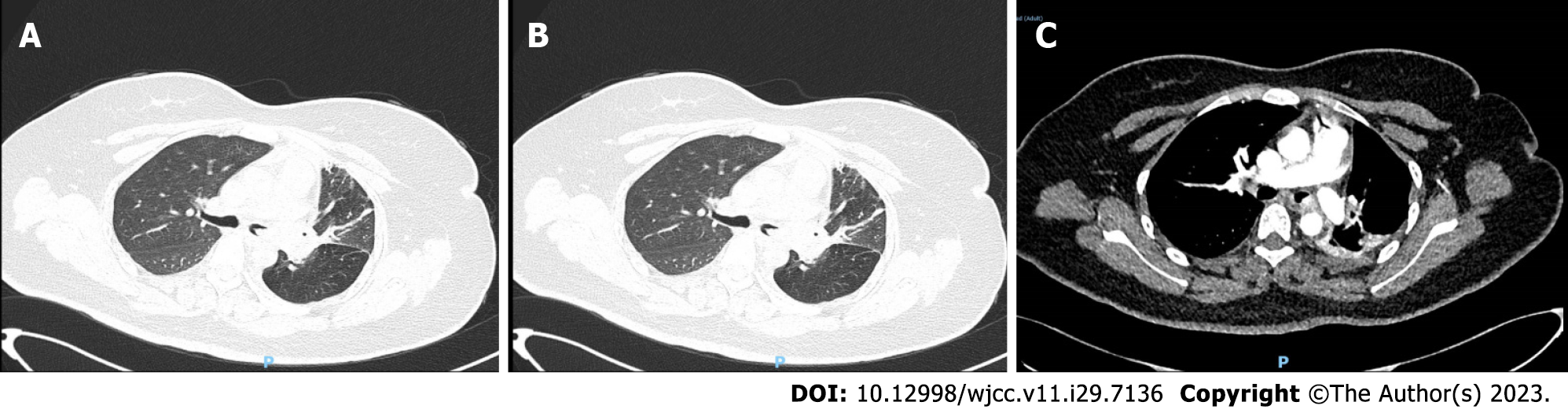
Chest computed tomography (CT) revealed left lung emphysema, atelectasis of part of the left lung, and part of the bronchus unclear (Figure 1).
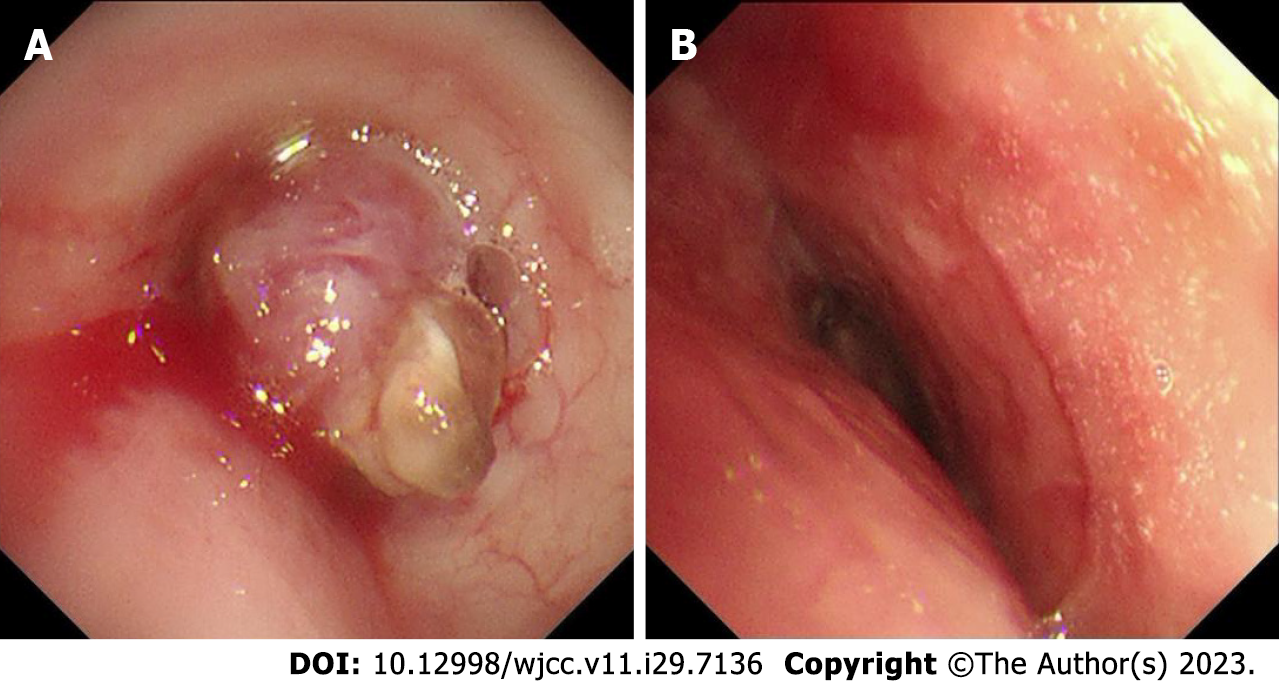
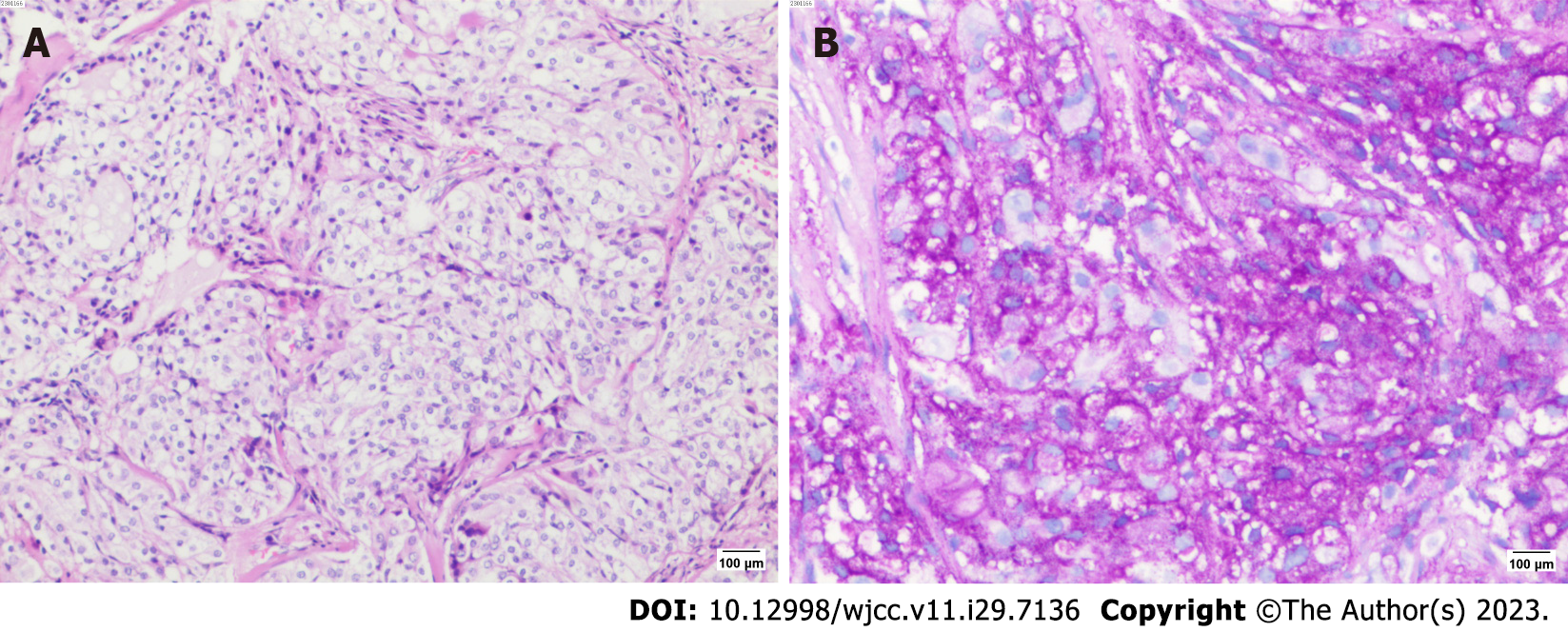
After admission, the patient was treated by hemostasis and anti-infection therapy, and no hemoptysis occurred. Contrast-enhanced CT of the chest showed that a left main bronchial nodule was seen (Figure 1). Increased soft tissue density shadows appeared in the left hilar area of the lung, and fiberoptic bronchoscopy was recommended. Left pulmonary obstructive emphysema and obstructive atelectasis and obstructive inflammation of the left lung were noted. Further electronic bronchoscopy showed that the main bronchus had a spherical lesion 1.5 cm from the protuberance, with pulsation and slight bleeding on the surface. The considerations were as follows: (1) The lesion in the left main bronchus was suspected to be a tumor; and (2) malignant stenosis of the left main bronchus (Figure 2) was present. The lung tumor markers improved and showed no abnormalities. Cytopathology and DNA ploidy analysis of alveolar lavage fluid (left main bronchoalveolar lavage fluid) showed no definite malignant cells. The results of bronchial tissue biopsy showed that the mucosa presented acute and chronic inflammatory changes, mucinous gland hyperplasia in the lamina mucosa, squamation of the columnar epithelium, and active proliferation of epithelial cells. Some necrotic tissues were also observed, among which small clusters of proliferative glandular epithelial cells were found, the cytoplasm was vacuolar, nucleoli were visible, and no mitotic image was observed. The immunohistochemical results were as follows: Carcinoembryonic antigen (CEA) (+), cytokeratin (CK) (+), CK5/6 (partially +), CK7 (+), P40 (-), TTF1 (-), and NapsinA (-). The automatic immunohistochemical results were as follows: CD117 (-) and Ki67 (5% +). Antacid staining was negative, while periodic acid-Schiff (PAS) was positive (Figure 3).
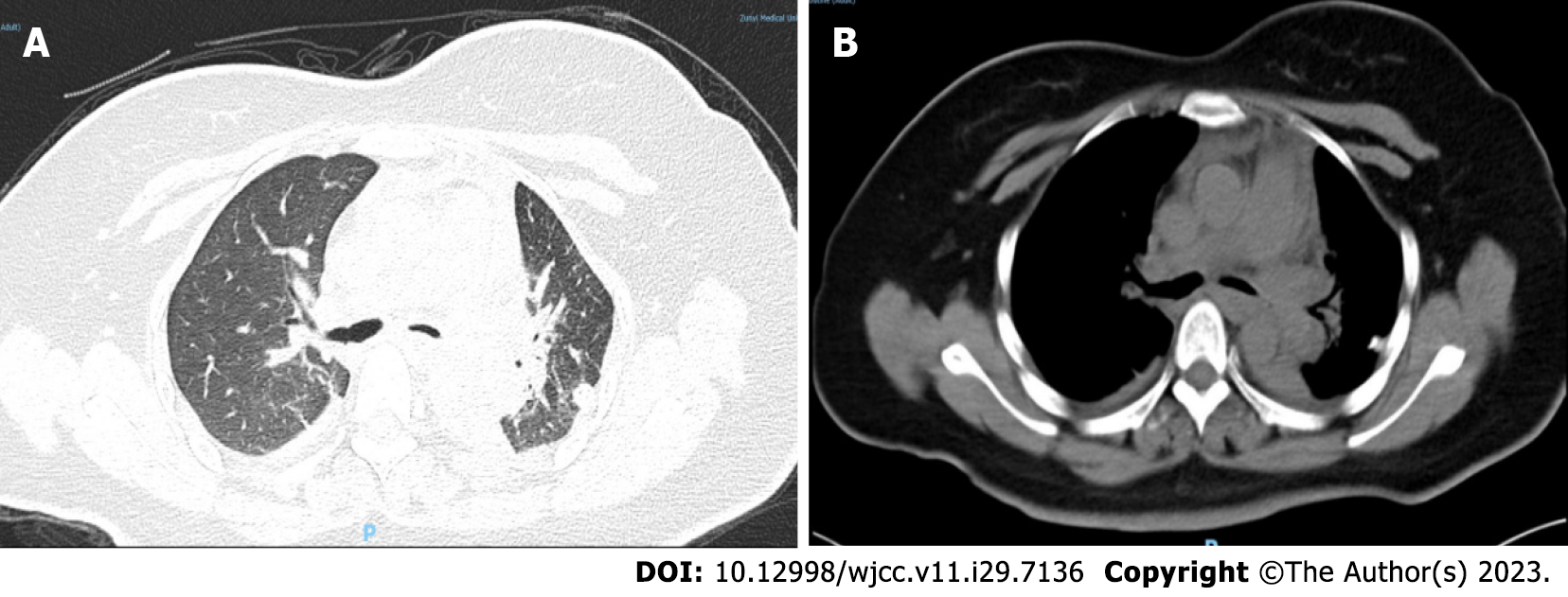
Based on the immunohistochemistry findings, the patient was transferred to the Department of Thoracic Surgery for surgical treatment on the 16th day after admission. After the exclusion of surgical contraindications, the patient underwent resection of the tumor in the left main bronchus by single-pore video-assisted thoracic surgery on the 19th day of admission (thoracotomy of the left main bronchus and trachea window for tumor resection and anastomosis). Rapid intraoperative pathological examination revealed neoplastic lesions (in the left main bronchus), which were likely to be malignant tumors from the salivating glands. Postoperative histopathological results showed the following: Lung mucoepidermoid carcinoma (in the left main bronchus), without vascular invasion or nerve invasion; group 4 lymph nodes (0/3), group 5 lymph nodes (0/1), group 10 lymph nodes (0/2), and no tumor metastasis; pTNM stage pT1bNOMX. Immunohistochemical results were: CK (+), CK5/6 (partially +), P40 (partially +), P63 (partially +), CK7 (+), CK-H (+), TTF1 (-), NapsinA (-), and CEA (focally +). The automatic immunohistochemical results included CD117 (partially +) and Ki67 (10% +). PAS staining was positive (Figure 4). Head MRI plain + enhanced scans and upper abdominal CT plain + enhanced scans showed no obvious abnormalities. After the operation, the patient was treated with anti-infection, hemostasis, cough suppressant and expectorant, anti-inflammatory treatment, and lung function recovery exercises. After repeat chest CT (Figure 5), the patient was discharged from the hospital on the 8th day after surgery.
After considering the pathological results of the patient's two tissue biopsies and imaging data, the pathologist believed that the lesion was a moderately differentiated pulmonary epidermal muxoid carcinoma, and the relevant genetic examination can be further performed to guide subsequent treatment. The radiologist believed that the imaging data of the patient in this case were not consistent with typical adenocarcinoma manifestations, and considering the rarity of the disease, the diagnosis was mainly based on tissue biopsy pathological nodes. The thoracic surgeon considered the patient to be a young woman, and the mass was removed locally. The lung tissue was preserved as much as possible, and close follow-up was performed to determine whether there was metastasis or recurrence. The respiratory surgeon believed that the patient should have a complete bronchoscopy at regular intervals after discharge to clarify the bronchial cavity, and reoperation could have been considered if necessary.
The final diagnosis of the presented case was PMEC.
After surgical treatment, anti-infection, hemostasis, cough suppressant and expectorant; anti-inflammatory treatments; lung function recovery exercises and other treatments were given, the patient improved and was discharged on the 8th day after surgery.
After surgical treatment, the patient was treated with anti-infection, hemostasis, cough suppressant and expectorant, anti-inflammatory treatments, lung function recovery exercises, etc., the patient improved and was discharged from the hospital on the 8th day after surgery. During the follow-up period, the patient experienced shortness of breath after feeling active and no special discomfort.
As the most commonly principal salivary gland cancer in the lungs, PMEC originates from the respiratory tract[2]. As a rare malignant lung tumor, it was first reported by Smetana et al[1] as early as 1952, and it was found that it accounted for less than 1% of malignant lung tumors[3-5]. Due to its rarity and lack of specific clinical and radiological features, the diagnosis is largely dependent on pathological examination. In addition, PMEC should be distinguished from adenosquamous carcinoma, especially in tiny biopsy specimens obtained by fibrobronchoscopy or lung puncture. It is defined as a tumor composed of mucoepidermoid cells, epidermoid cells, and intermediate cells[6]. At present, there is no unified standard for treatment, and surgical resection is considered the main treatment[7].
As the most general malignant tumor in the salivary glands, mucoepidermoid carcinoma has an incidence of 0.44/100000 people[8]. Although it can be appeared at every age, it occurs mostly between the ages of 35 and 65, with approximately 60% of cases occurring in women. The large salivary glands are frequently involved and very rarely (less than 1%) it occurs in the lung[9]. Patients with PMEC often have no specific clinical symptoms. Obstructive airway symptoms, mainly cough, dyspnea, or asthma, are usually manifested in tumors located in the central bronchus; 85% of PMECs are reported to be peripheral to the lung, characterized by cough, chest pain, and lung inflammation. Some asymptomatic patients are found during physical examination[7]. Therefore, there are challenges in the diagnosis of this disease. It is therefore necessary to improve the understanding of clinicians to achieve early detection and early and accurate treatment.
In this case, hemoptysis was the first symptom, and chronic inflammatory changes were considered after the first bronchial histopathologic biopsy. However, the immunohistochemical results suggested CEA (focally +), CK (+), CK5/6 (partially +), CK7 (+), and Ki67 (5% +), and PAS staining was positive. Considering the abnormal immunohistochemistry findings, a tumor could not be excluded. PMEC was clearly diagnosed after surgical resection and pathological biopsy. Immunohistochemistry plays an important role in the diagnosis of this disease.
The diagnosis of PMEC mainly depends on histopathological and immunohistochemical examination, and it is histopathologically composed of squamous epithelial cells, mucous cells, and intermediate cells with keratosis defects. PMEC is classified into low-grade and high-grade tumors based on histological features, mitotic frequency, cell atypia, and degree of necrosis[5]. The survival rate of high-grade mucoepidermoid carcinoma was significantly lower than that of low-grade mucoepidermoid carcinoma, and the possibility of metastasis to lymph nodes of high-grade mucoepidermoid carcinoma was found to be 10 times higher than that of low-grade mucoepidermoid carcinoma[10]. In general, PMEC tumors appear as tan or light brown polypoid masses. The central bronchus may exhibit exoplastic tumors that almost completely obstruct the bronchial lumen[11]. The immunohistochemical characteristics of PMEC were retrospectively analyzed and summarized, and the positive rates of P63, CK7, MUC5AC, P40, and CK5/6 were 58/58 (100%), 33/33 (100%), 26/26 (100%), 52/54 (96.3%), and 3/6 (50%), respectively[7]. However, some studies do not support this conclusion. Zhang et al[12] reported that TTF-1 and Napsin A were positive in some PMEC cases, and one paper reported that trastuzumab treatment was effective in metastatic PMEC patients with positive HER2 expression[13]. In addition, some scholars found that the Ki-67 index in low-grade cases was lower than that in high-grade cases and proposed that the Ki-67 index might be used as one of the indicators to distinguish PMEC malignancy[14]. At present, most studies are mainly case reports or case series and there are little case data, so the number of studies needs to be expanded for further research.
Studies have shown that the tumor is associated with the t(11;19)(q21-22;p13) translocation, which is associated with the MECT1-MAML2 fusion[15], and some scholars have found that this fusion gene can not only activate HES1 transcription, thus destroying the Notch signaling pathway, but it also activates the protein CREB, thus simulating the activation of cyclic adenosine monophosphate signaling[16,17]. Some scholars proposed that gene fusion could be used as a diagnostic basis for PMEC because it was found to exist in most patients with this disease[7]. In addition, some studies found that gene fusion was often found in low-grade groups[18,19]. At present, most of the genes related to this disease are limited to fusion genes. There are also studies using comprehensive genome amplification to study a small number of high-grade PMECs, and it was found that most patients have at least one gene mutation, and the most common genomic changes occur in CDKN2A and TP53. However, the reliability of this study remains questionable due to the small sample size[20].
More interestingly, EGFR overexpression is present in most PMEC cases, but amplification or mutation of the tyrosine kinase region of the EGFR gene is rarely reported[18].
At present, there is no unified standard for the treatment of PMEC, and the main treatment is surgical excision. The effect of chemoradiotherapy is controversial. There have also been reports of cases effectively treated by chemotherapy, such as apatinib combined with graded stereotaxic radiotherapy[14], carboplatin combined with paclitaxel[21], and EGFR-tyrosine kinase inhibitor drug therapy[22]. However, there are individual differences due to the majority of the literature being case reports, and further research is required to define the specific curative effect. Studies show that PMEC is a fairly inert tumor with a relatively optimistic prognosis, which has a better survival rate compared with small cell lung cancer and non-small cell lung cancer. The 5-year survival rate of PMEC is approximately 45%–70%[5]. Mucoepidermoid carcinoma usually involves the proximal bronchus. Therefore, the typical symptoms of mucoepidermoid carcinoma are bronchial obstruction, resulting in such as cough, hemoptysis, asthma, and fever[23]. Salivary gland tumors are rare primary lung lesions. The most common primary salivary gland tumors in the lung mainly include mucoepidermoid carcinoma, adenoid cystic carcinoma, and epithelial-myoepithelial carcinoma. Their morphology, immunophenotype, and molecular characteristics are similar to those in the head and neck or other sites. Because of their rarity, research is often limited, and relevant studies are usually small or limited to individual cases. Fortunately, molecular changes such as MAML2 rearrangement in mucoepidermoid carcinoma of the lung, MYB rearrangement in adenoid cystic carcinoma and clear cell carcinoma, and EWSR1 rearrangement in myoepithelial tumors have been found[2]. These molecular changes help to distinguish salivary gland tumors from other lung tumors to a certain extent and will provide great help for the diagnosis and treatment of this disease.
PMEC is a rare malignant tumor with no specific clinical symptoms. The diagnosis of PMEC mainly relies on pathological and immunohistochemical examination. The diagnosis of the patient in this case was confirmed by two biopsies. Reporting this case is expected to improve the diagnosis rate of this disease to achieve early treatment.
PMEC is a rare malignant tumor, and its clinical symptoms often have no specific manifestations. Diagnosis of the disease mainly relies on pathological and immunohistochemical examination. In this case, two tissue biopsies were performed to confirm the disease. When there is unexplained hemoptysis in a young patient, the disease should be considered, and diagnostic work-up should be carried out as soon as possible to confirm the diagnosis.
First of all, I would like to thank the thoracic surgeons and endoscopy doctors for their help in the treatment of the patient. I would also like to thank the pathologists and imaging department for their relevant information. Last but not least, I would like to thank the patient for her trust in our hospital.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Respiratory system
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hasan A, Egypt S-Editor: Liu JH L-Editor: Wang TQ P-Editor: Liu JH
| 1. | Smetana HF, Iverson L, Swan LL. Bronchogenic carcinoma; an analysis of 100 autopsy cases. Mil Surg. 1952;111:335-351. [PubMed] |
| 2. | Roden AC. Recent updates in salivary gland tumors of the lung. Semin Diagn Pathol. 2021;38:98-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 3. | Song Z, Liu Z, Wang J, Zhu H, Zhang Y. Primary tracheobronchial mucoepidermoid carcinoma--a retrospective study of 32 patients. World J Surg Oncol. 2013;11:62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 4. | Wang Y, Cai S, Xue Q, Mu J, Gao Y, Tan F, Mao Y, Wang D, Zhao J, Gao S, He J. Treatment outcomes of patients with tracheobronchial mucoepidermoid carcinoma compared with those with adenoid cystic carcinoma. Eur J Surg Oncol. 2020;46:1888-1895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Hsieh CC, Sun YH, Lin SW, Yeh YC, Chan ML. Surgical outcomes of pulmonary mucoepidermoid carcinoma: A review of 41 cases. PLoS One. 2017;12:e0176918. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 6. | Shen W, Yang T, Fan Y, Li X, Ai C, Wang X, Wang D, Zhou X. Pulmonary mucoepidermoid carcinoma: A clinicopathological study of 45 patients. Thorac Cancer. 2022;13:2385-2389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 7. | Hu S, Gong J, Zhu X, Lu H. Pulmonary Salivary Gland Tumor, Mucoepidermoid Carcinoma: A Literature Review. J Oncol. 2022;2022:9742091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 8. | Devaraju R, Gantala R, Aitha H, Gotoor SG. Mucoepidermoid carcinoma. BMJ Case Rep. 2014;2014:bcr-2013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 9. | Falk N, Weissferdt A, Kalhor N, Moran CA. Primary Pulmonary Salivary Gland-type Tumors: A Review and Update. Adv Anat Pathol. 2016;23:13-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 81] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 10. | Park G, Lee SW. Postoperative radiotherapy for mucoepidermoid carcinoma of the major salivary glands: long-term results of a single-institution experience. Radiat Oncol J. 2018;36:317-324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Ishizumi T, Tateishi U, Watanabe SI, Matsuno Y. Mucoepidermoid carcinoma of the lung: high-resolution CT and histopathologic findings in five cases. Lung Cancer. 2008;60:125-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Zhang XP, Hu PZ, Shen SS, Li XY. [Clinical characteristics and prognostic analyses of 87 patients with pulmonary mucoepidermoid carcinoma]. Zhonghua Zhong Liu Za Zhi. 2018;40:452-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 13. | Haddad R, Colevas AD, Krane JF, Cooper D, Glisson B, Amrein PC, Weeks L, Costello R, Posner M. Herceptin in patients with advanced or metastatic salivary gland carcinomas. A phase II study. Oral Oncol. 2003;39:724-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 183] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 14. | Yan H, Li X, Peng Y, Zhang P, Zou N, Liu X. Apatinib and fractionated stereotactic radiotherapy for the treatment of limited brain metastases from primary lung mucoepidermoid carcinoma: A case report. Medicine (Baltimore). 2020;99:e22925. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Achcar Rde O, Nikiforova MN, Dacic S, Nicholson AG, Yousem SA. Mammalian mastermind like 2 11q21 gene rearrangement in bronchopulmonary mucoepidermoid carcinoma. Hum Pathol. 2009;40:854-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 57] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 16. | Tonon G, Modi S, Wu L, Kubo A, Coxon AB, Komiya T, O'Neil K, Stover K, El-Naggar A, Griffin JD, Kirsch IR, Kaye FJ. t(11;19)(q21;p13) translocation in mucoepidermoid carcinoma creates a novel fusion product that disrupts a Notch signaling pathway. Nat Genet. 2003;33:208-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 431] [Cited by in RCA: 415] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 17. | Wu L, Liu J, Gao P, Nakamura M, Cao Y, Shen H, Griffin JD. Transforming activity of MECT1-MAML2 fusion oncoprotein is mediated by constitutive CREB activation. EMBO J. 2005;24:2391-2402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 115] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 18. | Huo Z, Wu H, Li J, Li S, Wu S, Liu Y, Luo Y, Cao J, Zeng X, Liang Z. Primary Pulmonary Mucoepidermoid Carcinoma: Histopathological and Moleculargenetic Studies of 26 Cases. PLoS One. 2015;10:e0143169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 19. | Salem A, Bell D, Sepesi B, Papadimitrakopoulou V, El-Naggar A, Moran CA, Kalhor N. Clinicopathologic and genetic features of primary bronchopulmonary mucoepidermoid carcinoma: the MD Anderson Cancer Center experience and comprehensive review of the literature. Virchows Arch. 2017;470:619-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 20. | Wang K, McDermott JD, Schrock AB, Elvin JA, Gay L, Karam SD, Raben D, Somerset H, Ali SM, Ross JS, Bowles DW. Comprehensive genomic profiling of salivary mucoepidermoid carcinomas reveals frequent BAP1, PIK3CA, and other actionable genomic alterations. Ann Oncol. 2017;28:748-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 21. | Sonobe S, Inoue K, Tachibana S, Shiojiri M, Maeda T, Nakanishi N, Moritaka T, Ikura Y, Kawaguchi T. A case of pulmonary mucoepidermoid carcinoma responding to carboplatin and paclitaxel. Jpn J Clin Oncol. 2014;44:493-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Rossi G, Sartori G, Cavazza A, Tamberi S. Mucoepidermoid carcinoma of the lung, response to EGFR inhibitors, EGFR and K-RAS mutations, and differential diagnosis. Lung Cancer. 2009;63:159-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Alsidawi S, Morris JC, Wikenheiser-Brokamp KA, Starnes SL, Karim NA. Mucoepidermoid carcinoma of the lung: a case report and literature review. Case Rep Oncol Med. 2013;2013:625243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |