Published online Oct 16, 2023. doi: 10.12998/wjcc.v11.i29.7091
Peer-review started: August 21, 2023
First decision: September 4, 2023
Revised: September 12, 2023
Accepted: September 22, 2023
Article in press: September 22, 2023
Published online: October 16, 2023
Processing time: 53 Days and 4.9 Hours
The treatment of multiple myeloma has significantly progressed over the past half-century. The purpose of this study was to perform a systematic review and meta-analysis in order to explore the efficacy and safety of daratumumab in treating multiple myeloma.
To explore the efficacy and safety of daratumumab in treating multiple myeloma.
A systematic literature search was performed using Chinese and English databases, including the China National Knowledge Infrastructure, Wanfang, China Biology Medicine, VIP, the Cochrane Library, Embase, and PubMed. The search encompassed studies in treating multiple myeloma with daratumumab, spanning from the inception of the database to June 2023. Revman 5.1 software was used for analysis.
Our analysis included eight English articles and one Chinese article of high quality. The meta-analysis results indicated that compared to other therapies, daratumumab could improve the overall response rate (ORR) [odds ratio (OR) = 2.67, 95% confidence interval (CI) = 2.01, 3.53, Z = 6.85, P < 0.00001], complete remission (CR) (OR = 2.87, 95%CI = 2.16, 3.83, Z = 7.23, P < 0.00001) and progression-free survival (PFS) time (hazard ratio = 0.48, 95%CI = 0.38,0.60, Z = 6.54, P < 0.00001) in patients with multiple myeloma. These differences were statistically significant. Additionally, these results suggested that daratumumab increases the risk of neutropenia and thrombocytopenia with minimal effect on the incidences of anemia and upper respiratory tract infections.
Daratumumab can improve ORR, CR rate, and PFS in patients with multiple myeloma. It also increases the risk of neutropenia and thrombocytopenia, necessitating careful monitoring during its clinical application.
Core Tip: Daratumumab demonstrates promising efficacy in treating multiple myeloma, improving the overall response rate, complete remission, and progression-free survival time, compared to other therapies. However, it is also associated with an increased risk of neutropenia and thrombocytopenia. Clinicians should closely monitor their patients for these adverse effects. Further studies are needed to explore optimal dosing and combination therapies to maximize the benefits of daratumumab in treating multiple myeloma.
- Citation: Wang P, Jin SY. Meta-analysis of the efficacy and safety of daratumumab in the treatment of multiple myeloma. World J Clin Cases 2023; 11(29): 7091-7100
- URL: https://www.wjgnet.com/2307-8960/full/v11/i29/7091.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i29.7091
Multiple myeloma (MM) is characterized by kidney damage, hypercalcemia, bone destruction, and anemia, collectively abbreviated as CRAB. At present, it has become the second most common hematological disease, after lymphoma, with an incidence rate of 1% among all tumors and affecting 4.5-6 individuals per 100000 people. Furthermore, the incidence rate continues to rise annually, and it is increasingly affecting younger individuals. MM is a malignant hematological tumor that originates from the accumulation of monoclonal plasma cells in the bone marrow. Currently, numerous developed and developing countries are experiencing a gradual shift towards aging societies, which has inevitably led to the rising incidence of MM. Recent years have seen significant advancements in tumor-related research with the development of social, scientific, and technological progress and continuous improvements in medical technology. This progress in tumor-related research has focused on exploring and elucidating the specific components and mechanisms of action of clinical drugs to promote the development of modern medicine. MM is associated with several risk factors, including sex, race, age, and family history. Treatment options for MM are diverse, including medication, surgery, and immunotherapy, with medication being the most commonly employed approach.
In the early 1960s, MM was mainly treated with cyclophosphamide, glucocorticoids, alkylating agents, and other drugs, and the median survival time of patients during that era was short. Since the 20th century, the emergence of immunomodulators and proteasome inhibitors has effectively extended the median survival time of patients with MM. Notably, after 2015, the United States Food and Drug Administration successively approved a range of drugs, including CD38 antibody (daratumumab), CS1 monoclonal antibody, and second-generation proteasome inhibitors, ushering in a new era in MM treatment. Daratumumab, a human monoclonal antibody, targets CD38 on the surface of malignant plasma cells in patients with MM and effectively destroys tumor cells through various mechanisms[1]. In July 2019, the Chinese Drug Administration approved the use of daratumumab in the country. With the continuous emergence of relevant research, we have systematically organized relevant clinical data on the use of daratumumab for treating MM from both domestic and international sources to conduct a systematic evaluation. Our aim was to establish a definitive guiding framework for the clinical application of daratumumab in MM treatment.
Two researchers conducted a comprehensive search of the Chinese and English databases of the China National Knowledge Infrastructure (CNKI), Wanfang, China Biology Medicine (CBM), VIP, Embase, PubMed, and Cochrane Library using computerized methods to search for clinical randomized controlled trials (RCTs) involving the use of daratumumab in MM treatment. The search encompassed studies available from the inception up to June 2023. Additionally, the included references were reviewed to identify any relevant studies that might have been missed. The search terms used were “Darzalex,” “Daratumumab,” and “Multiple Myeloma.” The primary screening of the retrieved articles was performed using the Endnote X9 software. Literatures that did not meet the inclusion criteria were excluded after reviewing their titles and abstracts. To prevent missed detections, full texts were read to determine their suitability for inclusion. When the two researchers had disagreements regarding the inclusion of an article, a third researcher was consulted to resolve the disagreement.
(1) Randomized controlled trial that investigated the use of daratumumab in MM treatment; (2) Research participants comprised patients diagnosed with MM, with no limitations based on their age, sex, and ethnicity; (3) The intervention measures for the experimental group should include daratumumab, whereas those for the control group should not be limited (immunomodulators, proteasome inhibitors, or hormonal drugs may be used); (4) Outcome indicators included: overall response rate (ORR), complete response (CR) rate, progression-free survival (PFS) time, or the incidence of related adverse reactions during treatment; (5) Studies were conducted on duplicate articles or reports with the most comprehensive data included; and (6) The original data were recorded in detail; there was no difference in the general situation.
(1) Non-RCT studies, such as case-control studies, reviews, animal experiments, and case reports; (2) Repetitively published data or articles; and (3) Studies that did not include the required outcome indicators.
The included articles were independently reviewed by two researchers, and extracted the following literature information: title, first author's name, clinical trial launch time, publication time, names, age, treatment method, and outcome indicators.
Two reviewers independently used Cochrane Rob 2.0 Bias Risk Assessment Tool to evaluate the quality and crosscheck the included RCT studies. The assessment included the following aspects: (1) Bias in the randomization process reflecting the generation of random sequences of articles and the concealment of allocation; (2) bias related to blinding, indicating whether the tested patients and intervention executors were adequately blinded to the intervention; (3) bias in outcome measurement, reflecting whether the outcome evaluators were sufficiently blinded; (4) bias related to missing outcome data reflecting the presence of follow-up bias and completeness of outcome data; (5) bias in selective reporting of results; and (6) overall bias of the risk assessment for individual RCT outcomes based on the results of the above five bias assessments.
Handle methods of data loss: If data were not available, the original author was contacted to obtain the required data, or the study was excluded if contact with the author was unsuccessful or the required data remained unavailable.
The included studies were subjected to meta-analysis using Revman 5.1 software, and a forest plot was generated to visually represent the results of the meta-analysis. A funnel plot was used to assess the presence of publication bias and the sensitivity analysis was used to assess the stability of the results. A P value of < 0.05 indicated that the results had statistically significant differences. For counting data, the odds ratio (OR) and 95% confidence interval (CI) were used to determine the magnitude of the effect. A quantitative evaluation of heterogeneity was conducted based on I2. The low, medium, and high heterogeneities were defined as 25%, 50%, and 75%, respectively[2]. An I2 < 50% indicated no significant heterogeneity, and a fixed-effects model was used for the meta-analysis. For an I2 > 50%, a sensitivity analysis was used to rule out individual studies to investigate the source of heterogeneity. When the number of studies was less than three, a corresponding qualitative description was conducted[3].
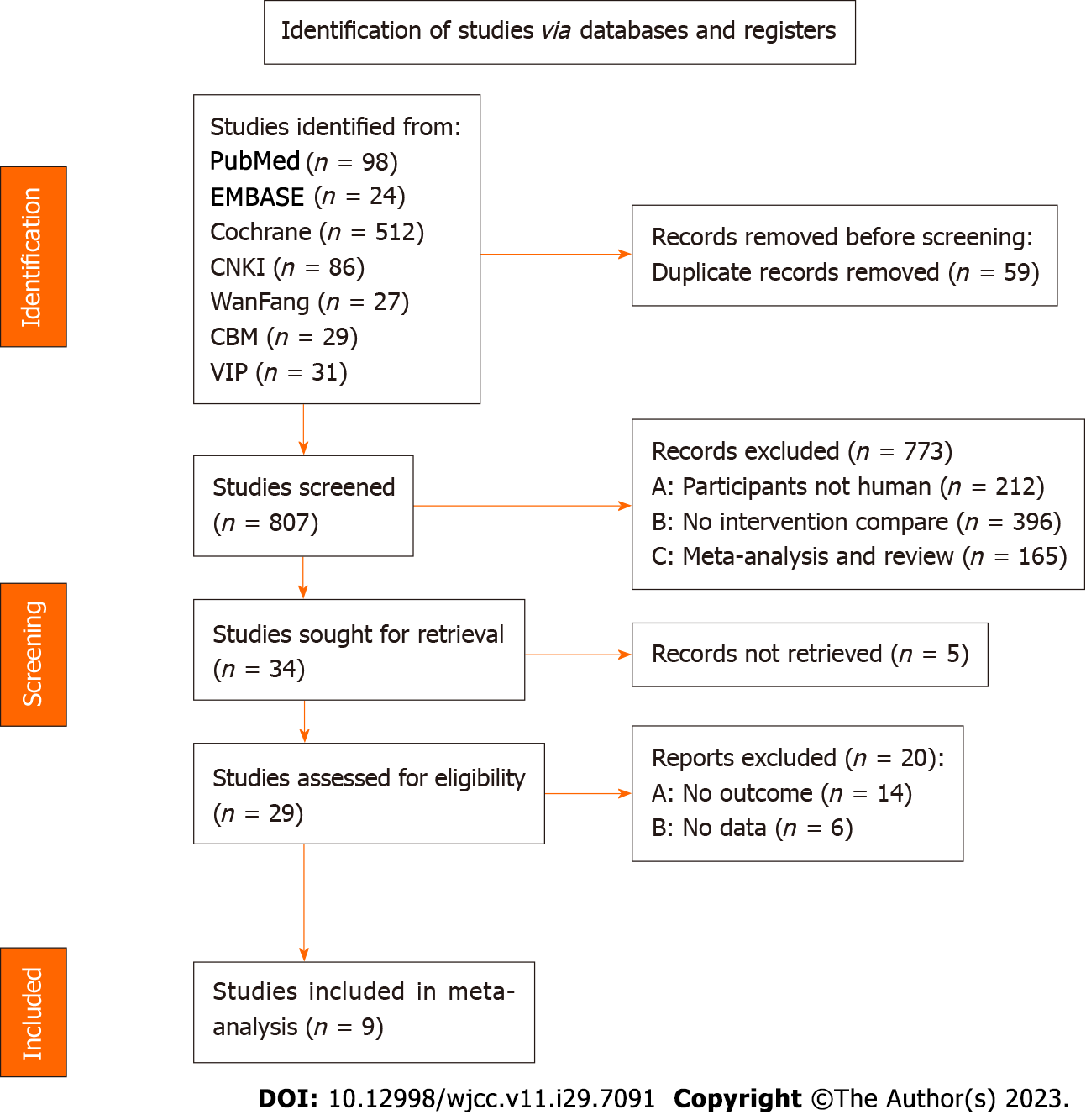
According to the established search terms and strategies, 807 studies were retrieved from seven literature databases, including 86 studies from CNKI, 27 from Wanfang, 31 from VIP, 29 from CBM, 98 from PubMed, 512 from Cochrane Library, and 24 from Embase. The remaining 34 studies were subjected to re-examination and preliminary screening, and 9 studies were included after the full text was read. A screening flowchart is depicted in Figure 1, and the baseline date of the literature is presented in Table 1[4-12].
| Ref. | Year | Interventions | Number of examples | Outcome indicators | ||
| Experimental | Control | Experimental | Control | |||
| Bahlis et al[4], POLLUX | 2020 | Lenalidomide + dexamethasone + Daratumumab | Lenalidomide + dexamethasone | 368 | 369 | ORR, CR, adverse reactions |
| Dimopoulos et al[5], CANDOR | 2020 | Caffezomib + Dex + Daratumumab | Caffezomib + Dex | 312 | 154 | PFS, ORR, CR, adverse reactions |
| Dimopoulos et al[6], APOLLO | 2021 | Poloxamer + dexamethasone + Daratumumab | Poloxamer + Dex | 151 | 153 | PFS, ORR, CR, adverse reactions |
| Facon et al[7], MAIA | 2021 | Lenalidomide + dexamethasone + Daratumumab | Lenalidomide + dexamethasone | 368 | 369 | PFS, ORR, CR, adverse reactions |
| Mateos et al[8], CASTOR | 2020 | Bortezomib + dexamethasone + Daratumumab | Bortezomib + dexamethasone | 251 | 247 | PFS, ORR, CR, adverse reactions |
| Mateos et al[9], ALCYONE | 2020 | Bortezomib + melphalan + prednisone + Daratumumab | Bortezomib + melphalan + prednisone | 346 | 354 | PFS, ORR, CR, adverse reactions |
| Moreau et al[10], CASSIOPEIA | 2019 | Bortezomib + thalidomide + dexamethasone + Daratumumab | Bortezomib + thalidomide + dexamethasone | 543 | 542 | PFS, ORR, CR, adverse reactions |
| Voorhees et al[11], GRIFFIN | 2020 | Lenalidomide + bortezomib + dexamethasone + Daratumumab | Lenalidomide + bortezomib + dexamethasone | 99 | 97 | ORR, CR, adverse reactions |
| Yan et al[12] | 2021 | Bortezomib + cyclophosphamide/doxorubicin + dexamethasone + Daratumumab | Bortezomib + cyclophosphamide/doxorubicin + dexamethasone | 64 | 51 | CR, adverse reactions |
One of the nine studies adopted a good randomization method, whereas the other eight studies did not specifically describe the randomization method. None of the nine studies reported a blinded intervention, which may have introduced a bias in the results. As eight studies provided comprehensive information on the loss of follow up, the bias associated with data loss and selective reporting was low. Overall, the bias assessment suggested that the included studies had a general level of bias; the detailed bias risks are depicted in Figure 2.
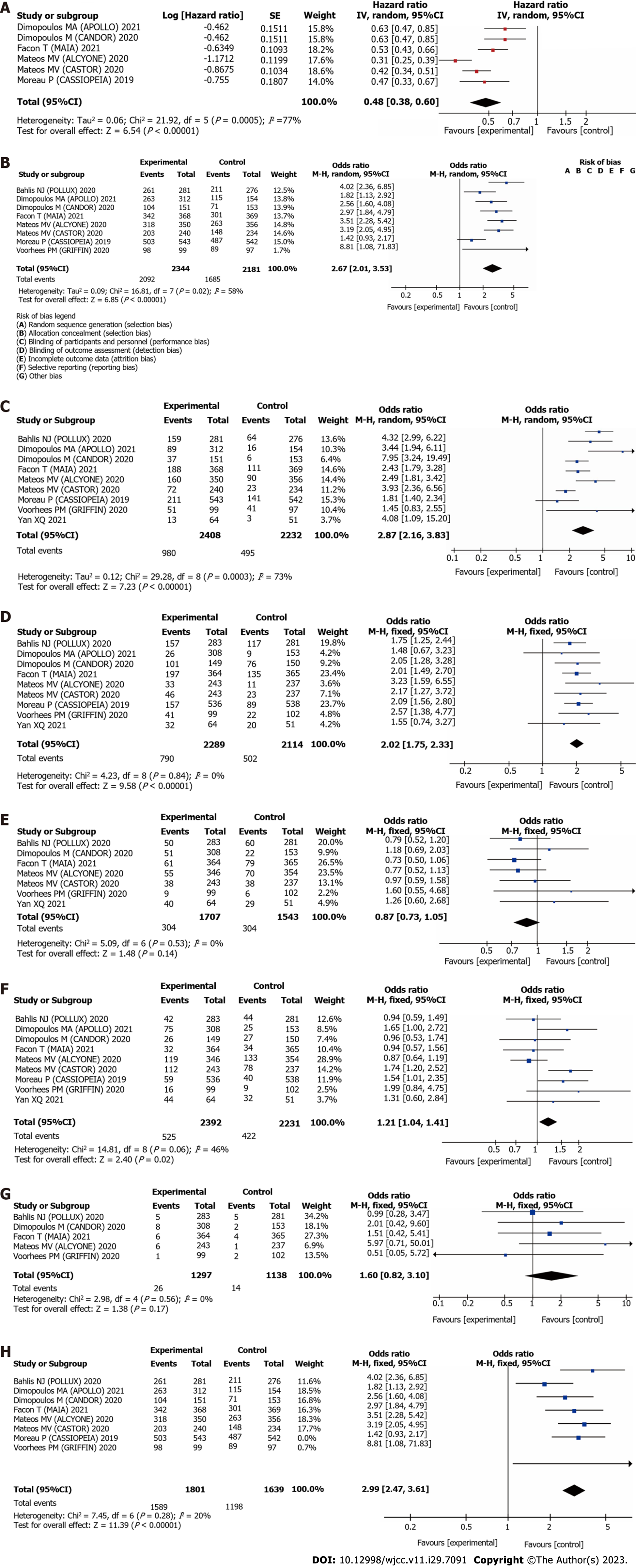
PFS: Six studies reported the effects of daratumumab on post-treatment PFS. The meta-analysis results indicated that the use of daratumumab could prolong PFS of patients with MM compared to conventional therapy alone (HR = 0.48, 95%CI = 0.38–0.60), as well as reduce the risk of MM by 52%, indicating a statistically significant difference (P < 0.00001), with I2 = 77%, exceeding the threshold of 50%. Using random effects model to analyze, as depicted in Figure 3A.
ORR: Eight studies reported the impact of daratumumab on ORR. The meta-analysis results indicated that the use of daratumumab in the experimental group significantly improved ORR of patients with MM compared with that of patients in the control group (OR = 2.67, 95%CI = 2.01–3.53), indicating a statistically significant difference (P < 0.00001), with I2 = 58%, exceeding the threshold of 50%. Using random effects model to analyze, as depicted in Figure 3B.
CR: Nine studies reported the impact of daratumumab on CR. The meta-analysis results revealed that compared with conventional treatment, daratumumab significantly improved CR among patients with MM (OR = 2.87, 95%CI = 2.16–3.83), indicating a statistically significant difference (P < 0.00001), with I2 = 73%, exceeding the threshold of 50%. Using random effects model to analyze, as depicted in Figure 3C.
Reduction incidence of neutrophils: Nine articles reported the impact of daratumumab on the incidence of neutropenia. The meta-analysis results revealed a statistically significant difference (OR = 2.02, 95%CI = 1.75–2.33, P < 0.00001), with I2 = 0%, below the threshold of 50%. Using fixed effects model to analyze, as depicted in Figure 3D.
Anemia: Seven studies reported the impact of daratumumab on the occurrence of anemia. The meta-analysis results revealed a statistically significant difference (OR = 0.87, 95%CI = 0.73–1.05, P = 0.14 > 0.05], with I2 = 0%, below the threshold of 50%. Using fixed effects model to analyze, as depicted in Figure 3E.
Incidence of thrombocytopenia: Nine articles reported the impact of daratumumab on the incidence of thrombocytopenia. The meta-analysis results revealed a statistically significant difference (OR = 1.21, 95%CI = 1.04–1.41, P = 0.02 < 0.05, with I2 = 46%, below the threshold of 50%. Using fixed effects model to analyze, as depicted in Figure 3F.
Incidence of upper respiratory tract infection: Five articles reported the impact of daratumumab on the occurrence of upper respiratory tract infections. The meta-analysis results revealed a statistically significant difference (OR = 1.60, 95%CI = 0.82–3.10, P = 0.17 > 0.05, with I2 = 0%, below the threshold of 50%. Using fixed effects model to analyze, as depicted in Figure 3G.
Sensitivity analysis: In the meta-analysis of ORR and CR results, I2 exceeded 50%, indicating a significant level of heterogeneity, and a sensitivity analysis was performed to investigate the source of heterogeneity. The results revealed that upon excluding the study conducted by Moreau P[10], I2 decreased to 20%, suggesting that heterogeneity stemmed from the concerned study. Further analysis indicated that the relevant studies included in this analysis were in their early stages, and the outcome data were incomplete. In the sensitivity analysis of CR, heterogeneity was not significantly reduced upon the exclusion of individual studies; however, the results remained stable. Considering the degree of clinical homogeneity, the results were considered credible and reliable (Figure 3H).
Publication bias detection: The funnel plots of all results revealed that the scattered points were uniform and symmetrical, indicating that there was minimal possibility of publication bias in the results (Figure 4).
MM is a common malignant tumor typically diagnosed in older patients, and its pathogenesis varies widely. Some scholars believe that its pathogenesis is attributed to the genetic mutations occurring during the differentiation of plasma cells derived from B lymphocytes[13]. In patients with MM, approximately 90% of the plasma cells exhibit cellular genetic abnormalities, including chromosomal translocation and abnormal gene fusion resulting from deoxyribonucleic acid strand breaks, leading to the pathological state of MM. These genetic abnormalities usually disrupt the normal functioning of the signaling pathways. Notably, the nuclear factor-kappaB (NF-κB) pathway directly affects the lymphocyte development, and the formation of lymphoma is closely related to the disorder of this pathway[14]. Unlike other types of malignant B-cell tumors, MM is most closely associated with the non-tumor-related NF-κB pathway. Genetic changes in MM have been reported to be closely associated with the activation of the mitogen-activated protein kinases pathway[15]. Bone marrow microenvironment is a functional network system composed of hematopoietic and non-hematopoietic cells. The pathogenesis of MM affects the relationship between the bone marrow microenvironment and MM cells, promoting the activation of signaling pathways and influencing the survival, development, migration, and drug resistance of MM cells[16]. This bone marrow invasion leads to anemia, and the weakened immune response can lead to recurrent infections[17].
Monoclonal antibodies have been recognized to play a role in treating various hematological malignancies[18], and anti-CD38 monoclonal antibodies have shown significant efficacy in MM treatment. CD38 is a transmembrane glycoprotein that carries several extracellular enzymes. Owing to its unique expression, CD38 serves as a phenotypic marker of complete differentiation. It also exhibits receptor and enzyme activities, as well as adhesion properties[19,20]. In addition, CD38 is not only expressed in T cells, natural killer cells, hematopoietic stem cells, and dendritic cells[21], but is also expressed in myeloma cells[22], which is an important reason why CD38 can become a therapeutic target for MM. Daratumumab, a human CD38 IgG1 monoclonal antibody, is capable of inducing apoptosis in MM cells through an Fc-dependent immune response mechanism, including complement-dependent cytotoxicity, antibody-dependent phagocytosis, antibody-dependent cell-mediated cytotoxicity, and direct apoptosis triggered by cross-linking reaction[23-25]. In addition, the inhibition of CD38 extracellular enzyme function induces direct apoptosis in MM cells. Furthermore, daratumumab modulates patients’ immune function and enhances the immune system’s ability to eliminate MM cells[22,26].
This meta-analysis included nine clinical studies, and the assessments of publication bias indicated minimal possibility of bias among these studies. The combined analysis of the results of PFS, ORR, and CR revealed that intervention with daratumumab could effectively improve the efficacy of treatment outcomes for patients in the experimental group, offering them significant benefits. For the evaluation of safety indicators, we focused on hematological and non-hematological adverse reactions. The incidences of neutropenia, anemia, and thrombocytopenia were selected as indicators for hematological adverse reactions, and upper respiratory tract infection was selected as a representative indicator for non-hematological adverse reactions. After conducting a meta-analysis for safety indicators, the findings suggested that the use of daratumumab increased the incidence of neutropenia and thrombocytopenia, but did not have a significant effect on the incidence of anemia and upper respiratory tract infections.
In this study, the efficacy and safety of daratumumab in treating MM were systematically evaluated. The results revealed that daratumumab could effectively prolong PFS of patients and improve ORR and CR rate, as well as lead to an increased incidence of neutropenia and thrombocytopenia. Therefore, appropriate preparatory measures should be taken during daratumumab administration. Most of the studies included in this meta-analysis had the characteristics of unclear randomization methods, unclear use of blind methods, and a small number of included studies. In the future, high-quality RCT trials will be required to provide supporting conclusions.
Daratumumab is a humanized anti-CD38 monoclonal antibody with broad-spectrum killing activity. Related studies have confirmed that Daratumumab can induce the rapid death of multiple myeloma (MM) cells through multiple pathways.
Nowadays, there are many studies on the clinical application of daratumumab in MM treatment, but there are few reviews and analyses, and the safety is still inconclusive.
To investigate the efficacy and safety of Daratumumab in treating multiple myeloma by meta-analysis.
A few relevant articles of this subject have been searched from the public databases. The data of the outcome indicators have been extracted from the articles. A meta-analysis has been performed for the pooling results.
The use of Daratumumab in the patients of multiple myeloma can improve the overall response rate and complete remission rate, and effectively increase the progression-free survival time of patients. It is noteworthy that the use of Daratumumab also increases the risk of neutrophils and thrombocytopenia, which should be noticed during clinical use.
Daratumumab has a good effect in treating multiple myeloma, but the safety needs to be confirmed by more studies.
To determine the efficacy indicator and safety indicator of Daratumumab in treating multiple myeloma, and make a meta-analysis of the summary results of the indicators.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Hematology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Palosaari S, Finland S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Afifi S, Michael A, Lesokhin A. Immunotherapy: A New Approach to Treating Multiple Myeloma with Daratumumab and Elotuzumab. Ann Pharmacother. 2016;50:555-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 2. | Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39087] [Cited by in RCA: 46536] [Article Influence: 2115.3] [Reference Citation Analysis (3)] |
| 3. | Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA; Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18487] [Cited by in RCA: 24848] [Article Influence: 1774.9] [Reference Citation Analysis (3)] |
| 4. | Bahlis NJ, Dimopoulos MA, White DJ, Benboubker L, Cook G, Leiba M, Ho PJ, Kim K, Takezako N, Moreau P, Kaufman JL, Krevvata M, Chiu C, Qin X, Okonkwo L, Trivedi S, Ukropec J, Qi M, San-Miguel J. Daratumumab plus lenalidomide and dexamethasone in relapsed/refractory multiple myeloma: extended follow-up of POLLUX, a randomized, open-label, phase 3 study. Leukemia. 2020;34:1875-1884. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 173] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 5. | Dimopoulos M, Quach H, Mateos MV, Landgren O, Leleu X, Siegel D, Weisel K, Yang H, Klippel Z, Zahlten-Kumeli A, Usmani SZ. Carfilzomib, dexamethasone, and daratumumab vs carfilzomib and dexamethasone for patients with relapsed or refractory multiple myeloma (CANDOR): results from a randomised, multicentre, open-label, phase 3 study. Lancet. 2020;396:186-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 311] [Article Influence: 62.2] [Reference Citation Analysis (0)] |
| 6. | Dimopoulos MA, Terpos E, Boccadoro M, Delimpasi S, Beksac M, Katodritou E, Moreau P, Baldini L, Symeonidis A, Bila J, Oriol A, Mateos MV, Einsele H, Orfanidis I, Ahmadi T, Ukropec J, Kampfenkel T, Schecter JM, Qiu Y, Amin H, Vermeulen J, Carson R, Sonneveld P; APOLLO Trial Investigators. Daratumumab plus pomalidomide and dexamethasone vs pomalidomide and dexamethasone alone in previously treated multiple myeloma (APOLLO): an open-label, randomised, phase 3 trial. Lancet Oncol. 2021;22:801-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 212] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 7. | Facon T, Kumar SK, Plesner T, Orlowski RZ, Moreau P, Bahlis N, Basu S, Nahi H, Hulin C, Quach H, Goldschmidt H, O'Dwyer M, Perrot A, Venner CP, Weisel K, Mace JR, Raje N, Tiab M, Macro M, Frenzel L, Leleu X, Ahmadi T, Wang J, Van Rampelbergh R, Uhlar CM, Tromp B, Delioukina M, Vermeulen J, Usmani SZ. Daratumumab, lenalidomide, and dexamethasone vs lenalidomide and dexamethasone alone in newly diagnosed multiple myeloma (MAIA): overall survival results from a randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22:1582-1596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 234] [Article Influence: 58.5] [Reference Citation Analysis (0)] |
| 8. | Mateos MV, Dimopoulos MA, Cavo M, Suzuki K, Jakubowiak A, Knop S, Doyen C, Lucio P, Nagy Z, Kaplan P, Pour L, Cook M, Grosicki S, Crepaldi A, Liberati AM, Campbell P, Shelekhova T, Yoon SS, Iosava G, Fujisaki T, Garg M, Chiu C, Wang J, Carson R, Crist W, Deraedt W, Nguyen H, Qi M, San-Miguel J; ALCYONE Trial Investigators. Daratumumab plus Bortezomib, Melphalan, and Prednisone for Untreated Myeloma. N Engl J Med. 2018;378:518-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 640] [Cited by in RCA: 736] [Article Influence: 105.1] [Reference Citation Analysis (0)] |
| 9. | Mateos MV, Cavo M, Blade J, Dimopoulos MA, Suzuki K, Jakubowiak A, Knop S, Doyen C, Lucio P, Nagy Z, Pour L, Cook M, Grosicki S, Crepaldi A, Liberati AM, Campbell P, Shelekhova T, Yoon SS, Iosava G, Fujisaki T, Garg M, Krevvata M, Chen Y, Wang J, Kudva A, Ukropec J, Wroblewski S, Qi M, Kobos R, San-Miguel J. Overall survival with daratumumab, bortezomib, melphalan, and prednisone in newly diagnosed multiple myeloma (ALCYONE): a randomised, open-label, phase 3 trial. Lancet. 2020;395:132-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 324] [Article Influence: 64.8] [Reference Citation Analysis (0)] |
| 10. | Moreau P, Attal M, Hulin C, Arnulf B, Belhadj K, Benboubker L, Béné MC, Broijl A, Caillon H, Caillot D, Corre J, Delforge M, Dejoie T, Doyen C, Facon T, Sonntag C, Fontan J, Garderet L, Jie KS, Karlin L, Kuhnowski F, Lambert J, Leleu X, Lenain P, Macro M, Mathiot C, Orsini-Piocelle F, Perrot A, Stoppa AM, van de Donk NW, Wuilleme S, Zweegman S, Kolb B, Touzeau C, Roussel M, Tiab M, Marolleau JP, Meuleman N, Vekemans MC, Westerman M, Klein SK, Levin MD, Fermand JP, Escoffre-Barbe M, Eveillard JR, Garidi R, Ahmadi T, Zhuang S, Chiu C, Pei L, de Boer C, Smith E, Deraedt W, Kampfenkel T, Schecter J, Vermeulen J, Avet-Loiseau H, Sonneveld P. Bortezomib, thalidomide, and dexamethasone with or without daratumumab before and after autologous stem-cell transplantation for newly diagnosed multiple myeloma (CASSIOPEIA): a randomised, open-label, phase 3 study. Lancet. 2019;394:29-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 680] [Cited by in RCA: 707] [Article Influence: 117.8] [Reference Citation Analysis (0)] |
| 11. | Voorhees PM, Rodriguez C, Reeves B, Nathwani N, Costa LJ, Lutska Y, Bobba P, Hoehn D, Pei H, Ukropec J, Qi M, Lin TS, Richardson PG. Daratumumab plus RVd for newly diagnosed multiple myeloma: final analysis of the safety run-in cohort of GRIFFIN. Blood Adv. 2021;5:1092-1096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 12. | Yan XQ, Yu J, Zhou X, Shen HY, Yang M. Curative effect of daratumumab on recurrent and refractory multiple myeloma and its influences on level of CD38 in marrow fluid. Xibu Yixue. 2021;33:1482-1485 Available from: http://www.xbyxqk.cn/xbyx/article/abstract/20211015?st=search. |
| 13. | Bianchi G, Munshi NC. Pathogenesis beyond the cancer clone(s) in multiple myeloma. Blood. 2015;125:3049-3058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 217] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 14. | Wang PP, Zhu DQ, Yang XY. Progress in Pathogenesis and Treatment of Multiple Myeloma. Zhongguo Yixue Chuangxin. 2023;20:164-168 Available from: http://www.zgyxcx.com/CN/abstract/abstract1594.shtml. |
| 15. | Walker BA, Boyle EM, Wardell CP, Murison A, Begum DB, Dahir NM, Proszek PZ, Johnson DC, Kaiser MF, Melchor L, Aronson LI, Scales M, Pawlyn C, Mirabella F, Jones JR, Brioli A, Mikulasova A, Cairns DA, Gregory WM, Quartilho A, Drayson MT, Russell N, Cook G, Jackson GH, Leleu X, Davies FE, Morgan GJ. Mutational Spectrum, Copy Number Changes, and Outcome: Results of a Sequencing Study of Patients With Newly Diagnosed Myeloma. J Clin Oncol. 2015;33:3911-3920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 403] [Cited by in RCA: 446] [Article Influence: 44.6] [Reference Citation Analysis (0)] |
| 16. | Hamedi KR, Harmon KA, Goodwin RL, Arce S. Autophagy and the Bone Marrow Microenvironment: A Review of Protective Factors in the Development and Maintenance of Multiple Myeloma. Front Immunol. 2022;13:889954. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 17. | Brigle K, Rogers B. Pathobiology and Diagnosis of Multiple Myeloma. Semin Oncol Nurs. 2017;33:225-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 122] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 18. | Lu RM, Hwang YC, Liu IJ, Lee CC, Tsai HZ, Li HJ, Wu HC. Development of therapeutic antibodies for the treatment of diseases. J Biomed Sci. 2020;27:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 698] [Cited by in RCA: 1296] [Article Influence: 259.2] [Reference Citation Analysis (0)] |
| 19. | van de Donk NWCJ, Richardson PG, Malavasi F. CD38 antibodies in multiple myeloma: back to the future. Blood. 2018;131:13-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 333] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 20. | Funaro A, Malavasi F. Human CD38, a surface receptor, an enzyme, an adhesion molecule and not a simple marker. J Biol Regul Homeost Agents. 1999;13:54-61. [PubMed] |
| 21. | Malavasi F, Deaglio S, Funaro A, Ferrero E, Horenstein AL, Ortolan E, Vaisitti T, Aydin S. Evolution and function of the ADP ribosyl cyclase/CD38 gene family in physiology and pathology. Physiol Rev. 2008;88:841-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 691] [Cited by in RCA: 660] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 22. | Krejcik J, Casneuf T, Nijhof IS, Verbist B, Bald J, Plesner T, Syed K, Liu K, van de Donk NW, Weiss BM, Ahmadi T, Lokhorst HM, Mutis T, Sasser AK. Daratumumab depletes CD38+ immune regulatory cells, promotes T-cell expansion, and skews T-cell repertoire in multiple myeloma. Blood. 2016;128:384-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 651] [Cited by in RCA: 736] [Article Influence: 81.8] [Reference Citation Analysis (0)] |
| 23. | Overdijk MB, Verploegen S, Bögels M, van Egmond M, Lammerts van Bueren JJ, Mutis T, Groen RW, Breij E, Martens AC, Bleeker WK, Parren PW. Antibody-mediated phagocytosis contributes to the anti-tumor activity of the therapeutic antibody daratumumab in lymphoma and multiple myeloma. MAbs. 2015;7:311-321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 381] [Cited by in RCA: 421] [Article Influence: 42.1] [Reference Citation Analysis (0)] |
| 24. | Hogan KA, Chini CCS, Chini EN. The Multi-faceted Ecto-enzyme CD38: Roles in Immunomodulation, Cancer, Aging, and Metabolic Diseases. Front Immunol. 2019;10:1187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 248] [Article Influence: 41.3] [Reference Citation Analysis (0)] |
| 25. | Overdijk MB, Jansen JH, Nederend M, Lammerts van Bueren JJ, Groen RW, Parren PW, Leusen JH, Boross P. The Therapeutic CD38 Monoclonal Antibody Daratumumab Induces Programmed Cell Death via Fcγ Receptor-Mediated Cross-Linking. J Immunol. 2016;197:807-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 185] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 26. | van de Donk NWCJ. Reprint of "Immunomodulatory effects of CD38-targeting antibodies". Immunol Lett. 2019;205:71-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |