Published online Oct 6, 2023. doi: 10.12998/wjcc.v11.i28.6967
Peer-review started: August 13, 2023
First decision: August 30, 2023
Revised: August 31, 2023
Accepted: September 12, 2023
Article in press: September 12, 2023
Published online: October 6, 2023
Processing time: 42 Days and 11.6 Hours
Gastric hamartomatous inverted polyps (GHIPs) are benign polyps of the gastric submucosal layer. Currently there are 52 reported cases in the English literature. According to a literature review, approximately 27% of GHIPs show a coexisting carcinoma.
A 66-year-old man was referred to our institution with ulcerative lesions detected on esophagogastroduodenoscopy (EGD) during a regular check-up. Other medical findings were nonspecific. The lesions had borderline histologic features that could not exclude malignancy and were followed up with three EGDs and biopsies at intervals of 3 mo. The latest biopsy was revealed as an adenocarcinoma. A total gastrectomy was performed to remove the tumor. The surgical specimen revealed a 6.9 cm × 4.5 cm sized GHIP with a coexisting 1.6 cm sized well-differentiated adenocarcinoma which extended to the muscularis propria. The malignancy did not originate from the GHIP but showed an overlap.
A large GHIP, which was unusually presented as an ulcerative lesion, was surgically removed, and was accompanied by advanced gastric cancer. Regular follow-up and thorough examinations of ulcerative lesions with equivocal biopsy have resulted in appropriate diagnosis and treatment. Therefore, aggressive intervention may be beneficial if GHIP is suspected.
Core Tip: Gastric hamartomatous inverted polyps (GHIPs) are benign lesions that mostly present as small submucosal tumors or polyps that are easily removed by endoscopic procedures. We report a rare case of GHIP that presented as a large ulcero-infitrative mass that was removed by total gastrectomy and confirmed to be accompanied by gastric cancer. Our case highlights that GHIPs may be a predisposing factor for malignancy due to ulcerative changes. Furthermore, the unusual endoscopic features of GHIP broaden the outlook of physicians and emphasize the need for a thorough investigation of GHIPs including the importance of complete resection.
- Citation: Park G, Kim J, Lee SH, Kim Y. Large gastric hamartomatous inverted polyp accompanied by advanced gastric cancer: A case report. World J Clin Cases 2023; 11(28): 6967-6973
- URL: https://www.wjgnet.com/2307-8960/full/v11/i28/6967.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i28.6967
Gastric hamartomatous inverted polyps (GHIPs) are benign polyps in which a foveolar, pyloric, or fundic glandular epithelium densely proliferates downward in the gastric submucosal layer. GHIPs have been observed as solitary lesions in patients without hereditary diseases. To the best of our knowledge, no more than 52 cases have been reported in the English literature since it was first described in 1993[1-4].
The pathogenesis of GHIP has not been established, but it has been widely hypothesized that repeated erosion and conversion of gastric glands into the submucosa lead to thinning and rupturing of the muscularis mucosa[5]. Several studies have reported the coexistence of carcinoma[3,6,7]. Kono et al[2] suggested that p53 alterations might be key to the carcinogenesis of GHIP, but this has not been substantiated[2]. Herein, we report a case of a large GHIP with coexisting gastric cancer that was initially considered an advanced gastric carcinoma.
A 66-year-old man was referred to the hospital due to ulcerated lesions incidentally found on an esophagogastroduodenoscopy (EGD), which is a regular health screening process provided by the Korean National Health Insurance Service for all individuals aged ≥ 40 years[8].
The patient was a 40 pack-years smoker and a heavy alcohol user who was taking medication for hypertension.
No previous signs or symptoms were observed.
No previous personal or family history was recorded.
Vital signs and physical examination results were within normal limits.
Laboratory findings were nonspecific except for slightly elevated liver enzyme levels.
No imaging was done before treatment plan was established.
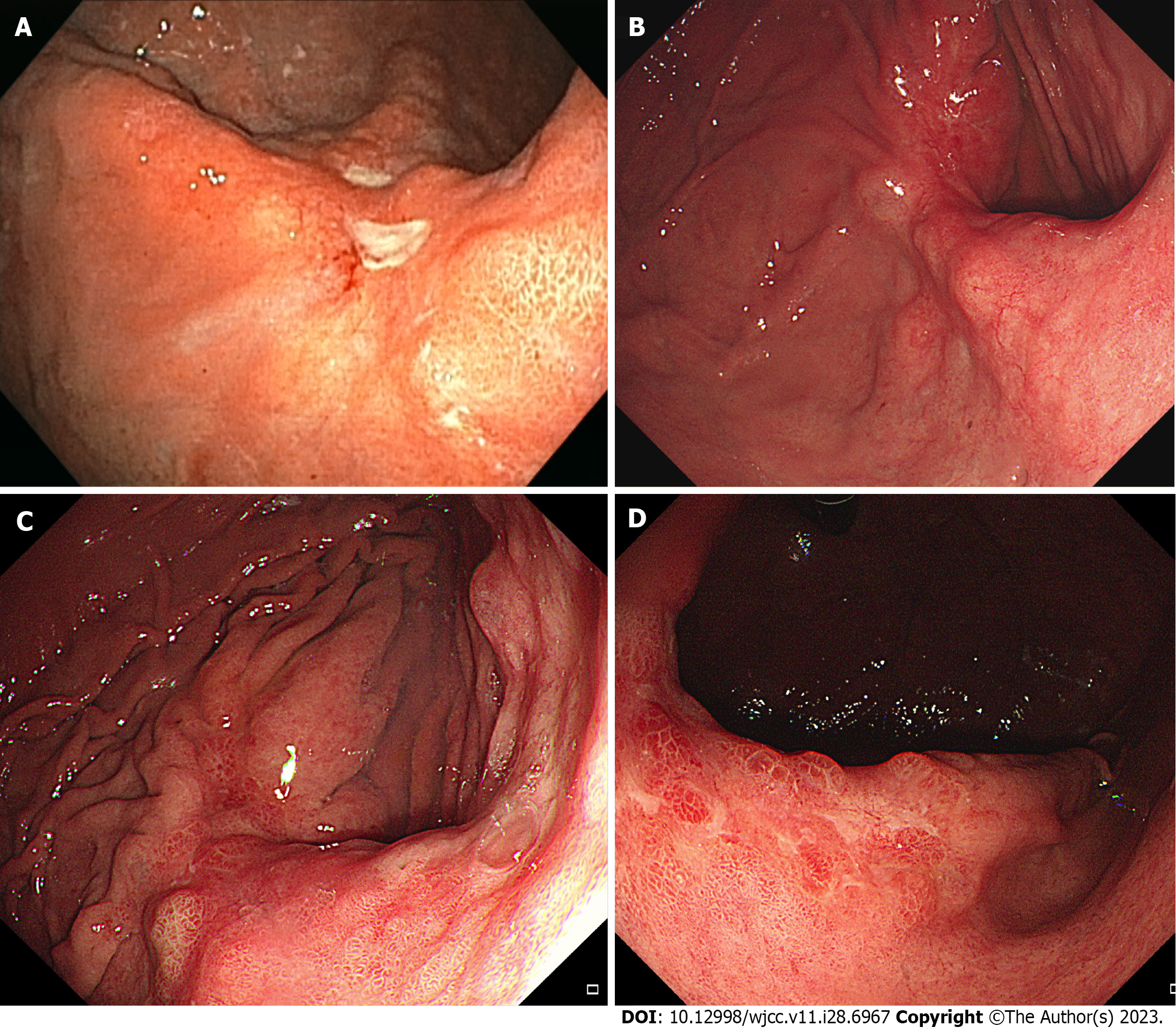
The initial EGD showed two healing-stage ulcer craters located at the posterior wall of the mid-body (Figure 1A). Abnormal fold changes including clubbing and fusion were observed around the ulcer craters. An initial biopsy was diagnosed as atypical glands that could not exclude malignancy. After 3 mo, during the first follow-up EGD, the ulcer healed and was covered with a reddish scar. Several bulging areas of the normal mucosa were observed around the ulcer scar (Figure 1B). Biopsy of the ulcer scar was not definitive for malignancy. The second follow-up EGD was performed after 3 mo with repeated biopsies. Histological results were the same as those of the previous biopsy. The third follow-up EGD performed 3 mo later revealed a huge ulcero-infiltrative lesion measuring up to 4.5 cm with an irregular margin in the posterior wall of the mid-body (Figure 1C). This lesion was covered with an unevenly distributed regenerative epithelium and a whitish discoloration (Figure 1D). The endoscopic impression was advanced gastric cancer with a gross feature of Bormann type 3. Multiple biopsies were performed to rule out malignancy and histological examination revealed a well-differentiated tubular adenocarcinoma.
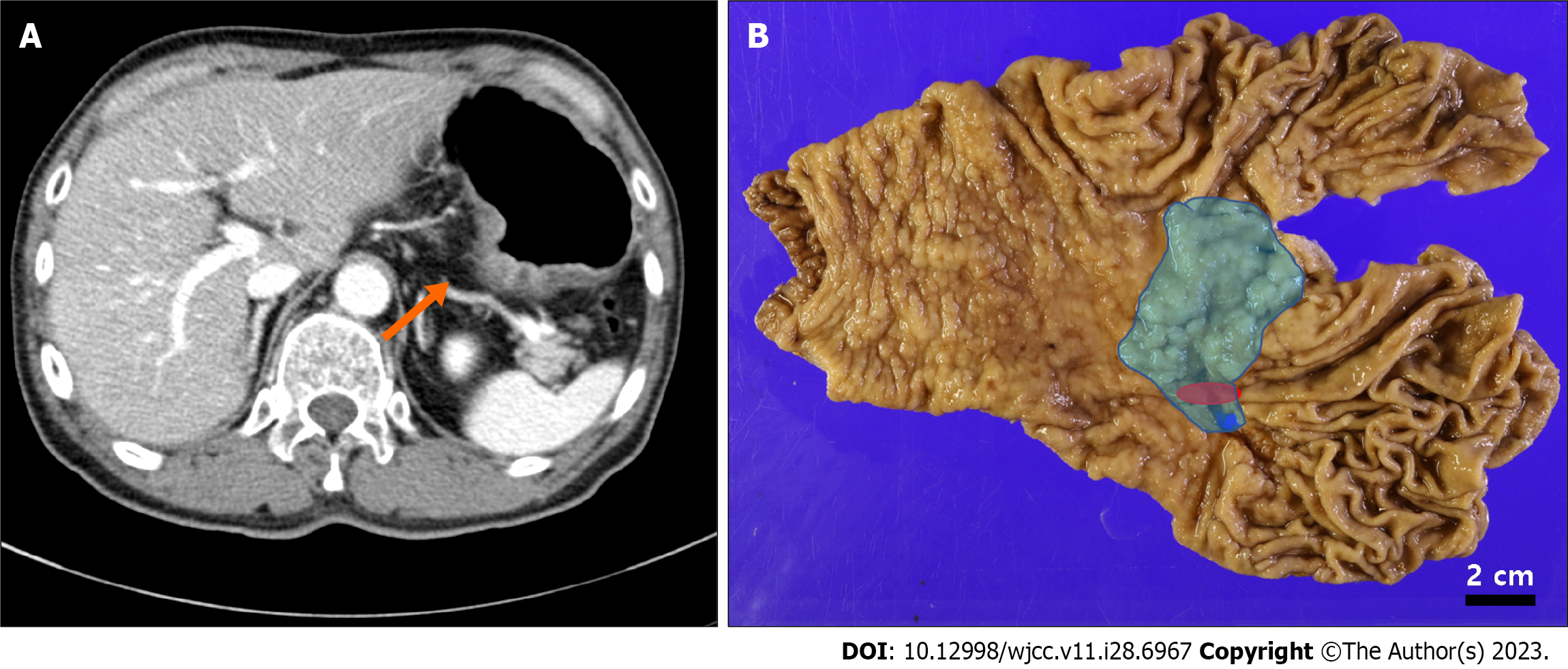
Preoperative computed tomography of the stomach revealed a 4.6 cm-sized lesion at the posterior wall of the mid-body (Figure 2A). Differential diagnosis included a large ulcerative lesion with focal involvement of carcinoma or a 4.6 cm-sized Bormann type 3 cancer. No lymph node or distant metastases were observed.
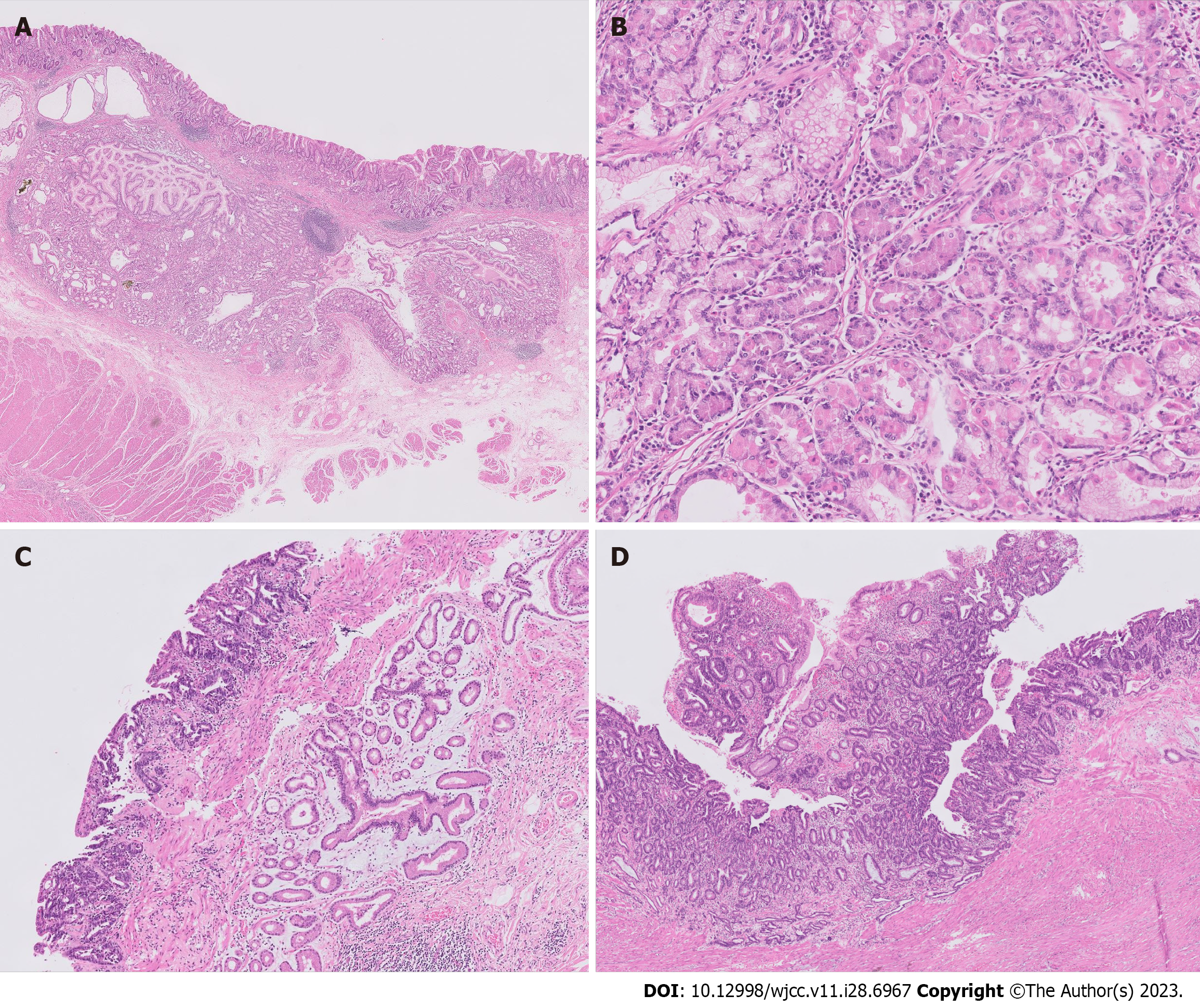
The surgical specimen revealed a 6.9 cm × 4.5 cm-sized ulcero-infiltrative lesion, in which the epicenter was located at the posterior wall of the body (Figure 2B, blue area). Histopathological findings demonstrated a diffuse distribution of submucosal glands within the lesion (Figure 3A and B). These glands were composed of foveolar, fundic, pyloric, and intestinal type cells with focal cystic dilations and surrounded by smooth muscle bundles. The lesion was diagnosed as GHIP. At the periphery of the GHIP, a 1.6 cm-sized well differentiated tubular adenocarcinoma was detected. The malignancy did not originate from the GHIP, but mostly overlapped [Figures 2B (red area) and 3C]. Despite its relatively small size, the depth of the tumor extended to the muscularis propria (pT2 according to the American Joint Committee on Cancer 8th edition), with a steep decline from the mucosa to the ulcerative proportion probably due to ulceration caused by the GHIP (Figure 3D).
The patient underwent laparoscopic total gastrectomy.
The patient was discharged without complications and is under close follow-up.
We report a rare case of a large GHIP that was treated surgically due to a coexisting carcinoma. Reviewing 52 previous cases, the median size of the GHIP was 2.1 cm (range: 0.1-4.5 cm)[2-4,7,9-16], which demonstrates that the current case was exceptionally large (6.9 cm) (Supplementary Table 1). Kim et al[3] reported 12 cases of GHIP and classified them into three histopathological types. Endoscopically, 12 cases were described as submucosal tumors (9 cases, 81.8%) or pedunculated polyps (2 cases, 18.2%). Type 1 GHIP is characterized by a mucosal communicating structure at the center and a well-defined smooth muscle boundary, accounting for half of all cases. Type 2 GHIP has a similar morphology to type 1 GHIP, but does not have a central communicating structure. Type 3 GHIP does not have a mucosal communicating structure or smooth muscle. Overall, GHIP cases have been described as submucosal tumors (43 cases, 82.7%) or pedunculated polyps (6 cases, 11.5%) covered with a normal gastric mucosa on EGD[3]. Most cases are treated by endoscopic or wedge resection[17]. However, the present GHIP showed a distinct ulcerative feature in which the differential diagnosis was an extensive advanced gastric cancer that was treated by total gastrectomy.
Several GHIPs have been reported to be accompanied by a carcinoma. Kim et al[3] reported that all three cases with coexisting carcinoma were type 1 GHIP. The authors hypothesized that the central communicating structure of type 1 GHIP allows the continuous exposure of regional epithelial cells to luminal carcinogens and mechanical stress. In contrast, the current case corresponding to type 2 presented as an ulcero-infiltrative lesion and still included a carcinoma portion. Overall, among the previously reported 52 GHIP cases, seven were accompanied by early gastric cancer (13.5%) and seven by advanced carcinoma (13.5%). Altogether, 26.9% (14 out of 52 cases) of GHIPs showed a coexisting carcinoma, which warrants caution and intensive checkup if GHIP is suspected. Compared to the reported rate of malignant change in low-grade adenomas, which is less than 10%[18], the possibility of finding carcinoma with GHIP is relatively high. Since a biopsy cannot contain a substantial amount of the submucosal layer, additional diagnostic examinations, such as endoscopic ultrasound and CT, could be performed. Considering the possibility of a coexisting carcinoma in GHIP, complete resection of the lesion and thorough histologic examination are required. According to a previous study, it is recommended that a GHIP larger than 2 cm should be treated by endoscopic submucosal dissection[19].
The pathogenesis of carcinomas arising from GHIPs remains elusive. Gastritis cystica profundas (GCPs), which are often encountered in microscopic examination of type 2 GHIP, are cystically dilated organizations in the submucosa or muscularis mucosa, consisting of gastric glands[3]. GCPs may develop from chronic inflammation and ischemia resulting in an interruption of the muscularis mucosa followed by subsequent gastric gland migration into the submucosa[20]. Several studies have reported that GCP can coexist with GHIP and GHIP may be derived from GCP[6,21,22]. Although GHIPs are frequently associated with malignancies, it remains unclear whether GHIP is a direct aggravating factor for carcinomas. However, since GCPs are strong predisposing factors for gastric carcinoma, GHIP, which may have arisen from GCP, could also lead to the development of carcinoma via chronic inflammation[23]. In the current case, an extensive and aggressive form of GHIP could have affected not only carcinogenesis but also played a substantial role in aggravating the tumor to an advanced stage.
The current case revealed a large GHIP that overlapped with a small advanced gastric carcinoma. Unlike previous reports, in which most GHIPs were identified as submucosal tumors or pedunculated polyps, this GHIP was displayed as an ulcero-infiltrative type. When GHIP is suspected, clinicians should assertively intervene because the possibility of a coexisting carcinoma is relatively high. Complete resection by endoscopy or surgery and thorough histologic examination should be considered.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Vyshka G, Albania S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Zhao S
| 1. | Kamata Y, Kurotaki H, Onodera T, Nishida N. An unusual heterotopia of pyloric glands of the stomach with inverted downgrowth. Acta Pathol Jpn. 1993;43:192-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 2. | Kono T, Imai Y, Ichihara T, Miyagawa K, Kanemitsu K, Ajiki T, Kawasaki K, Kamigaki T, Ikuta H, Ohbayashi C, Yokozaki H, Fujimori T, Kuroda Y. Adenocarcinoma arising in gastric inverted hyperplastic polyp: a case report and review of the literature. Pathol Res Pract. 2007;203:53-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 3. | Kim JY, Ahn S, Kim KM, Chang SH, Kim HS, Lee JH, Kim JJ, Sohn TS, Kang HJ, Joo M. Gastric Inverted Polyps-Distinctive Subepithelial Lesions of the Stomach: Clinicopathologic Analysis of 12 Cases With an Emphasis on Neoplastic Potential. Am J Surg Pathol. 2021;45:680-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 4. | Ohtsu T, Takahashi Y, Tokuhara M, Tahara T, Ishida M, Miyasaka C, Tsuta K, Naganuma M. Gastric hamartomatous inverted polyp: Report of three cases with a review of the endoscopic and clinicopathological features. DEN Open. 2023;3:e198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 5. | Itoh K, Tsuchigame T, Matsukawa T, Takahashi M, Honma K, Ishimaru Y. Unusual gastric polyp showing submucosal proliferation of glands: case report and literature review. J Gastroenterol. 1998;33:720-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Lee SJ, Park JK, Seo HI, Han KH, Kim YD, Jeong WJ, Cheon GJ, Eom DW. A case of gastric inverted hyperplastic polyp found with gastritis cystica profunda and early gastric cancer. Clin Endosc. 2013;46:568-571. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (111)] |
| 7. | Yamashita S, Nishi M, Yoshikawa K, Nakao T, Tokunaga T, Takasu C, Kashihara H, Wada Y, Yoshimoto T, Iwakawa Y, Oya T, Tsuneyama K, Shimada M. Gastric carcinoma with lymphoid stroma derived from hamartomatous inverted polyp with osteoclast-like giant cells: a case report. Int Cancer Conf J. 2022;11:196-200. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 8. | Kang HT. Current Status of the National Health Screening Programs in South Korea. Korean J Fam Med. 2022;43:168-173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 36] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 9. | Yang L, Li J, Jin P. Gastric inverted hyperplastic polyp with typical endoscopic feature. Dig Liver Dis. 2023;55:559-560. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 10. | Ng HI, Li ZQ, Zhang YM, Hu CF, Wang GQ. Gastric inverted hyperplastic polyp, an exceptional case diagnosed after endoscopic submucosal dissection. Clin Res Hepatol Gastroenterol. 2022;46:101890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 11. | Okamura T, Iwaya Y, Nagaya T, Muranaka F, Ota H, Umemura T. Gastric adenocarcinoma arising from hamartomatous inverted polyp during 8-year follow-up. DEN Open. 2022;2:e16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 12. | Hou JZ, Dong NN, Yue B, Meng FD, Wang YJ. Autoimmune gastritis with a gastric hamartomatous inverted polyp and two hyperplastic polyps: a case report. J Int Med Res. 2023;51:3000605231162451. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 13. | Ma Q, Gao L, Sun N, Chen Y, Liu L, Guo W, Yang X. Gastric inverted hyperplastic polyp: A case report. Exp Ther Med. 2023;25:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 14. | Han YP, Min CC, Li YB, Chen YQ, Liu H, Tian ZB, Yin XY. Diagnosis and treatment of gastric hamartomatous inverted polyp (GHIP) by endoscopic submucosal dissection: A case report. Medicine (Baltimore). 2023;102:e33443. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 15. | Miyamoto R, Takigawa H, Kotachi T, Kadota H, Yuge R, Hayashi R, Urabe Y, Ishikawa A, Sentani K, Oka S. Synchronous gastric MALT lymphoma and gastric adenocarcinoma of fundic gland type arising from a hamartomatous inverted polyp in a Helicobacter pylori naive patient. Clin J Gastroenterol. 2023;16:521-526. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 16. | Zeng XY, Zheng YS, Huang WF. A gastric hamartomatous inverted polyp mimicking submucosal tumor. Am J Med Sci. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 17. | Hayase S, Sakuma M, Chida S, Saito M, Ami H, Koyama Y, Ohki S, Kono K. Diagnosis and treatment of gastric hamartomatous inverted polyp (GHIP) using a modified combination of laparoscopic and endoscopic approaches to neoplasia with a non-exposure technique (modified CLEAN-NET): a case report. Surg Case Rep. 2020;6:200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Taniyama D, Taniyama K, Kuraoka K, Zaitsu J, Saito A, Nakatsuka H, Sakamoto N, Sentani K, Oue N, Yasui W. Long-term follow-up study of gastric adenoma; tumor-associated macrophages are associated to carcinoma development in gastric adenoma. Gastric Cancer. 2017;20:929-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Kim HS, Hwang EJ, Jang JY, Lee J, Kim YW. Multifocal Adenocarcinomas Arising within a Gastric Inverted Hyperplastic Polyp. Korean J Pathol. 2012;46:387-391. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Itami H, Morita K, Nakai T, Uchiyama T, Sugimoto S, Sasaki S, Matsuoka M, Myojin T, Nitta Y, Okabe F, Fujii T, Hatakeyama K, Mitoro A, Sho M, Ohbayashi C. Gastritis cystica profunda is associated with aberrant p53 and Epstein-Barr virus in gastric cancer: A clinicopathological, immunohistochemical and in situ hybridization study. Pathol Int. 2021;71:42-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (1)] |
| 21. | Yamashita M, Hirokawa M, Nakasono M, Kiyoku H, Sano N, Fujii M, Koyama T, Yoshida S, Sano T. Gastric inverted hyperplastic polyp. Report of four cases and relation to gastritis cystica profunda. APMIS. 2002;110:717-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 22. | Cho H, Hashimoto T, Naka T, Yatabe Y, Oda I, Saito Y, Yoshikawa T, Sekine S. Activating KRAS and GNAS mutations in heterotopic submucosal glands of the stomach. J Gastroenterol. 2022;57:333-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 23. | Kuwahara N, Kitazawa R, Fujiishi K, Nagai Y, Haraguchi R, Kitazawa S. Gastric adenocarcinoma arising in gastritis cystica profunda presenting with selective loss of KCNE2 expression. World J Gastroenterol. 2013;19:1314-1317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (111)] |