Published online Oct 6, 2023. doi: 10.12998/wjcc.v11.i28.6961
Peer-review started: July 30, 2023
First decision: August 9, 2023
Revised: August 20, 2023
Accepted: September 6, 2023
Article in press: September 6, 2023
Published online: October 6, 2023
Processing time: 57 Days and 4.2 Hours
Salmonella derby (S. derby) is a Gram-negative diplococcus that is common in the digestive tract. Infected patients generally experience symptoms such as fever and diarrhea. Mild cases are mostly self-healing gastroenteritis, and severe cases can cause fatal typhoid fever. Clinical cases are more common in children. The most common form of S. derby infection is self-healing gastroenteritis, in which, fever lasts for about 2 d and diarrhea for < 7 d. S. derby can often cause bacterial conjunctivitis, pneumonia, endocarditis, peritonitis and urethritis. However, intracranial infections in infants caused by S. derby are rare in clinical practice and have not been reported before in China.
A 4-mo-old female infant had recurrent fever for 2 wk, with a maximum body temperature of around 39.4°C. Treatment for infectious fever in a local hospital was ineffective, and she was admitted to our hospital. Before admission, there was one sudden convulsion, characterized by unclear consciousness, limb twitching, gaze in both eyes, and slight cyanosis on the face. Cerebrospinal fluid (CSF) culture was positive for Gram-negative bacilli, which conformed to S. derby. After treatment with meropenem and ceftriaxone antibiotics, the patient was discharged home in a clinically stable state after 4 wk of treatment.
We reported a rare case of S. derby cultured in CSF. S. derby enters the CSF through the blood–brain barrier, causing purulent meningitis. If not treated timeously, it can lead to serious, life-threatening infection.
Core Tip:Salmonella spp. are common foodborne pathogens that causes various infections through contaminated food or water through the mouth. Among them, Salmonella derby is an important zoonotic pathogen, and healthy individuals can also be carriers, often causing symptoms such as sepsis and food poisoning. In rare cases, patients can develop bacterial encephalitis. Antimicrobial treatment should be given based on bacterial culture and antimicrobial susceptibility testing, and take into consideration any contraindications.
- Citation: Yu JL, Jiang LL, Dong R, Liu SY. Intracranial infection and sepsis in infants caused by Salmonella derby: A case report. World J Clin Cases 2023; 11(28): 6961-6966
- URL: https://www.wjgnet.com/2307-8960/full/v11/i28/6961.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i28.6961
Salmonella can cause various infections through consumption of contaminated food or water, and infected patients generally experience symptoms such as fever and diarrhea. Mild cases are mostly self-healing gastroenteritis and severe cases can cause fatal typhoid fever. Clinical cases are more common in children[1-5]. The most common form of Salmonella infection is self-healing gastroenteritis, in which, fever lasts for about 2 d and diarrhea for < 7 d. The classification of Salmonella is mainly based on its antigenic structure. More than 2000 serotypes have been identified, and approximately 100 have been identified in China[6]. Salmonella derby (S. derby) in group B is an important zoonotic pathogen that can cause symptoms such as sepsis and food poisoning, posing a serious threat to human health[7]. In recent years, the isolation of S. derby has become most frequent in the pork production chain, and the carrier rate among healthy populations is also high[8].
A 4-mo-old baby girl had recurrent fever for > 2 wk, but anti-infective treatment was ineffective, so she was admitted to our hospital.
The patient developed a fever without obvious cause 2 wk previously, with a maximum body temperature of 39.4°C and no chills or convulsions. She was treated with oral paracetamol to reduce the fever. Her temperature rose again after 3–4 h, and it fluctuated three or four times a day. She had been to another hospital several times and was diagnosed with infectious fever. Anti-infective treatment with oseltamivir granules and cefoperazone injection was ineffective. One hour before transfer to our hospital, she had a sudden convulsion, presenting as unclear consciousness (Glasgow Coma Scale score of 12 points), limb twitching, staring with both eyes, and slight cyanosis on the face.
The patient’s personal and family history was unremarkable. She denied a history of hepatitis, tuberculosis and other infectious diseases, diabetes, cardiovascular and cerebrovascular disease, and had no family history of malignant tumors.
A child with purulent meningitis and sepsis was admitted to our hospital on June 29, 2022. Physical examination showed: Unclear consciousness, body temperature 37.2°C, binocular gaze, bilateral pupils of equal size and approximately 3 mm in diameter, insensitivity to light, anterior fontanel about 1.5 cm × 1.5 cm, anterior fontanelle protrusion, anterior fontanel high tension, slightly pale complexion, thick breathing sounds in both lungs, without rales, high muscle tension in the limbs, neck resistance (convulsive state), lack of coordination in knee tendon reflexes, and positive bilateral Pap’s sign. She had diarrhea, yellow–green loose stools five or six times a day, with mucus but no blood, acid or odor.
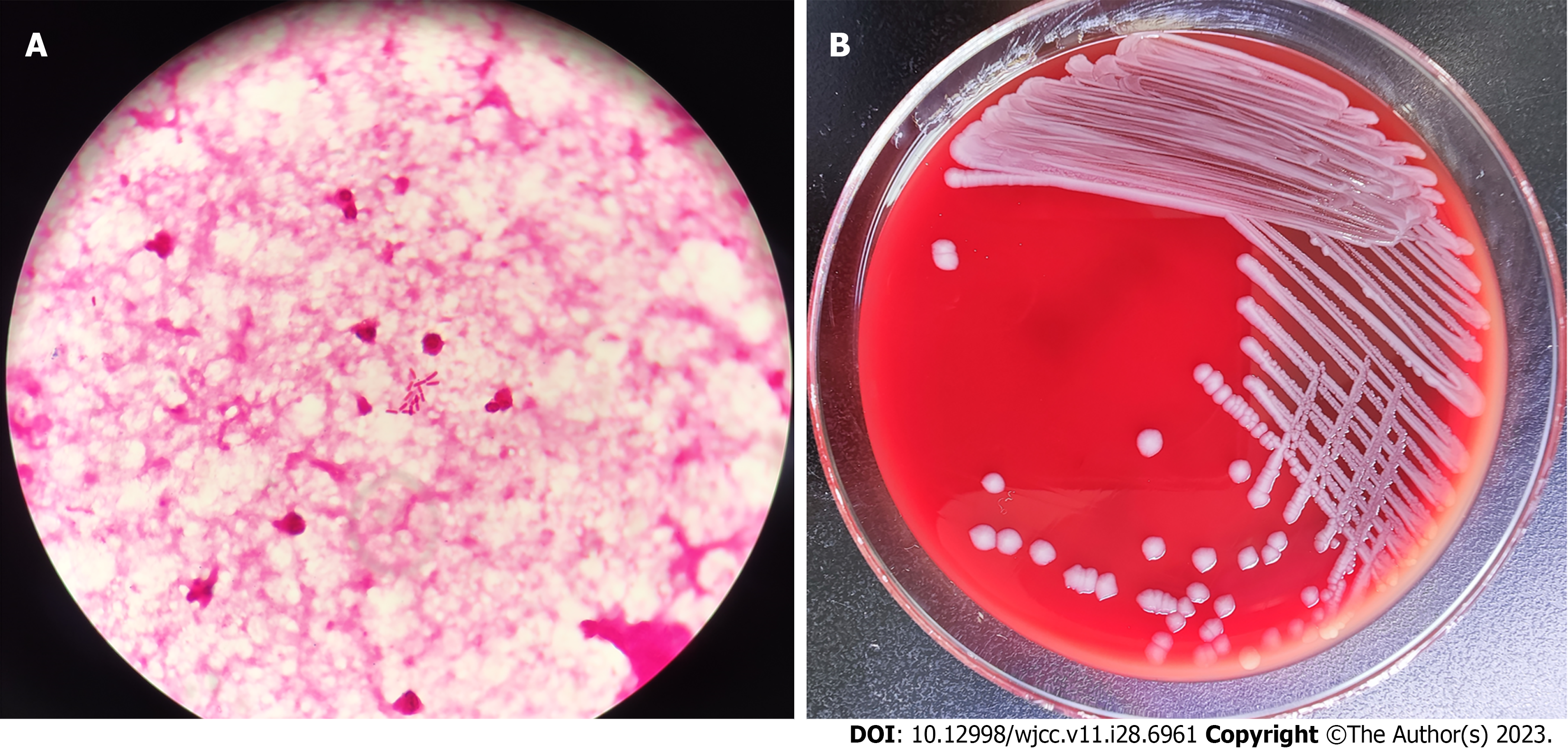
White blood cells 16.8 × 109/L (normal: 3.5 × 109-9.5 × 109/L), percentage of neutrophils 71.5% (normal: 40.0%–75.0%), percentage of lymph nodes 19.5% (normal: 20.0%–50.0%), hemoglobin 98 g/L (normal: 115–150 g/L), platelets 100 × 109/L (normal: 125 × 109–350 × 109/L). Rapid hypersensitivity C-reactive protein: 318.2 mg/L (normal: 0.0–8.0 mg/L). Blood gas analysis showed: pH 7.29 (normal: 7.35–7.45), oxygen partial pressure 24 mmHg (normal: 83–108 mmHg), carbon dioxide partial pressure 54 mmHg (normal: 35–45 mmHg), bicarbonate 26.0 mmol/L (normal: 21.0–28.0 mmol/L), potassium 4.7 mmol/L (normal: 3.5–5.3 mmol/L), sodium 133 mmol/L (normal: 137–147 mmol/L), glucose 6.8 mmol/L (normal: 3.9–6.1 mmol/L), lactic acid 4.1 mmol/L (normal: 0.5–1.7 mmol/L), cerebrospinal fluid (CSF) examination showed white blood cell count of 8000×106/L (normal: 0-20 × 106/L), mainly neutrophils. Pan's test was positive, with an increase in protein content of 4.45 g/L (normal: 0.15-0.45 g/L) and a decrease in sugar content of < 0.28 mmol/L (normal: 2.5-4.4 mmol/L), which meets the diagnostic criteria for bacterial meningitis. CSF bacterial smear examination detected Gram-negative bacteria (Figure 1A). Blood and CSF culture were used to identify pathogenic bacteria. Blood, cerebrospinal fluid, and feces of the child were cultured at the same time, and the results showed Gram negative bacteria and negative oxidase, and positive contact enzyme. They grew well on blood agar and chocolate agar plates, with 1–2 mm, gray, round, moist and protruding colonies. The Salmonella Shigella culture medium was transparent with black H2S-producing colonies in the center (Figure 1B). The bacteria were identified as Salmonella by microbial mass spectrometry. Further identification by Salmonella factor diagnostic serum revealed that the results were agglutinated with multivalent A to F group serum and O4, Hf, g, without the presence of H phase 2, and ultimately confirmed as B group Delphi Salmonella. Drug sensitivity test indicated sensitivity to carbapenems and third-generation cephalosporins. The pure culture was identified as S. derby by Vitek MS mass spectrometry. To obtain accurate identification, 16s RNA sequencing was performed. The results showed that the sequence was 99.85% consistent with the sequence of S. derby.
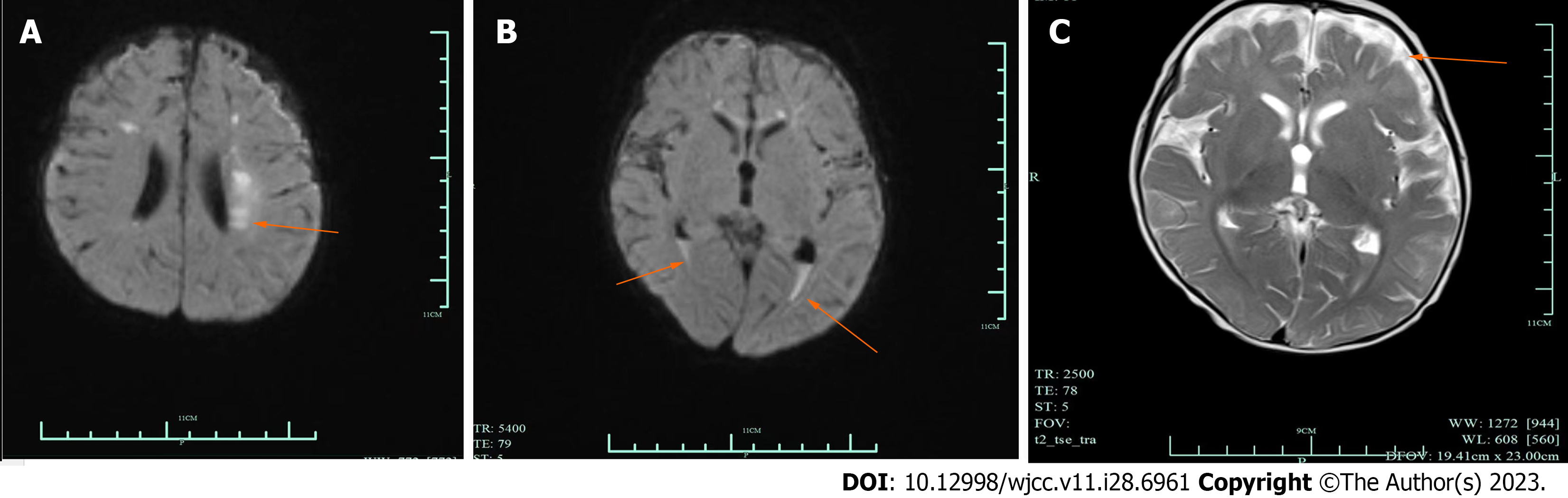
Brain magnetic resonance imaging (MRI) showed abnormal signals in bilateral paraventricular, frontal and parietal lobes, which indicated subacute cerebral infarction. The space outside the brain widened, and a small amount of blood accumulated in the posterior horn of both ventricles (Figure 2).
The final diagnosis was intracranial infection and sepsis caused by S. derby.
The patient was treated for 2 wk with meropenem 0.25 g q8h, which was ineffective. The treatment was changed to intravenous ceftriaxone 3 g for 2 wk, and the patient’s condition improved.
After treatment, the patient had a clear mind, good spirits, no fever, rosy complexion, flat breathing, clear respiratory sounds in both lungs, no rales, consistent heart rhythm and no murmurs, no cough or nasal congestion, no rash, no convulsions, good breastfeeding, good sleep, and normal bowel movements. The abdomen was soft without bloating, the liver and spleen were not enlarged, bowel sounds were 5 times/ min, and there were no abnormal positive signs in the nervous system examination. Re-examination of head MRI showed improvement; CSF was clear and transparent, with negative culture; blood culture was negative and stool culture was negative twice; inflammatory indicators decreased; clinical symptoms disappeared; and anti-infective treatment was effective. The patient was discharged.
The clinical manifestations of human Salmonella infection are complex, which can be divided into asymptomatic and symptomatic infection[9]. According to symptom severity, it can be divided into typhoid fever, gastroenteritis, local suppurative infection, and sepsis types[10]. Nontyphoid Salmonella infection generally manifests as mild symptoms of self-limited diarrhea in healthy individuals, and rare bloodstream or focal infections may only occur in individuals with specific risk factors[11,12]. Salmonella can cause various infections, ranging from self-healing gastroenteritis to fatal typhoid fever[13]. Clinically, it is common in children, and in infants and young children, it can seriously affect their physical and mental health[14,15]. Therefore, the cultivation and isolation of Salmonella and identification of the species are important. Different specimens are taken according to different disease courses. Usually, blood is taken in the first to second weeks, feces or urine is taken in the second to third weeks, and vomit and residual food are taken for detection of acute enteritis. Blood is taken for culture in suspected sepsis. According to literature reports, the isolation rate of Salmonella delbrueckii in the pork production chain tends to be the highest, and the carrier rate is also among the highest in healthy populations[16].
Currently, there are only a few reports of Salmonella cultured from CSF. From 2001 to 2022, three cases of Salmonella enteritidis, two each of Salmonella dublin and Salmonella newport, and one each of Salmonella essen, Salmonella para-typhi B and Salmonella typhimurium were cultured in CSF samples[17-21]. There was no relevant report on S. derby, of which 9 cases were in infants and one was in an adult trauma patient[14]. The reason may be that the blood–brain barrier of infants is not perfect, Salmonella enteric infection induces sepsis, and the bacteria in the blood enter the CSF through the blood-brain barrier, causing purulent meningitis.
This is the first case of S. derby cultured in the CSF of infants and young children in our hospital. The child lived in a remote rural areas with poor hygiene, mainly breastfeeding, and incomplete disinfection of hands or utensils may have caused infection. However, the initial route of infection still needs confirmation. Although drug sensitivity testing indicates that S. derby is sensitive to chloramphenicol and fluoroquinolones, the former is difficult to metabolize and eliminate in newborns, and the latter has an impact on the bone development of infants. The clinical use of antibiotics should be cautious. In this case, meropenem and ceftriaxone were selected for anti-infective treatment, with obvious effects and good prognosis. This report summarizes the clinical data of a case of S. derby infection to deepen clinicians’ understanding of the intestinal and bloodstream infection in infants. Bacteria can cross the blood–brain barrier and cause intracranial infection. Clinical attention should be paid to the isolation and identification of pathogens, drug sensitivity and individual factors to select appropriate and effective drugs.
We present an infant with intracranial infection and sepsis caused by S. derby. This infection is rare, and if not treated in a timely manner, it may become serious and even life-threatening, which should alert clinicians.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kenzaka T, Japan; Oley MH, Indonesia S-Editor: Lin C L-Editor: A P-Editor: Cai YX
| 1. | Sévellec Y, Granier SA, Le Hello S, Weill FX, Guillier L, Mistou MY, Cadel-Six S. Source Attribution Study of Sporadic Salmonella Derby Cases in France. Front Microbiol. 2020;11:889. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 2. | Li R, Lai J, Wang Y, Liu S, Li Y, Liu K, Shen J, Wu C. Prevalence and characterization of Salmonella species isolated from pigs, ducks and chickens in Sichuan Province, China. Int J Food Microbiol. 2013;163:14-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 142] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 3. | Simon S, Trost E, Bender J, Fuchs S, Malorny B, Rabsch W, Prager R, Tietze E, Flieger A. Evaluation of WGS based approaches for investigating a food-borne outbreak caused by Salmonella enterica serovar Derby in Germany. Food Microbiol. 2018;71:46-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 4. | Mohammed M, Thapa S. Evaluation of WGS-subtyping methods for epidemiological surveillance of foodborne salmonellosis. One Health Outlook. 2020;2:13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 5. | Chang YJ, Chen MC, Feng Y, Su LH, Li HC, Yang HP, Yu MJ, Chen CL, Chiu CH. Highly antimicrobial-resistant Nontyphoidal Salmonella from retail meats and clinical impact in children, Taiwan. Pediatr Neonatol. 2020;61:432-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 6. | Liao J, Orsi RH, Carroll LM, Kovac J, Ou H, Zhang H, Wiedmann M. Serotype-specific evolutionary patterns of antimicrobial-resistant Salmonella enterica. BMC Evol Biol. 2019;19:132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 7. | Wang Y, Chen Q, Cui S, Xu X, Zhu J, Luo H, Wang D, Li F. Enumeration and characterization of Salmonella isolates from retail chicken carcasses in Beijing, China. Foodborne Pathog Dis. 2014;11:126-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 8. | Hayward MR, AbuOun M, La Ragione RM, Tchórzewska MA, Cooley WA, Everest DJ, Petrovska L, Jansen VA, Woodward MJ. SPI-23 of S. Derby: role in adherence and invasion of porcine tissues. PLoS One. 2014;9:e107857. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Pornsukarom S, van Vliet AHM, Thakur S. Whole genome sequencing analysis of multiple Salmonella serovars provides insights into phylogenetic relatedness, antimicrobial resistance, and virulence markers across humans, food animals and agriculture environmental sources. BMC Genomics. 2018;19:801. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 95] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 10. | Mohammed M, Cormican M. Whole genome sequencing provides insights into the genetic determinants of invasiveness in Salmonella Dublin. Epidemiol Infect. 2016;144:2430-2439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Litrup E, Torpdahl M, Malorny B, Huehn S, Christensen H, Nielsen EM. Association between phylogeny, virulence potential and serovars of Salmonella enterica. Infect Genet Evol. 2010;10:1132-1139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 12. | Ao TT, Feasey NA, Gordon MA, Keddy KH, Angulo FJ, Crump JA. Global burden of invasive nontyphoidal Salmonella disease, 2010(1). Emerg Infect Dis. 2015;21:941-949. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 279] [Cited by in RCA: 346] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 13. | Kariuki S, Gordon MA, Feasey N, Parry CM. Antimicrobial resistance and management of invasive Salmonella disease. Vaccine. 2015;33 Suppl 3:C21-C29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 192] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 14. | MacLennan CA, Msefula CL, Gondwe EN, Gilchrist JJ, Pensulo P, Mandala WL, Mwimaniwa G, Banda M, Kenny J, Wilson LK, Phiri A, MacLennan JM, Molyneux EM, Molyneux ME, Graham SM. Presentation of life-threatening invasive nontyphoidal Salmonella disease in Malawian children: A prospective observational study. PLoS Negl Trop Dis. 2017;11:e0006027. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Jiang M, Wang HM, Zhou GL, Chen YS, Deng JK. Invasive Salmonella Infections Among Children in Shenzhen, China: A Five-year Retrospective Review. Pediatr Infect Dis J. 2022;41:684-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 16. | González-Santamarina B, García-Soto S, Hotzel H, Meemken D, Fries R, Tomaso H. Salmonella Derby: A Comparative Genomic Analysis of Strains From Germany. Front Microbiol. 2021;12:591929. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Mohan A, Munusamy C, Tan YC, Muthuvelu S, Hashim R, Chien SL, Wong MK, Khairuddin NA, Podin Y, Lau PS, Ng DC, Ooi MH. Invasive Salmonella infections among children in Bintulu, Sarawak, Malaysian Borneo: a 6-year retrospective review. BMC Infect Dis. 2019;19:330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 18. | Uche IV, MacLennan CA, Saul A. A Systematic Review of the Incidence, Risk Factors and Case Fatality Rates of Invasive Nontyphoidal Salmonella (iNTS) Disease in Africa (1966 to 2014). PLoS Negl Trop Dis. 2017;11:e0005118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 147] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 19. | Lee WS, Puthucheary SD, Omar A. Salmonella meningitis and its complications in infants. J Paediatr Child Health. 1999;35:379-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Bay C, Jofré M, Kuzmanic D, Aguirre C, Gutiérrez V. [Salmonella Enteritidis meningitis in an infant. Case report and literature review]. Rev Chilena Infectol. 2020;37:470-476. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 21. | Yang MT, Chi CS. Salmonella infections in infants and children. Zhonghua Yi Xue Za Zhi (Taipei). 1994;54:38-43. [PubMed] |