Published online Oct 6, 2023. doi: 10.12998/wjcc.v11.i28.6938
Peer-review started: July 18, 2023
First decision: August 15, 2023
Revised: August 21, 2023
Accepted: September 11, 2023
Article in press: September 11, 2023
Published online: October 6, 2023
Processing time: 68 Days and 21.3 Hours
Immune checkpoint inhibitor-associated interstitial lung disease (ICI-ILD) and opportunistic pneumonias are the main pulmonary complications during immunotherapy for malignancies. The organizing pneumonia (OP) pattern is one of the common radiological manifestations of ICI-ILD, and OP is the most common cause of reversed halo cycles and consolidations. However, opportu
In this report, we described a case of a 44-year-old man with esophageal cancer who showed multiple reversed-halo cycles and consolidations on chest computed tomography (CT) after he had a cold during immunotherapy. He was diagnosed with esophageal squamous-cell cancer (T2NIM0) after surgery. Then, he was successfully treated with 6 cycles of chemotherapy plus tislelizumab, one cycle of radiotherapy and 9 cycles of tislelizumab. Two months later, he complained of low-grade fever and cough with nonpurulent sputum after he had a cold. Community-acquired pneumonia was considered, but moxifloxacin was ineffective. Chest CT showed multiple reversed-halo cycles and consolidations. Mycobacterium tuberculosis was identified with next-generation sequence analysis of bronchoalveolar lavage fluid (BALF). Two months later, he improved with standard anti-tuberculosis medications. Both the cycles and consolidations disappeared in the repeat CT after 6 mo of medications.
When chest CT shows reversed-halo cycles and consolidations in patients during anticancer immunotherapy, both ICI-ILD and infectious pneumonia should be considered. BALF microbiological analysis was helpful to differentiate them.
Core Tip: Immune checkpoint inhibitor-associated interstitial lung disease and variable opportunistic pneumonias are the main pulmonary complications of malignancies during immune treatment. Although organizing pneumonia is the most common cause of reversed-halo cycles and consolidations, opportunistic pneumonias should be excluded first. Bronchoalveolar lavage fluid microbiological analysis was a helpful diagnostic tool.
- Citation: Suo H, Shi YJ, Huang ZD, Xu K, Huang H. Pulmonary reversed halo cycles and consolidations after immunotherapy: A case report. World J Clin Cases 2023; 11(28): 6938-6942
- URL: https://www.wjgnet.com/2307-8960/full/v11/i28/6938.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i28.6938
Reversed halo signs and consolidations can be observed in various infectious and noninfectious pulmonary diseases[1-4]. Immune checkpoint inhibitor-associated interstitial lung disease (ICI-ILD) and variable opportunistic pneumonias are the main pulmonary complications in patients with malignancies during immune treatment[5]. The organizing pneumonia (OP) pattern is one of the common radiological manifestations of ICI-ILD[6]. Although OP is the most common cause of reversed halo signs and consolidations, opportunistic pneumonias should be considered in the differential diagnosis.
Here, we describe a man with esophageal cancer who showed multiple reversed halo signs and consolidation shadows on chest computed tomography (CT) after having a cold and while undergoing immunotherapy. Finally, he was diagnosed with tuberculosis, which improved after treatment with standard antituberculosis medications.
A 44-year-old man with esophageal cancer was admitted for 2 wk with a fever and cough. He complained of low-grade fever and cough with nonpurulent sputum on December 3, 2021, after catching a cold.
Community-acquired pneumonia was considered, but moxifloxacin was ineffective. Exertional dyspnea appeared 2 wk later. ICI-ILD was suspected, so he came to our hospital.
He was diagnosed with esophageal squamous-cell cancer (T2NIM0) after surgery in November 2020. Then, he was successfully treated with 6 cycles of chemotherapy plus tislelizumab (200 mg, every 3 wk), one cycle of radiotherapy, and 9 cycles of tislelizumab (the final cycle in September 2021).
The patient did not smoke or drink. He had no significant family history.
There were no palpably enlarged superficial lymph nodes. There were no abnormal signs in the chest (including the heart and lungs) or abdominal examinations. There was no edema in either lower extremity.
Both the complete blood count and biochemical analysis results were normal. The lymphocyte subgroup analysis showed that the peripheral lymphocyte count was 870/µL, neutrophil count was 6000/μL, and CD4+ lymphocyte count was 284/µL. The C-reactive protein level was 99 mg/mL, and the ESR was 86 mm/h. Both his procalcitonin and 1,3-β-glucanase levels were normal. His nasopharyngeal swabs for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RNA and influenza A and B RNA, which were tested by PCR, were negative. The repeated rapid detection of the SARS-CoV-2 antigen was also negative. Arterial blood gas analysis showed hypoxia during inspiration of room air at rest: pH was 7.429, PCO2 was 37.4 mmHg and PO2 was 66.5 mmHg. Both ICI-ILD and infectious pulmonary diseases were suspected. Therefore, he underwent bronchoscopy. There were no significant abnormal manifestations during bronchoscopy. However, Mycobacterium tuberculosis was identified by next-generation sequencing (NGS) analysis of bronchoalveolar lavage fluid (BALF), which was harvested from the lingular lobe. The patient was still negative for SARS-CoV-2 RNA and influenza A and B RNA in the BALF NGS analysis.
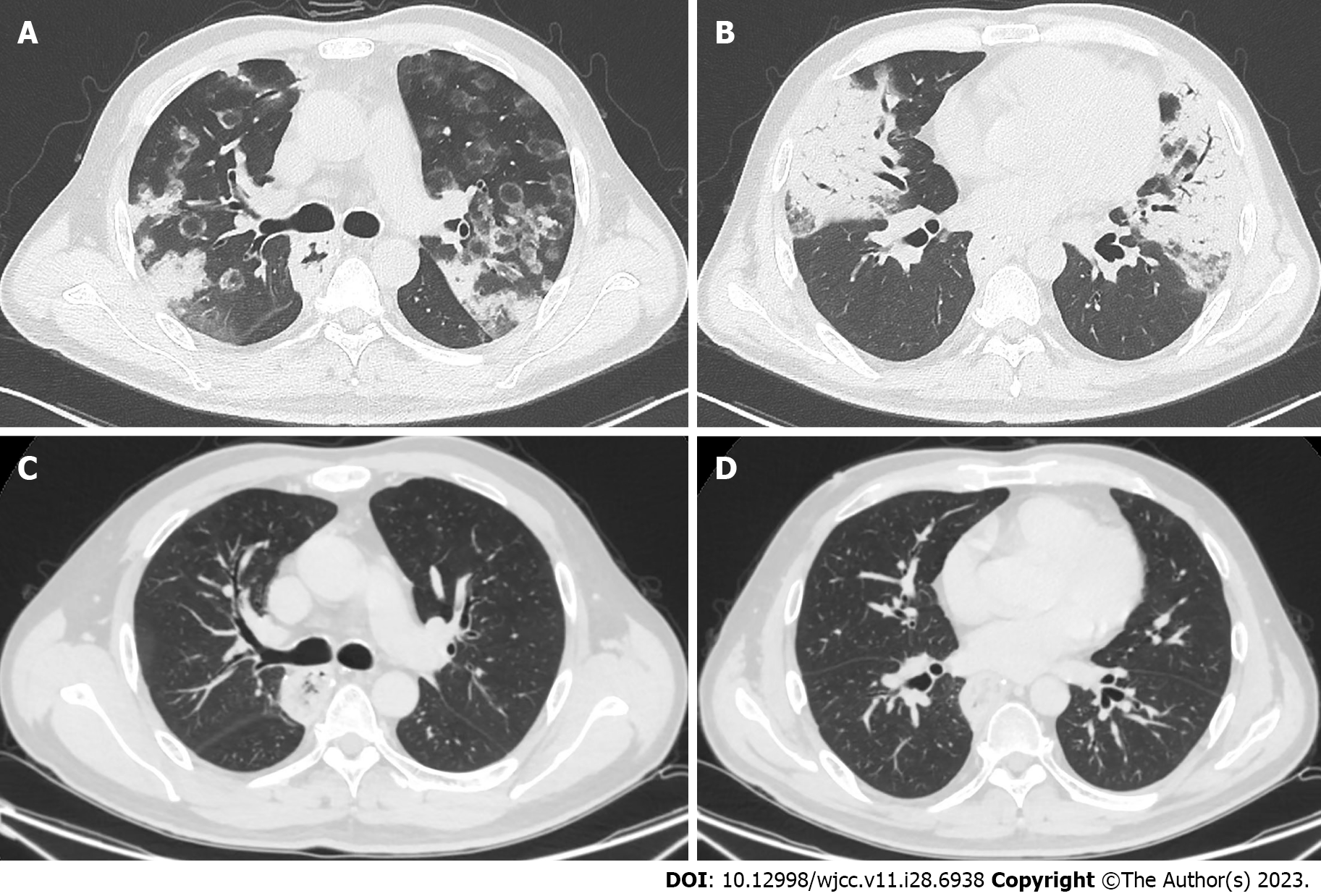
Chest CT showed multiple reversed-halo cycles and consolidations (Figure 1A and B) in both lungs.
The patient was diagnosed with pulmonary tuberculosis.
Isoniazid (0.3 qd), rifampin (0.45 qd), ethambutol (0.75 qd), and pyrazinamide (0.5 tid) were prescribed.
He improved with standard anti-tuberculosis medications two months later. Both the cycles and consolidations disappeared in the repeat CT after 6 mo of medications (Figure 1C and D).
Reversed halo signs and consolidations can be observed in various infectious and noninfectious pulmonary diseases. Cryptogenic or secondary OP is the most common noninfectious pulmonary disease; however, active pulmonary tuberculosis, coronavirus disease 2019 pneumonia, and fungal pneumonia are also common infectious pulmonary diseases[1-4]. ICI-ILD and infectious pulmonary diseases, especially opportunistic pneumonias, are the main pulmonary complications of anticancer immunotherapy[5]. The OP pattern is a common radiological manifestation in drug-related pneumonitis during treatment with molecular targeting agents and immune checkpoint inhibitors[6,7]. For immunocompromised patients, fungal and bacterial pneumonia are more common than OP when reversed halo signs are observed on chest CT[8]. The peripheral neutrophil count, rim thickness of the reversed halo sign, and concurrent effusion help to differentiate infectious diseases from noninfectious diseases[8]. Pulmonary tuberculosis is the most common cause of granulomatous reversed halo signs[9]. Nodular walls or nodules inside the reversed halo signs seem to be associated with active pulmonary tuberculosis[3]. Our patient’s neutrophil count was greater than 500/µl, and the rim was less than 1 cm and without effusion. Furthermore, concurrent significant consolidations were observed on his chest CT scans. These signs indicated that he might have OP pattern ICI-ILD. However, opportunistic pneumonia needed to be excluded for our patient, as he was treated with cancer immunotherapy and because there were ground-glass opacities inside the reversed halo signs[8]. BALF microbiological analysis was able to differentiate the two conditions. Finally, the patient recovered after 6 mo of regular antituberculosis treatment.
There were several limitations to this case report. First, the tuberculosis culture was negative 6 wk later. Therefore, the drug sensitivity test could not be ordered. Second, a lung biopsy was not arranged for him.
As chest CT showed reversed halo signs and consolidations during anticancer immunotherapy, both ICI-ILD and opportunistic pulmonary infectious diseases were considered. Differential diagnosis was more important than empirical therapy. BALF microbiological analysis was a helpful diagnostic tool.
We would like to thank the patient and his family for their assistance.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Respiratory system
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chien CR, Taiwan; Shalaby MN, Egypt S-Editor: Yan JP L-Editor: A P-Editor: Yan JP
| 1. | Marchiori E, Zanetti G, Escuissato DL, Souza AS Jr, de Souza Portes Meirelles G, Fagundes J, Souza CA, Hochhegger B, Marom EM, Godoy MCB. Reversed halo sign: high-resolution CT scan findings in 79 patients. Chest. 2012;141:1260-1266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 2. | Kim SJ, Lee KS, Ryu YH, Yoon YC, Choe KO, Kim TS, Sung KJ. Reversed halo sign on high-resolution CT of cryptogenic organizing pneumonia: diagnostic implications. AJR Am J Roentgenol. 2003;180:1251-1254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 207] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 3. | Piponnier M, Druart G, Guérineau N, de Bougrenet JL, Primot J. Optimal conditions for using the binary approximation of continuously self-imaging gratings. Opt Express. 2011;19:23054-23066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 4. | Aslan S. Multiple Reversed Halo Sign on Chest CT in COVID-19 Pneumonia. Arch Bronconeumol. 2021;57:69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Morelli T, Fujita K, Redelman-Sidi G, Elkington PT. Infections due to dysregulated immunity: an emerging complication of cancer immunotherapy. Thorax. 2022;77:304-311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 63] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 6. | Naidoo J, Wang X, Woo KM, Iyriboz T, Halpenny D, Cunningham J, Chaft JE, Segal NH, Callahan MK, Lesokhin AM, Rosenberg J, Voss MH, Rudin CM, Rizvi H, Hou X, Rodriguez K, Albano M, Gordon RA, Leduc C, Rekhtman N, Harris B, Menzies AM, Guminski AD, Carlino MS, Kong BY, Wolchok JD, Postow MA, Long GV, Hellmann MD. Pneumonitis in Patients Treated With Anti-Programmed Death-1/Programmed Death Ligand 1 Therapy. J Clin Oncol. 2017;35:709-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 605] [Cited by in RCA: 820] [Article Influence: 91.1] [Reference Citation Analysis (1)] |
| 7. | Johkoh T, Lee KS, Nishino M, Travis WD, Ryu JH, Lee HY, Ryerson CJ, Franquet T, Bankier AA, Brown KK, Goo JM, Kauczor HU, Lynch DA, Nicholson AG, Richeldi L, Schaefer-Prokop CM, Verschakelen J, Raoof S, Rubin GD, Powell C, Inoue Y, Hatabu H. Chest CT Diagnosis and Clinical Management of Drug-Related Pneumonitis in Patients Receiving Molecular Targeting Agents and Immune Checkpoint Inhibitors: A Position Paper From the Fleischner Society. Chest. 2021;159:1107-1125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 62] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 8. | Thomas R, Madan R, Gooptu M, Hatabu H, Hammer MM. Significance of the Reverse Halo Sign in Immunocompromised Patients. AJR Am J Roentgenol. 2019;213:549-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 9. | Zhan X, Zhang L, Wang Z, Jin M, Liu M, Tong Z. Reversed Halo Sign: Presents in Different Pulmonary Diseases. PLoS One. 2015;10:e0128153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |