Published online Oct 6, 2023. doi: 10.12998/wjcc.v11.i28.6931
Peer-review started: July 14, 2023
First decision: August 24, 2023
Revised: September 4, 2023
Accepted: September 14, 2023
Article in press: September 14, 2023
Published online: October 6, 2023
Processing time: 73 Days and 8 Hours
Although intestinal obstruction is one of the most common surgical emergencies in an infant, it is difficult to diagnose neonatal enteric duplication cysts (EDC) preoperatively owing to their rarity as a cause of intestinal obstruction. We describe a case report of a neonatal EDC presenting intestinal obstruction and shock.
A 32-d-old male infant with a prenatal sonographic finding of bladder distension was admitted to our hospital for a severely distended abdomen, fever, and oliguria. The first diagnostic hypothesis was septic shock and intestinal obstruction. The patient’s symptoms worsened; following an emergency surgical exploratory laparotomy and histopathological findings, the final diagnosis of cecal duplication cyst was confirmed. The patient’s postoperative course was uneventful, and on the fifth postoperative day, oral feeding restarted. Twenty days later, the patient was discharged from the hospital.
Although EDC located in the cecum is exceptional, it should be considered when evaluating suspected intestinal obstruction and shock.
Core Tip: Duplication cysts can present at any age with non-specific symptoms. An early diagnosis can help alleviate the symptoms with proper management, else these symptoms may progress to serious complications. This study presents a 32-d-old male child who presented with a severely distended abdomen with concomitant fever and oliguria. The provisional diagnosis was of septic shock which was later confirmed as a final diagnosis of cecal duplication cyst upon an emergency surgical laparotomy and histopathological findings of the excised mass. Although enteric duplication cyst located in the cecum is exceptional, it should be considered when evaluating suspected intestinal obstruction and shock.
- Citation: Kim SM, Lee SH, Park GY, Kim SS, Lee CG, Jin SJ. Cecal duplication cyst in an infant presenting as shock: A case report. World J Clin Cases 2023; 11(28): 6931-6937
- URL: https://www.wjgnet.com/2307-8960/full/v11/i28/6931.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i28.6931
As a rare congenital anomaly, the enteric duplication cyst (EDC) can arise anywhere along the intestinal tract between the mouth and anus, with an incidence of 1/4500 autopsies. Although its etiology is not completely understood, it is believed to occur between the 4th and 8th weeks of embryonic life. Recently, the origin of EDC has been hypothesized to be multifactorial[1]. EDCs can be structurally classified into either cystic or tubular forms. The cystic form is the most common duplication, and does not communicate with the adjacent lumen. On the other hand, the tubular form tends to communicate with the gastrointestinal tract running parallel to it[1,2]. Another way of classifying EDCs by their relation to the vascular supply was proposed in 1998. This classification divides them into parallel type (type I) and intra-mesenteric type (type II), and was suggested to facilitate surgical management[3].
Prenatal EDC diagnosis is difficult and rarely reported. Furthermore, preoperative diagnosis of EDCs in neonates may be difficult, and often occurs during laparotomy or through postoperative histopathological study[4]. Detection of EDC through prenatal ultrasonography is rare; only 20%–30% of entire EDCs are detected prenatally, and these are often misdiagnosed as other cystic lesions. This malformation can cause various nonspecific symptoms that complicate preoperative diagnosis[5]. The most common presentation of EDC among infants is an intestinal obstruction, and, after early childhood, abdominal pain. However, it may present symptoms at any age, which may progress to serious complications such as perforation, gastrointestinal bleeding, or septic shock. On the other hand, in some patients, symptoms do not appear until adulthood[1].
A full-term 32-d-old male infant was admitted to the general ward for a one-day history of intermittent fever (peak temperature 38.5ºC).
The patient had intermittent fever, deterioration of abdominal distension, and lethargy for one day.
The patient was born at 40+4 wk’ gestational age via spontaneous vaginal delivery with a birth weight of 3240 g. He had a perinatal history of bladder distension as seen on fetal ultrasonography, as well as abdominal distension that improved and worsened repeatedly after birth. Additionally, the patient presented with watery diarrhea, poor appetite and a markedly decreased amount of urine output for one day, but not with bilious vomiting or hematochezia. He was admitted to the general ward for conservative treatment, but symptoms worsened abruptly. Thus, he was transferred to the intensive care unit (ICU), where he exhibited lethargy, grunting, and signs of shock.
The patient did not have any clinically relevant family history.
On admission, the patient’s vital signs were as follows: blood pressure (BP) of 80/58 mmHg, heart rate (HR) of 158 beats per minute (bpm), respiratory rate (RR) of 43 breaths/min, oxygen saturation of 99% in room air, and body temperature (BT) of 38.4ºC. A physical examination showed an intensely distended abdomen with no palpable mass. In the ICU, signs of shock were observed: BP, 71/44 mmHg; HR, 170 bpm; RR, 52 breaths/min; oxygen saturation of 99% in room air; and BT, 38.4℃.
Initial laboratory tests showed mild leukopenia with mild neutropenia (white blood cell count 3.23 × 103/µL, neutrophils 1260/µL, band neutrophil 6%), elevated acute inflammatory markers [C-reactive protein, 4.99 mg/dL (reference: < 0.5 mg/dL), procalcitonin, 14.4 ng/mL (reference: < 0.5 ng/mL)], mild hyponatremia [sodium, 133 mmol/L (reference range: 135.0-145.0 mmol/L)], and pre-renal azotemia [blood urea nitrogen, 20.8 mg/dL (reference range: 8.0-20.0 mg/dL), creatinine, 0.4 mg/dL (reference range: 0.5-1.2 mg/dL)]. No microorganisms were detected in blood culture, urine culture, cerebrospinal fluid culture and multiplex polymerase chain reaction performed on a nasopharyngeal specimen.
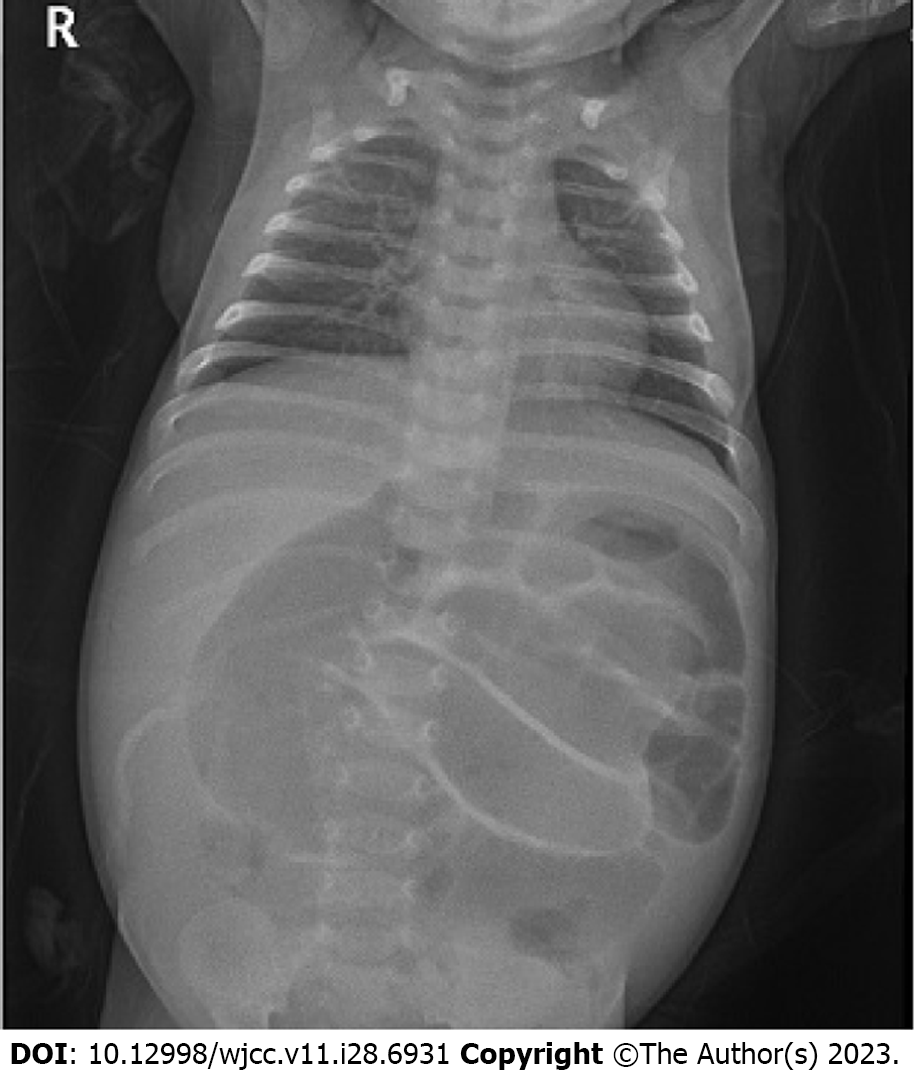
Plain abdominal radiographs showed diffuse gaseous distention of bowel loops (Figure 1). Due to the large amount of intra-intestinal gas accumulation, tracking the entire bowel in abdominal ultrasonography was restricted. In the visible area of sonography, there was no evidence of a focus of lesion that might cause mechanical obstruction. The patient also underwent an upper gastrointestinal series, which revealed that his junction between the duodenum and jejunum was normally placed on the left side of the vertebral body, suggesting that there was no bowel malrotation.
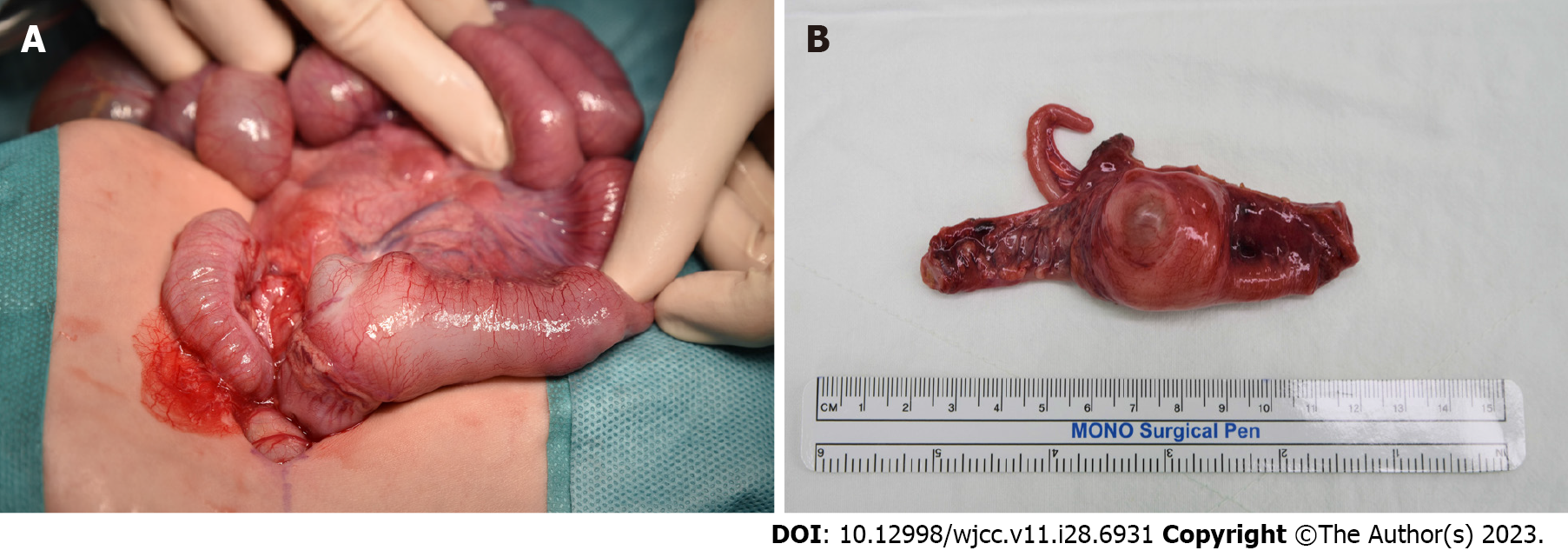
Symptoms of abdominal distension and oliguria progressively worsened. The patient was diagnosed with small bowel obstruction due to unspecified lesion and had to undergo surgery. During laparotomy, we observed a 4 cm × 3 cm solid structure in the cecum (Figure 2A). The small bowel was severely dilatated with no necrotic changes. The cyst and the contiguous portion of the ileum were resected (Figure 2B). Then, bowel continuity was restored by end-to-end anastomosis. When the cyst was opened, there was a cystic mass located at the cecum, measuring 2.5 cm × 2.0 cm × 1.0 cm which was found to be unilocular and contained a clear, light-yellowish, mucinous fluid, including gelatinous material. Histopathological examination of the cystic lesion revealed two distinct muscle layers on low magnification. The outer layer was continuous with the intestine. Immunohistochemical staining for desmin highlighted two separated muscle layers, and the cyst-lining epithelium consisting of low columnar epithelial cells or flattened cuboidal cells (Figure 3).
The findings of the investigations confirmed the diagnosis of a cecal duplication cyst.
The cyst and the contiguous portion of the ileum were resected and bowel continuity was restored by end-to-end anastomosis.
The patient’s postoperative healing was uneventful, and oral feeding resumed on the fifth postoperative day. Symptoms did not recur, and normal bowel gas patterns were observed on follow-up abdomen X-rays. The patient was discharged from the hospital 20 d later.
R H Fitz first reported the EDC in 1884; however, its etiology is not completely understood even now. The most important criterion when diagnosing EDC is the presence of a normal gastrointestinal epithelial lining. Other criteria include the presence of surrounding smooth muscles and continuity with the alimentary tract[6]. EDC can be found anywhere in the alimentary tract, though most cysts are found at the ileum[3,7]. Among all the locations of EDCs, duplication of the cecum as in our case, is rare. Oudshoorn et el[8] found only 16 cases of cecal duplication cysts among 362 cases (4.4%) of EDCs. Another single institutional review of 40 cases of EDCs revealed only 4 cases (10%) of cecal duplication cysts[9].
EDC can cause a variety of nonspecific symptoms that complicate preoperative diagnosis. Symptoms can occur by intra-abdominal EDCs at any time, but tend to occur in the early stages of life. The most common presentation of EDC among infants, as in our case, is intestinal obstruction[6,10,11]. Progression into serious complications such as perforation, gastrointestinal bleeding, or a septic shock is also possible[12]. In addition, EDCs may act as the regional point for volvulus or intussusceptions, or malignant neoplasm[13]. In some patients, however, EDC may not present any symptoms until adulthood, and gets detected incidentally[1,12].
Sonography is typically the first line modality in prenatal detection of abdominal masses, and prenatally detected cystic abdominal masses are usually renal or ovarian in origin. However, the less common causes such as hepatic, choledochal cysts, EDCs, hydrocolpos or malformations should be considered in the differential diagnoses[12]. EDC may be suspected when sonography detects an intra-abdominal cystic mass in the second or third trimester of gestation[13]. However, actual detection of EDC through prenatal ultrasonography is rare, and misdiagnosis with other cystic lesions is common[13,14].
Prenatal sonographic findings of an enlarged fetal bladder may simply be a transitory normal variant, but it may also be secondary to reflux or obstructive causes. When sonographic examination reveals an enlarged fetal bladder, the ureter, kidneys, genitalia, and sine should be evaluated carefully since serious complications can appear in infancy. In our case, bladder distension was observed during prenatal ultrasonography but was considered as a nonspecific finding at the time. It is assumed that the distended bladder found on prenatal ultrasonography was a duplication cyst. However, the importance of undergoing investigations for EDC in an asymptomatic infant with prenatal sonographic bladder distension is unclear, and requires more study.
Postnatal abdominal ultrasonography is a useful tool to evaluate EDC[1]. Five layered cyst wall, muscular rim sign, double-wall sign, and Y sign (splitting of shared muscularis propria between the cystic lesion and adjacent bowel) are sonographic features that can appear in EDC[1,15,16]. Among them, the “Y sign” is a unique finding of EDC that is absent in other abdominal cystic lesions[16,17]. Despite its sensitivity and usefulness, abdominal ultrasonography could not point out the focus of intestinal obstruction in our case. We assume that a large amount of air and fecal contents in the patient’s intestine kept EDC from detection. Therefore, it is important not to completely rule out the possibility of EDC when no specific lesion is detected in ultrasonography, especially when a clear view is unattainable.
Computed tomography (CT) is not typically used in diagnosing EDCs, especially in children, owing to the possibility of exposure to radiation. Moreover, magnetic resonance imaging (MRI) is not routinely used in younger children, due to the requirement of sedation. The patient in our case did not go through CT or MRI; since his symptoms and general condition worsened, he was deemed unable to endure sedation or medical procedure of CT or MRI[1,4].
End-to-end anastomosis with full EDC resection with an adjacent bowel has been recommended and used as the optimal management method[11,18]. Large duplication cysts, however, might be difficult to resect, since the risk of short-bowel syndrome increases as a greater portion of the bowel gets resected. In these cases, mucosal stripping offers a better surgical option, with the elimination of the possibility of subsequent peptic ulceration or carcinogenesis[18]. In our case, the patient went through a conventional full resection of EDC with adjacent bowel, since the size of the cyst was not large enough to cause postoperative short bowel syndrome.
We report a case of a full-term infant who experienced intestinal obstruction and shock; the infant was diagnosed with a cecal duplication cyst postoperatively. Although EDC rarely causes intestinal obstruction, and cecal location is extremely uncommon, it should be considered when evaluating suspected intestinal obstruction and shock in young children with prenatal sonographic findings of bladder distension without other urinary tract abnormalities. Furthermore, EDC should be considered during differential diagnoses when an infant with prenatal sonographic findings of abdominal cystic lesions shows signs of intestinal obstruction after birth.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Pediatrics
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Moshref L, Saudi Arabia; Tanpowpong P, Thailand S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Sangüesa Nebot C, Llorens Salvador R, Carazo Palacios E, Picó Aliaga S, Ibañez Pradas V. Enteric duplication cysts in children: varied presentations, varied imaging findings. Insights Imaging. 2018;9:1097-1106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 70] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 2. | Ben-Ishay O, Connolly SA, Buchmiller TL. Multiple duplication cysts diagnosed prenatally: case report and review of the literature. Pediatr Surg Int. 2013;29:397-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Li L, Zhang JZ, Wang YX. Vascular classification for small intestinal duplications: experience with 80 cases. J Pediatr Surg. 1998;33:1243-1245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Abdellaoui H, Mahmoudi A, Tazi Charki M, Bouabdallah Y. Caecal duplication cyst: a rare cause of neonatal intestinal obstruction. BMJ Case Rep. 2018;2018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 5. | Puligandla PS, Nguyen LT, St-Vil D, Flageole H, Bensoussan AL, Nguyen VH, Laberge JM. Gastrointestinal duplications. J Pediatr Surg. 2003;38:740-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 150] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 6. | Bond SJ, Graff DB, O Neil JA, Grosfeld JL, Tanskalsrud EW, Coran AG. Gastrointestinal duplications. In Paediatric Surgery, 5th ed.; Mosby, 1998; 1257-1263. [RCA] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Mehl SC, Anbarasu C, Sun R, Naik-Mathuria B. Cecal Duplication Cyst: A Rare Cause of Pediatric Bowel Obstruction. Am Surg. 2022;88:2068-2070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 8. | Oudshoorn JH, Heij HA. Intestinal obstruction caused by duplication of the caecum. Eur J Ped. 1996;155:338-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Erginel B, Soysal FG, Ozbey H, Keskin E, Celik A, Karadag A, Salman T. Enteric Duplication Cysts in Children: A Single-Institution Series with Forty Patients in Twenty-Six Years. World J Surg. 2017;41:620-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 10. | Gul A, Tekoglu G, Aslan H, Cebeci A, Erol O, Unal M, Ceylan Y. Prenatal sonographic features of esophageal and ileal duplications at 18 weeks of gestation. Prenat Diagn. 2004;24:969-971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Singh S, Gupta R, Mandal AK. Complete gastric duplication cyst. Indian J Gastroenterol. 2005;24:170-171. [PubMed] |
| 12. | Mandell J, Lebowitz RL, Peters CA, Estroff JA, Retik AB, Benacerraf BR. Prenatal diagnosis of the megacystis-megaureter association. J Urol. 1992;148:1487-1489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Chen M, Lam YH, Lin CL, Chan KW, Hui PW, Tang MH, Lee CP, Khong PL. Sonographic features of ileal duplication cyst at 12 weeks. Prenat Diagn. 2002;22:1067-1070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Foley PT, Sithasanan N, McEwing R, Lipsett J, Ford WD, Furness M. Enteric duplications presenting as antenatally detected abdominal cysts: is delayed resection appropriate? J Pediatr Surg. 2003;38:1810-1813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 79] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 15. | Silva CT, Engel C, Cross SN, Copel JE, Morotti RA, Baker KE, Goodman TR. Postnatal sonographic spectrum of prenatally detected abdominal and pelvic cysts. AJR Am J Roentgenol. 2014;203:W684-W696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Kumar D, Ramanathan S, Haider E, Khanna M, Otero C. Education and Imaging. Gastroenterology: Revisiting the forgotten sign: Five layered gut signature and Y configuration in enteric duplication cysts on high resolution ultrasound. J Gastroenterol Hepatol. 2015;30:1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Cheng G, Soboleski D, Daneman A, Poenaru D, Hurlbut D. Sonographic pitfalls in the diagnosis of enteric duplication cysts. AJR Am J Roentgenol. 2005;184:521-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 86] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 18. | Gálvez Y, Skába R, Kalousová J, Rousková B, Hríbal Z, Snajdauf J. Alimentary tract duplications in children: high incidence of associated anomalies. Eur J Pediatr Surg. 2004;14:79-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |