Published online Oct 6, 2023. doi: 10.12998/wjcc.v11.i28.6920
Peer-review started: June 30, 2023
First decision: July 28, 2023
Revised: August 9, 2023
Accepted: September 5, 2023
Article in press: September 5, 2023
Published online: October 6, 2023
Processing time: 86 Days and 23.1 Hours
Various treatment methods are available for the treatment of pancreatic arteriovenous malformation (P-AVM); however, there are no established treatment options for asymptomatic P-AVM.
A 47-year-old and a 50-year-old male patients sought treatment for P-AVM in the pancreas, which was incidentally detected during routine abdominal computed tomography and magnetic resonance imaging conducted as part of a health check-up. They underwent transcatheter arterial embolization (TAE), and over the course of a 9-year follow-up period, the AVM did not worsen and was asymp
TAE can be considered as an alternative treatment option for P-AVM in selective cases where patients are asymptomatic or have a high surgical risk.
Core Tip: Pancreatic arteriovenous malformation (P-AVM) is a rare condition characterized by symptoms like gastrointestinal bleeding and abdominal pain, with some cases being asymptomatic. Surgical intervention is commonly considered for symptomatic cases, but the treatment of asymptomatic P-AVMs is not well-established. Previous studies have reported various methods, including surgery and transcatheter arterial embolization (TAE). In selective cases, particularly for asymptomatic or high surgical risk patients, TAE may be an effective and safe treatment option.
- Citation: Shin SH, Cho CK, Yu SY. Pancreatic arteriovenous malformation treated with transcatheter arterial embolization: Two case reports and review of literature. World J Clin Cases 2023; 11(28): 6920-6930
- URL: https://www.wjgnet.com/2307-8960/full/v11/i28/6920.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i28.6920
Pancreatic arteriovenous malformation (P-AVM) is a rare condition characterized by abnormal blood flow resulting from anastomoses between arteries and veins[1]. Since its initial description by Halpern et al[1] in 1968, fewer than 100 cases of P-AVM have been reported in the literature[2]. P-AVM can present with various symptoms including abdominal pain, gastrointestinal (GI) hemorrhage, and jaundice, or it may be discovered incidentally without any specific symptoms[3].
P-AVM diagnosis can be confirmed with various imaging modalities such as contrast-enhanced computed tomography (CT), magnetic resonance imaging (MRI), and angiography. In contrast-enhanced CT and MRI scans, imaging findings often show the presence of dilated and tortuous feeding arteries that supply the abnormal vascular network within the pancreas[4]. Additionally, angiography is particularly useful for identifying the abnormal vascular network associated with P-AVM, with the early filling of draining veins (e.g., the portal vein) indicating the presence of P-AVM[5].
Surgery is often considered as the primary treatment option for symptomatic patients with P-AVM; nevertheless, other treatment modalities such as transcatheter arterial embolization (TAE), radiation therapy, and even conservative management have been considered by researchers[2,6-8].
Here, we present a report of two cases of asymptomatic P-AVM treated with TAE and an additional literature review on P-AVM.
Case 1: A 47-year-old male patient came to our hospital for treatment due to suspicion of P-AVM, which was identified during a health checkup at a different medical institution.
Case 2: A 50-year-old male patient came to our hospital for treatment due to suspicion of P-AVM, which was identified during a health checkup at a different medical institution.
Case 1: The patient, who was asymptomatic, underwent a routine health check-up at a local hospital. The laboratory tests performed during the check-up did not show any abnormalities. However, as the abdominal ultrasound showed abnormal findings in the pancreas, an abdominal CT was performed. Additionally, to obtain more detailed information, MRI was conducted, which detected P-AVM. For the treatment this lesion, the patient visited our hospital.
Case 2: The patient, who was asymptomatic, underwent a routine health check-up at a local hospital. The abdominal ultrasound showed abnormal findings in the pancreas, which led to the decision to perform MRI. Subsequently, abdominal CT was conducted, which revealed the presence of pancreatic neck and body AVM. The patient visited our hospital for the treatment of this lesion.
Case 1: The patient had hypertension (HTN) and chronic kidney disease (CKD) detected 4 months ago. HTN was well-controlled (mean systolic blood pressure 140 mmHg) with medication. CKD was diagnosed as advanced renal failure (Cr 5.1 mg/dL, eGFR 11.79 mL/min/1.73 m²), stage 5.
Case 2: The patient was diagnosed with diabetes mellitus 1 year ago, which was effectively managed with medication, resulting in well-controlled blood sugar levels.
Cases 1 and 2: The patients had no family history.
Cases 1 and 2: The patients' abdomen was soft upon palpation, without any abnormalities.
Cases 1 and 2: The laboratory tests were normal.
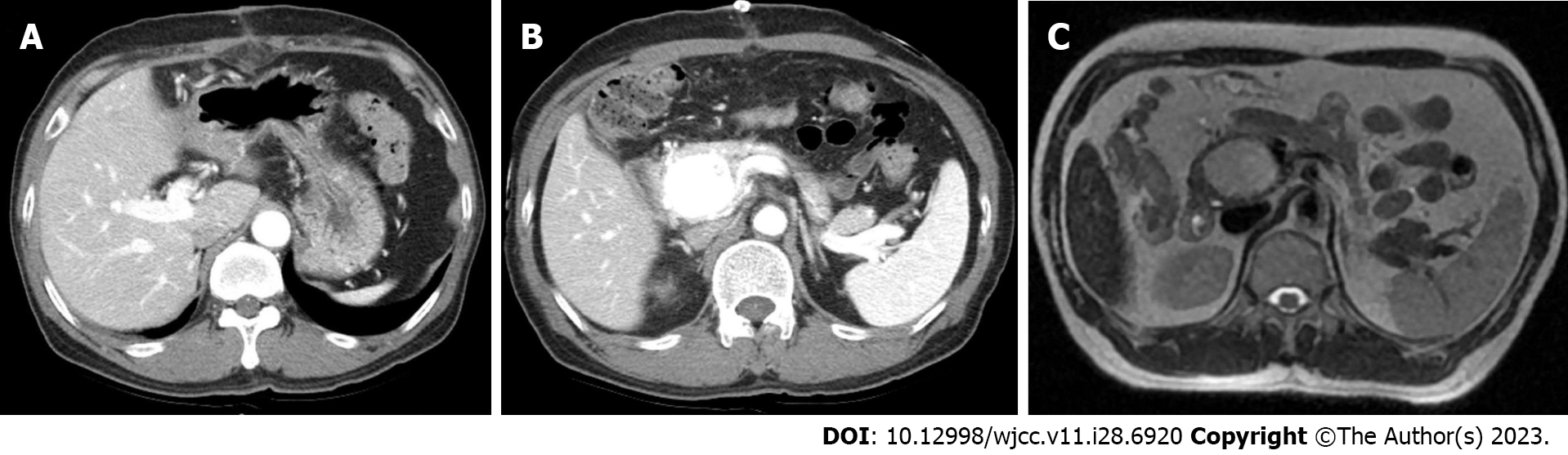
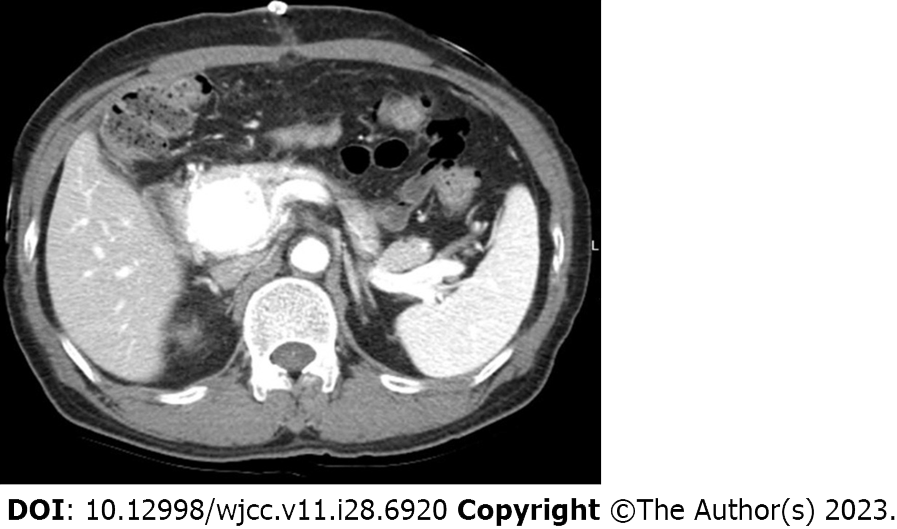
Case 1: Abdominal contrast-enhanced CT and MRI revealed a well-enhanced hypervascular lesion of around 4.0 cm located in the pancreatic head. The portal vein was filled early with arterial blood during the arterial phase, leading to the suspicion of P-AVM (Figure 1).
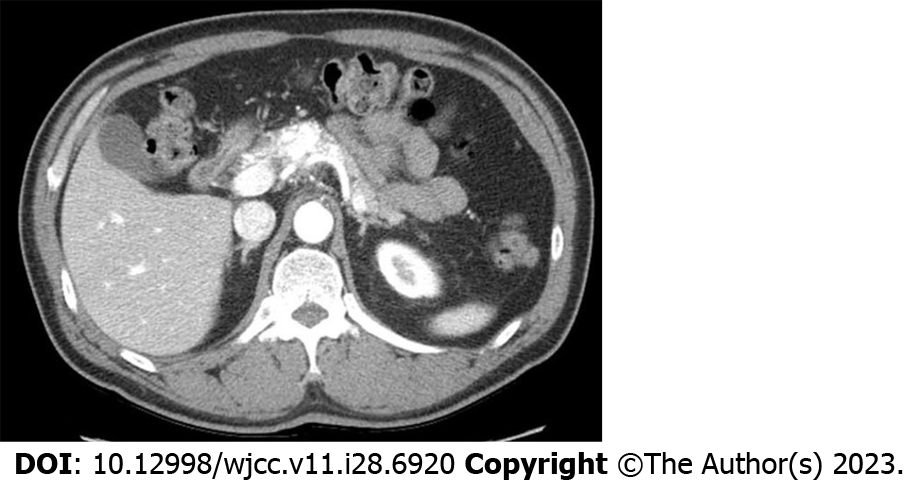
Case 2: Abdominal contrast CT and MRI scans showed a 5.2 cm × 4.0 cm dilated and twisted hypervascular lesion in the pancreas neck and body, with strong enhancement of the vessels and arterial blood presence in the portal vein during the arterial phase (Figure 2).
Case 1: Before intervention, the patient underwent follow-up laboratory tests. Based on the results, the patient had slightly elevated amylase (136 U/L) and lipase (109 U/L) levels, along with an increased CRP level (5.73 mg/dL); however, serum tumor marker levels were normal.
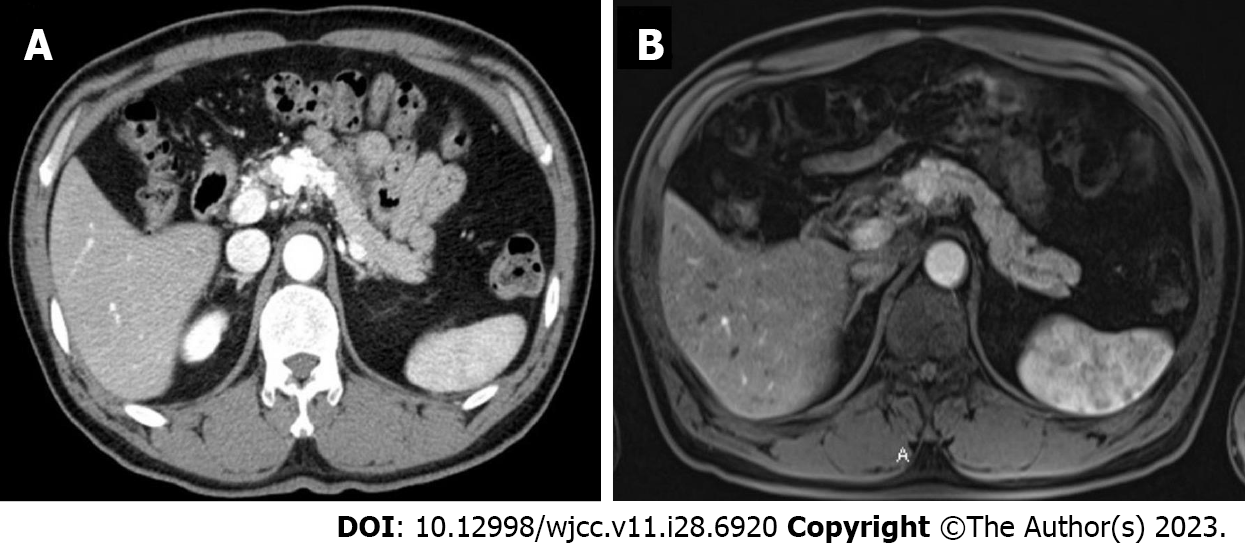
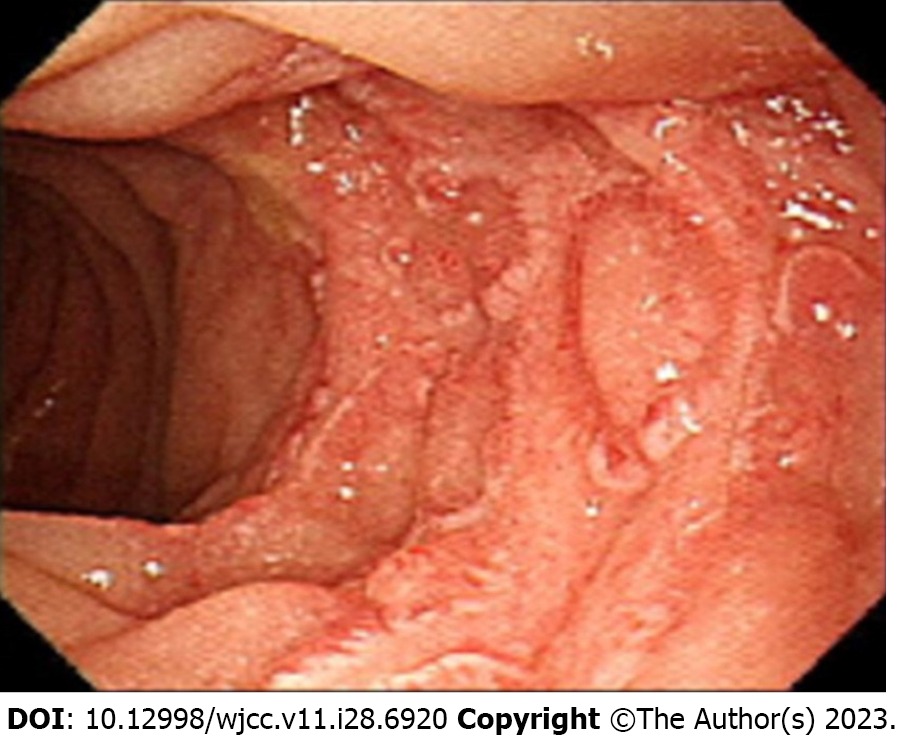
Case 2: Before intervention, all laboratory tests including tumor markers were within the normal range. Additionally, an irregular cystic lesion measuring approximately 2.0 cm was identified in the neck and body of the pancreas during abdominal endoscopic ultrasonography (Figure 3).
P-AVM.
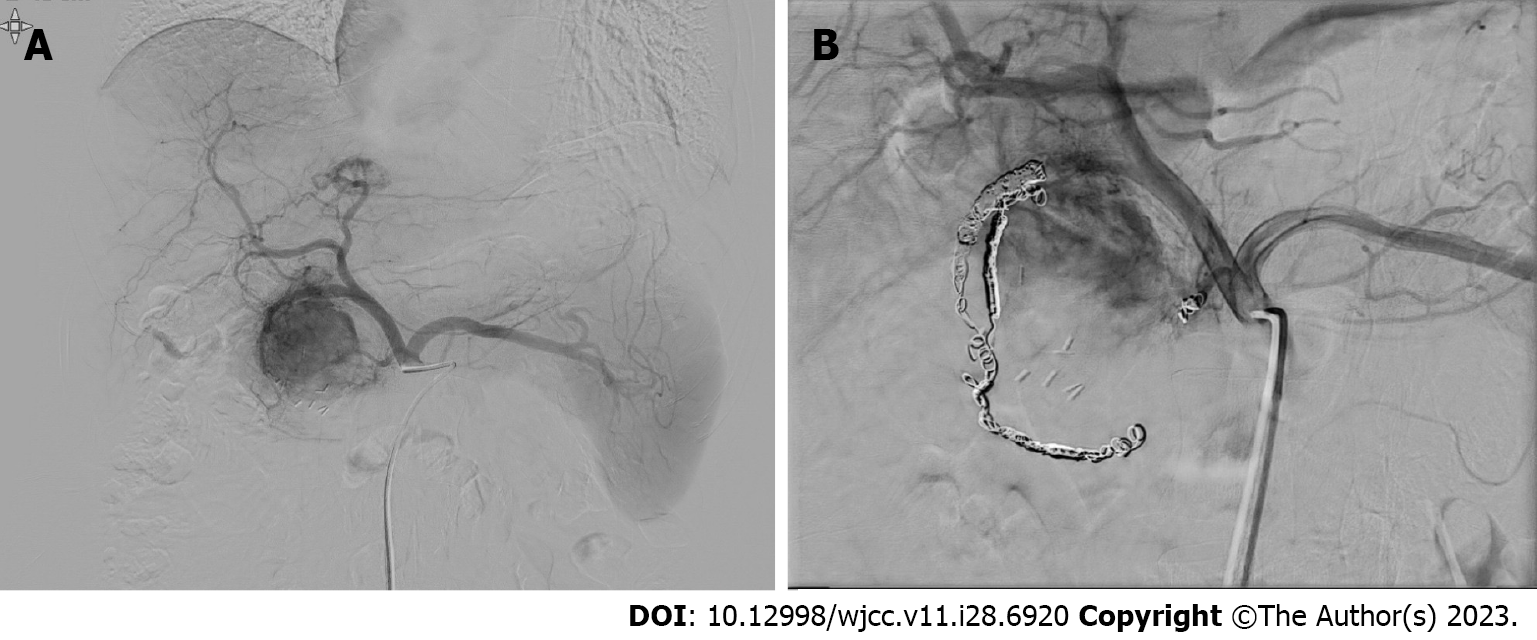
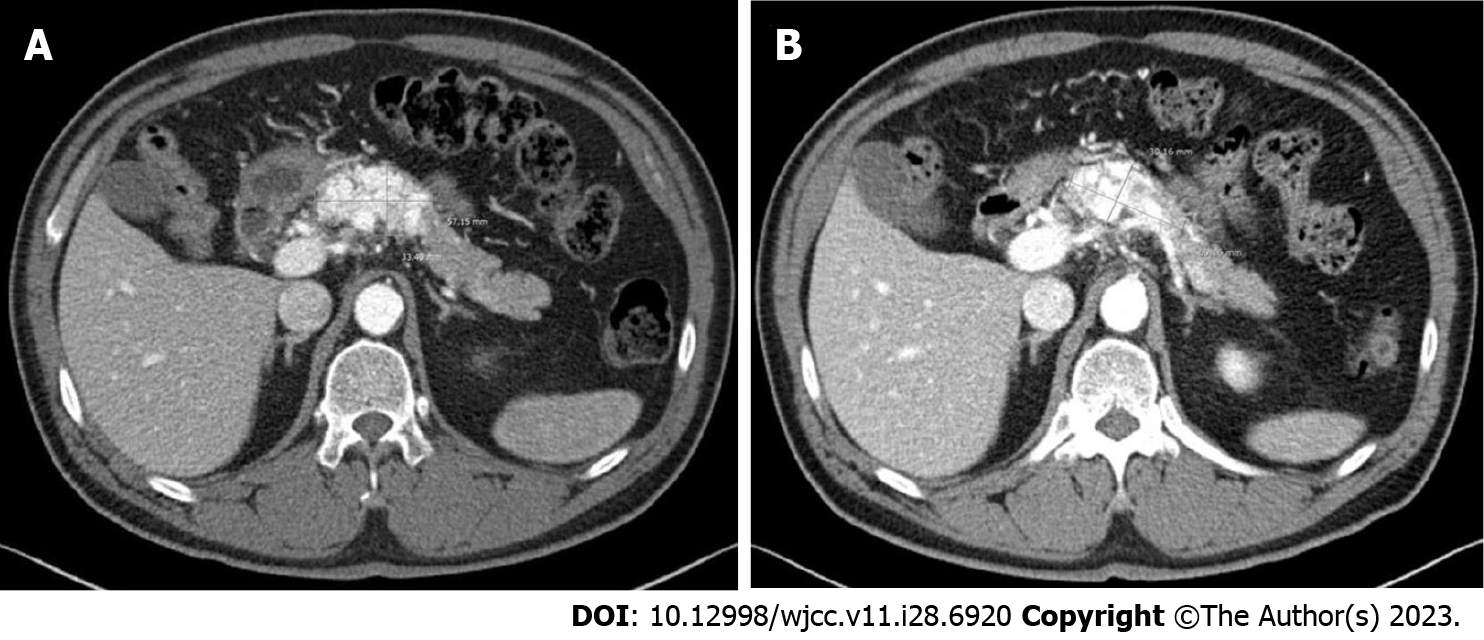
Considering the patient’s asymptomatic status and high surgical risk, we decided to perform TAE. Angiography of the SMA, celiac axis, and gastroduodenal artery was conducted, showing hypervascular staining of the tumor of the pancreatic head. Superselective catheterization of the gastroduodenal artery showed multiple feeding branches originating from the pancreaticoduodenal arcade. The pancreaticoduodenal arcade and dorsal pancreatic artery were treated using interlock coils. Interlock coils are specialized devices designed specifically for the purpose of occluding blood vessels (Figure 4).
Considering the patient’s asymptomatic status and diffuse lesion of the pancreas, we decided to perform TAE. Angiography of the SMA, celiac axis, and common hepatic artery was performed, which showed hypervascular staining in the pancreatic neck and body. The feeding branches from the proper hepatic artery and dorsal pancreatic artery were then embolized using interlock coils (Figure 5).
A follow-up abdominal computed tomography angiography performed on the 5th day after TAE showed no significant change in the size of the hypervascular lesion in the pancreatic head (Figure 6). The patient had persistent symptoms such as fever, chills, and upper abdominal pain beginning on the 5th day after the procedure. An esophagogastroduodenoscopy was performed to further evaluate the condition of the patient, which showed evidence of ischemic duodenitis and atrophic gastritis (Figure 7). After TAE, the patient received conservative treatment for approximately 1 week, during which the symptoms were gradually improved, and the patient was discharged. Conservative treatment included nutrition support, proton pump inhibitor medication, pain control, and hydration.
After discharge, the patient underwent regular follow-ups, including abdominal CT and laboratory tests, which were initially performed every 3 to 6 mo for 2 years before subsequently transitioning to an annual follow-up schedule.
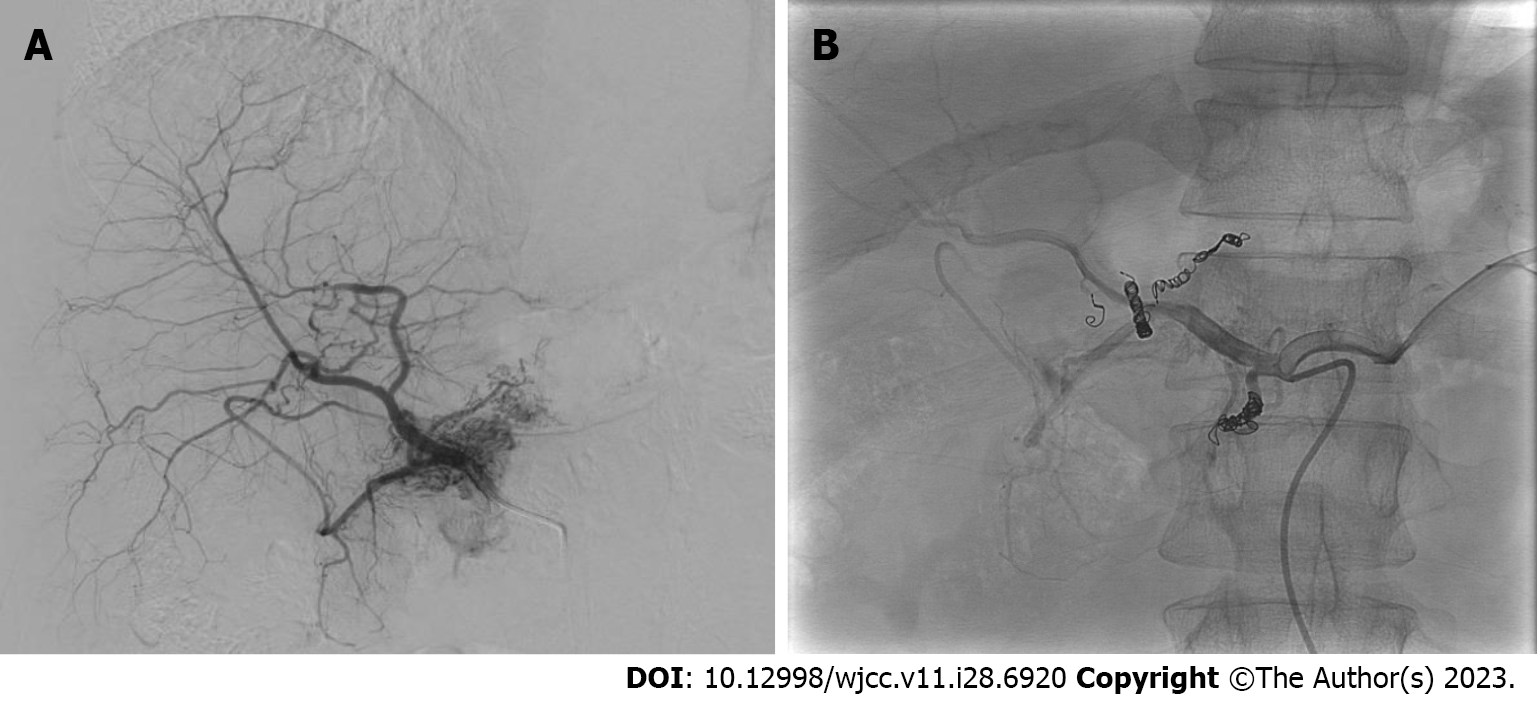
During the 9-year follow-up period, the AVM showed a significant improvement in hypervascularity, with the lesion size decreasing from 3.7 cm to 2.3 cm. The patient remained asymptomatic (Figure 8).
A follow-up CT scan on the 4th day after TAE showed reduced contrast enhancement and hypervascularity (Figure 9). However, during hospital stay, the symptoms of the patient gradually improved, and the patient was discharged.
After discharge, the patient underwent regular follow-ups, including abdominal CT and laboratory tests, which were initially performed every 3 to 6 mo for 2 years before subsequently transitioning to an annual schedule.
During the 3rd year of follow-up, no significant increase in the hypervascularity of the P-AVM was observed; however, the size of the lesion changed from 5.2 cm × 4.0 cm to 5.7 cm × 3.3 cm. No significant changes were observed in subsequent follow-up examinations over the next 9 years (Figure 10). During the 9-year follow-up period, the patient remained asymptomatic, and there were no significant changes in the size of the lesion in the pancreatic head, which remained at 6.0 cm × 3.0 cm.
P-AVM is a rare condition characterized by the abnormal connection between the arterial and portal systems in the pancreas, resulting in tumor formation and blood flow abnormalities[8]. Histological examinations usually identify dilated and twisted blood vessels within the pancreatic parenchyma as well as collections of irregularly tortuous blood vessels with thick walls[3,9,10].
Chou et al[11] (2013) conducted a study on 89 patients with P-AVM, which showed that it was more common among males (85.4%) than among females (14.6%). The median age of the patients in their study was 50 years. Furthermore, Wu et al[3] (2021) reported a male predominance (24 males, 2 females) among P-AVM patients, with an average age at diagnosis of 51.5 years.
AVM can occur in any part of the GI tract; however, the incidence of P-AVM is very low, accounting for only 0.9% of all AVM cases[11-15]. Several studies have reported that the majority of P-AVMs (48.3%-62.3%) are located in the pancreatic head, followed by the pancreatic body and tail and the entire pancreas[10,12,16].
Common symptoms of P-AVM include abdominal pain and GI bleeding; however, acute pancreatitis, portal HTN leading to ascites, esophageal variceal rupture, and severe GI bleeding are also associated with P-AVM[10]. Chou et al[11] (2013) showed that GI bleeding was the most common symptom (47.2%), followed by epigastric pain (46.1%) and back pain (9%), with a small percentage of patients (4.5%) being asymptomatic.
CT, MRI, and angiography are commonly used to diagnose P-AVM as they can detect dilated and tortuous feeding arteries supplying the abnormal vascular network in the pancreas[4]. Angiography can also provide a detailed visualization of blood vessels, with the early filling of veins confirming abnormal blood flow and the presence of P-AVM[5].
Angiography is valuable for obtaining detailed information about the extent and characteristics of P-AVM, including multiple lesions, localization, and spread. It also plays an important role in treatment planning by identifying feeding arteries and determining the optimal target for embolization[17,18]. According to Wu et al[3] (2021), Color Doppler ultrasonography is a useful diagnostic tool for P-AVM, particularly in identifying the characteristic "mosaic sign" of vascular malformation associated with P-AVM.
Yakes et al[19] (2014) developed a categorization system for AVM based on the inflow arteries, outflow veins, and AVM type. This classification includes five types of AVM. Type 1 has a single arterial input without early venous drainage. Type 2 involves multiple arterial inputs with no early venous drainage. Type 3 exhibits early venous drainage with a single draining vein (subdivided into Type 3a with low flow and a small-diameter draining vein and Type 3b with high flow and a large-diameter draining vein). Type 4 has multiple arterial inputs with early venous drainage. Our AVM cases showed multiple feeding branches and vein outflow on contrast-enhanced abdomen CT and arteriography, which could be classified as Type 2 AVMs.
P-AVM is normally treated with surgical resection or TAE; however, other treatment options such as radiotherapy, trans-jugular intrahepatic portosystemic shunt (TIPS), and conservative management may also be considered[2].
Surgical resection is considered as a curative treatment modality for P-AVM. However, it is important to carefully evaluate the potential risks associated with the procedure. Pancreatectomy for AVM carries the risk of significant bleeding during or after the operation, and the invasiveness of the procedure may lead to the development of diabetes as a potential side effect. Therefore, a thorough assessment of these risks is crucial when considering pancreatectomy as a treatment option for P-AVM[2].
Chou et al[11] (2013) reported that primary treatments for P- AVM were surgery (43.8%) and TAE (11.2%). A smaller proportion of cases involved a combination of surgery and TAE (10.1%), and a minority of patients were treated with radiotherapy (2.2%). However, a significant percentage of P-AVM patients (29.2%) were managed through conservative treatment without any intervention[8].
TAE is less invasive for P-AVM management but carries a higher risk of recurrence and potential complications requiring additional treatments. Hakoda et al[20] (2022) reported that TAE may be associated with a higher risk of AVM recurrence and the potential for embolization of other portal branches because of the migration of embolic agents. Additionally, achieving complete embolization may be difficult as the AVM is often supplied by multiple feeding arteries. Wu et al[3] (2021) found that 57.7% of the 26 patients in their study who underwent TAE for P-AVM were successfully treated, whereas the remaining 42.3% of patients required additional surgical intervention. They also reported that surgical resection may be the most effective treatment option for P-AVM, and alternative approaches such as TAE, TIPS, and radiotherapy may not be able to effectively manage the associated complications.
Other studies have investigated the effectiveness of TAE for P-AVM. Marcelin et al[21] (2022) conducted a study with 7 patients to evaluate the efficacy and safety of TAE for patients with P-AVM. A total of 5 of the 7 patients underwent TAE for symptomatic P-AVM, with 80% of patients experiencing complete resolution of P-AVM symptoms. Among patients with incomplete embolization, 25% of patients required additional surgery, and 75% of patients responded well to conservative treatment. They observed the progressive regression of P-AVM with no symptom recurrence despite incomplete embolization. Therefore, they suggested that TAE may be considered as a safe and effective treatment option for selected cases of symptomatic P-AVM, with surgical intervention reserved as a secondary option[20].
We have conducted a case review focusing on TAE as a treatment option for P-AVM, which included our cases. Of the 33 cases included in our review, the majority of cases involved males (29 cases, 87.9%). The most common initial symptom was abdominal pain, which was reported in 18 cases (54.5%). GI bleeding was observed in 9 cases (27.3%). There was 1 case (3%) of ascites, and the remaining 5 cases (15.2%) were asymptomatic. In 26 cases (78.8%), the pancreatic head was the predominant location for P-AVM, and the remaining 7 cases (21.2%) involved either the body or tail region. After TAE, most patients (17 cases, 51.5%) showed no post-procedure symptoms or complications; however, there were GI bleeding in 7 cases (21.2%), duodenal ulcers and duodenitis in 3 cases (9.1%), and abdominal pain in 1 case (3%). The remaining 5 cases (15.2%) had unknown complications (Table 1). These findings offer valuable insights into the clinical characteristics and outcomes of TAE as a treatment option for P-AVM[3].
| Ref. | Year | No. of cases | Age | Sex | Initial main symptoms | Location | Symptoms or complications after TAE | Additional treatment |
| Gomes et al[22] | 1982 | 1 | 52 | M | Abdominal pain | Head | GI bleeding | PD |
| Kato et al[23] | 1991 | 1 | 60 | M | Asymptomatic | Body or/and tail | None | None |
| Ishikawa et al[24] | 1993 | 1 | 66 | M | Abdominal pain | Head | None | None |
| Hirai et al[25] | 1995 | 1 | 67 | M | Asymptomatic | Body or/and tail | Unknown | Radiation |
| Hayashi et al[8] | 1998 | 1 | 45 | M | GI bleeding | Head | GI bleeding | TIPS |
| Uda et al[26] | 1999 | 1 | 48 | M | Abdominal pain | Head | Unknown | PPPD |
| Iwashita et al[27] | 2002 | 1 | 58 | M | GI bleeding | Head | None | None |
| Sato et al[7] | 2003 | 1 | 60 | M | GI bleeding | Head | GI bleeding | Radiation |
| Hosogi et al[28] | 2006 | 1 | 45 | M | GI bleeding | Head | Unknown | PPPD |
| Ogawa et al[29] | 2009 | 1 | 54 | M | Asymptomatic | Body or/and tail | None | None |
| Gincul et al[30] | 2010 | 1 | 55 | M | Abdominal pain | Head | None | None |
| Charalabopoulos et al[12] | 2011 | 1 | 64 | F | Abdominal pain | Body or/and tail | Abdominal pain | None |
| Sharma et al[5] | 2011 | 1 | 26 | M | Abdominal pain | Head | GI bleeding | PD |
| Qayed et al[31] | 2011 | 1 | 47 | M | GI bleeding | Head | GI bleeding | PPPD |
| Grasso et al[32] | 2012 | 1 | 48 | M | GI bleeding | Head | Duodenal ulcers | None |
| Song et al[6] | 2012 | 2 | 44 (1), 46 (1) | M (2) | Abdominal pain (2) | Head (2) | Unknown (2) | PPPD (2) |
| Arora et al[33] | 2013 | 1 | 37 | Male | Abdominal pain | Head | GI Bleeding | PD |
| Uojima et al[34] | 2014 | 1 | 49 | Male | Abdominal pain | Head | None | None |
| Tatsuta et al[35] | 2014 | 1 | 57 | Male | Abdominal pain | Head | None | None |
| Cassinotto et al[36] | 2015 | 1 | 56 | Male | Abdominal pain | Head | Duodenal ulcers | None |
| Fukami et al[37] | 2015 | 1 | 50 | Male | Abdominal pain | Head | None | PPPD |
| Vidmar et al[38] | 2016 | 1 | 54 | Male | Abdominal pain | Head and body | None | None |
| Kohan et al[39] | 2017 | 1 | 46 | Male | Abdominal pain | Body or/and tail | None | None |
| Gupta et al[14] | 2018 | 1 | 60 | Male | Abdominal pain | Head | None | PPPD |
| Yoon et al[2] | 2020 | 1 | 43 | Male | Abdominal pain | Body or/and tail | None | None |
| Marcelin et al[21] | 2022 | 5 | 61.1 (43-79) | M (1), F (4) | GI bleeding (3), abdominal pain (1), ascite (1) | Head (5) | GI bleeding (1), none (4) | None (4), PPPD (1) |
| Current studies | 2023 | 2 | 56 (1), 59 (1) | M (2) | Asymptomatic (2) | Head (1), body (1) | Ischemic duodenitis (1), none (1) | None (2) |
This study has some limitations, such as a small number of included patients and the use of a retrospective design. The limited sample size might not fully represent the entire population of patients with P-AVM. To strengthen the evidence on the effectiveness of TAE for P-AVM, prospective studies will be necessary in the future. Well-designed prospective studies can provide more reliable evidence on the outcomes of TAE. These studies may potentially involve a larger patient cohort with a longer follow-up period, allowing for a more comprehensive evaluation of treatment efficacy.
Despite these limitations, this study provides valuable insights into the management of P-AVM with TAE. The findings could contribute to a more comprehensive understanding of the efficacy and safety of TAE as a treatment option for P-AVM, ultimately leading to improved patient outcomes.
There are currently no established treatment guidelines for P-AVM; however, there are various treatment options available, including surgical resection and TAE. Surgical resection is the mainstay of treatment for symptomatic patients. Nevertheless, TAE can be considered as an alternative treatment option in selective cases where patients are asymptomatic or have a high surgical risk.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, general and internal
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Augustin G, Croatia; Toti L, Italy S-Editor: Yan JP L-Editor: A P-Editor: Yan JP
| 1. | Halpern M, Turner AF, Citron BP. Hereditary hemorrhagic telangiectasia. An angiographic study of abdominal visceral angiodysplasias associated with gastrointestinal hemorrhage. Radiology. 1968;90:1143-1149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 122] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 2. | Yoon SY, Jeon GS, Lee SJ, Kim DJ, Kwon CI, Park MH. Embolization of pancreatic arteriovenous malformation: A case report. World J Clin Cases. 2020;8:1471-1476. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
| 3. | Wu W, An FD, Piao CL, Tan MK, Si ZD, Xin L, Zhao N, Leng JJ. Management of pancreatic arteriovenous malformation: Case report and literature review. Medicine (Baltimore). 2021;100:e27983. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Ishigami K, Sakuma T, Saito M, Kawakami Y, Masaki Y, Murota A, Motoya M, Kimura Y, Nakase H. Arteriovenous malformation in pancreas mimicking hypervascular tumor. JGH Open. 2020;4:773-774. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Sharma M, Bedi MM, Mahesh S, Gandhi MD, Antony R, Mukkada RJ, Lekha V, Ramesh H. Arteriovenous malformation of the pancreatic head--difficulties in diagnosis and treatment. Indian J Gastroenterol. 2011;30:46-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Song KB, Kim SC, Park JB, Kim YH, Jung YS, Kim MH, Lee SK, Lee SS, Seo DW, Park DH, Kim JH, Han DJ. Surgical outcomes of pancreatic arteriovenous malformation in a single center and review of literature. Pancreas. 2012;41:388-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 7. | Sato M, Kishi K, Shirai S, Suwa K, Kimura M, Kawai N, Tanihata H, Yamada K, Terada M, Yamaue H. Radiation therapy for a massive arteriovenous malformation of the pancreas. AJR Am J Roentgenol. 2003;181:1627-1628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Hayashi N, Sakai T, Kitagawa M, Inagaki R, Yamamoto T, Ishii Y, Maehara M, Nakagawara G. Intractable gastrointestinal bleeding caused by pancreatic arteriovenous malformation: successful treatment with transjugular intrahepatic portosystemic shunt. Eur J Radiol. 1998;28:164-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Korai T, Kimura Y, Imamura M, Nagayama M, Kanazawa A, Miura R, Murakami T, Kyuno D, Yamaguchi H, Terai K, Sugita S, Nobuoka T, Hasegawa T, Takemasa I. Arteriovenous malformation in the pancreatic head initially mimicking a hypervascular mass treated with duodenum-preserving pancreatic head resection: a case report. Surg Case Rep. 2020;6:301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 10. | Kitazono M, Fujita M, Katsue K, Ikeda N, Oyama T, Eguchi M, Kamimura G, Sato R, Uchiyama S, Toyosaki R, Suenaga T. A case of emergency pancreatoduodenectomy for bleeding from the duodenal mucosa due to arteriovenous malformation of the pancreatic head. Clin Case Rep. 2021;9:e04824. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 11. | Chou SC, Shyr YM, Wang SE. Pancreatic arteriovenous malformation. J Gastrointest Surg. 2013;17:1240-1246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 12. | Charalabopoulos A, Macheras N, Krivan S, Petropoulos K, Misiakos E, Macheras A. Arteriovenous malformation of the pancreas. Case Rep Med. 2011;2011:612657. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Nikolaidou O, Xinou E, Papakotoulas P, Philippides A, Panagiotopoulou-Boukla D. Pancreatic arteriovenous malformation mimicking pancreatic neoplasm: a systematic multimodality diagnostic approach and treatment. Radiol Case Rep. 2018;13:305-309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Gupta V, Kedia S, Sonika U, Madhusudhan KS, Pal S, Garg P. A case of pancreatic AV malformation in an elderly man. Clin J Gastroenterol. 2018;11:212-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Shearer DD, Demos TC, Sichlau MJ. Pancreatic arteriovenous malformation: a case report and literature review. J Radiol Case Rep. 2011;5:8-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Meyer CT, Troncale FJ, Galloway S, Sheahan DG. Arteriovenous malformations of the bowel: an analysis of 22 cases and a review of the literature. Medicine (Baltimore). 1981;60:36-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 126] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 17. | Abe T, Suzuki N, Haga J, Azami A, Todate Y, Waragai M, Sato A, Takano Y, Kawakura K, Imai S, Sakuma H, Teranishi Y. Arteriovenous malformation of the pancreas: a case report. Surg Case Rep. 2016;2:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Soulez G, Gilbert Md Frcpc P, Giroux Md Frcpc MF, Racicot Md Frcpc JN, Dubois J. Interventional Management of Arteriovenous Malformations. Tech Vasc Interv Radiol. 2019;22:100633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 19. | Yakes W, Baumgartner I. Interventional treatment of arterio-venous malformations. Gefässchirurgie. 2014;19:325-330. |
| 20. | Hakoda H, Kawaguchi Y, Miyata Y, Togashi J, Nagai M, Suzuki Y, Nomura Y. Surgical resection of arteriovenous malformation of the pancreatic head with acute pancreatitis: a case report. J Surg Case Rep. 2022;2022:rjac427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 21. | Marcelin C, Park AW, Gilbert P, Bouchard L, Therasse E, Perreault P, Giroux MF, Soulez G. Management of Pancreatico-duodenal arterio-venous malformation. CVIR Endovasc. 2022;5:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 22. | Gomes AS, Mali WP, Oppenheim WL. Embolization therapy in the management of congenital arteriovenous malformations. Radiology. 1982;144:41-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Kato T, Takahashi M, Okawada T, Miyazaki Y, Kaneko M. Pancreatic arteriovenous malformation treated by transcatheter embolization: report of a case with hepatocellular carcinoma. Radiat Med. 1991;9:19-21. [PubMed] |
| 24. | Ishikawa M, Tanaka M, Ogawa Y, Chijiiwa K. Arteriovenous malformation at pancreatobiliary region causing hemobilia after cholecystectomy. Gastroenterology. 1993;105:1553-1556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | Hirai T, Ohishi H, Yamada R, Hirohashi S, Matsuo N, Uchida H. Color Doppler flow imaging of pancreatic arteriovenous malformation. J Ultrasound Med. 1995;14:393-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 26. | Uda O, Aoki T, Tsuchida A, Asami K, Inoue K, Masuhara S, Koyanagi Y, Hakamada Y, Yasuda D. Pancreatic arteriovenous malformation observed to bleed from the bile duct and a duodenal ulcer: report of a case. Surg Today. 1999;29:462-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Iwashita Y, Kawano T, Maeda T, Nagasaki S, Kitano S. Pancreatic arteriovenous malformation treated by transcatheter embolization. Hepatogastroenterology. 2002;49:1722-1723. [PubMed] |
| 28. | Hosogi H, Ikai I, Hatano E, Taura K, Fujii H, Yamamoto Y, Shimahara Y. Pancreatic arteriovenous malformation with portal hypertension. J Hepatobiliary Pancreat Surg. 2006;13:344-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 29. | Ogawa H, Itoh S, Mori Y, Suzuki K, Ota T, Naganawa S. Arteriovenous malformation of the pancreas: assessment of clinical and multislice CT features. Abdom Imaging. 2009;34:743-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 30. | Gincul R, Dumortier J, Ciocirlan M, Adham M, Queneau PE, Ponchon T, Pilleul F. Treatment of arteriovenous malformation of the pancreas: a case report. Eur J Gastroenterol Hepatol. 2010;22:116-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 31. | Qayed E, Willingham FF. An unusual cause of upper GI bleeding. Diagnosis: Pancreatic arteriovenous malformation. Gastroenterology. 2011;141:35-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 32. | Grasso RF, Cazzato RL, Luppi G, Faiella E, Del Vescovo R, Giurazza F, Borzomati D, Coppola R, Zobel BB. Pancreatic arteriovenous malformation involving the duodenum embolized with ethylene-vinyl alcohol copolymer (Onyx). Cardiovasc Intervent Radiol. 2012;35:958-962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 33. | Arora A, Tyagi P, Kirnake V, Singla V, Sharma P, Bansal N, Ghuman SS, Jain D, Kumar A. Unusual cause of massive upper gastrointestinal bleeding: a pancreatic arteriovenous malformation. JOP. 2013;14:292-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 34. | Uojima H, Tsukiyama T, Ichida C, Tsuchiya T, Itoi T. [A case of arteriovenous malformation of the pancreas treated with n-butyl-2-cyanoacrylate transcatheter arterial embolization]. Nihon Shokakibyo Gakkai Zasshi. 2014;111:2174-2180. [PubMed] |
| 35. | Tatsuta T, Endo T, Watanabe K, Hasui K, Sawada N, Igarashi G, Mikami K, Shibutani K, Tsushima F, Takai Y, Fukuda S. A successful case of transcatheter arterial embolization with n-butyl-2-cyanoacrylate for pancreatic arteriovenous malformation. Intern Med. 2014;53:2683-2687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 36. | Cassinotto C, Lapuyade B. Pancreatic arteriovenous malformation embolization with onyx. J Vasc Interv Radiol. 2015;26:442-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 37. | Fukami Y, Kurumiya Y, Mizuno K, Sekoguchi E, Kobayashi S. Pancreatic arteriovenous malformation with portal vein thrombosis. Surgery. 2015;157:171-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 38. | Vidmar J, Omejc M, Dežman R, Popovič P. Thrombosis of pancreatic arteriovenous malformation induced by diagnostic angiography: case report. BMC Gastroenterol. 2016;16:68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 39. | Kohan G, Provenzano M, Rosado M, Farfan G, Sánchez N, Fastman D. [Pancreatic arteriovenous malformation as cause of acute pancreatitis treated by endovascular access]. Medicina (B Aires). 2017;77:506-508. [PubMed] |