Published online Oct 6, 2023. doi: 10.12998/wjcc.v11.i28.6908
Peer-review started: June 29, 2023
First decision: August 9, 2023
Revised: August 18, 2023
Accepted: September 6, 2023
Article in press: September 6, 2023
Published online: October 6, 2023
Processing time: 88 Days and 0.5 Hours
Accumulating evidence demonstrates that autoimmune hematopoietic failure and myeloid neoplasms have an intrinsic relationship with regard to clonal hematopoiesis and disease evolution. In approximately 10%-15% of patients with severe aplastic anemia (SAA), the disease phenotype is transformed into myeloid neoplasms following antithymocyte globulin plus cyclosporine-based immuno
A middle-aged Chinese female had a 6-year history of non-SAA and a 2-year history of paroxysmal nocturnal hemoglobinuria (PNH). With aggravation of systemic inflammatory symptoms, severe pancytopenia developed, and her hemoglobinuria disappeared. Laboratory findings in cytological, immunological and cytogenetic analyses of bone marrow samples met the diagnostic criteria for “SAA.” Definitive diagnosis of disseminated tuberculosis was made in the search for infectious niches. Remarkable improvement in hematological parameters was achieved within 1 mo of anti-tuberculosis treatment, and complete hematological remission was achieved within 4 mo of treatment. Frustratingly, the hematological response lasted for only 3 mo, and pancytopenia reemerged. At this time, cytological findings (increased bone marrow cellularity and an increased percentage of myeloblasts that accounted for 16.0% of all nucleated hematopoietic cells), immunological findings (increased percentage of cluster of differentiation 34+ cells that accounted for 12.28% of all nucleated hematopoietic cells) and molecular biological findings (identification of somatic mutations in nucleophosmin-1 and casitas B-lineage lymphoma genes) revealed that “SAA” had transformed into acute myeloid leukemia with mutated nucleophosmin-1. The transformation process suggested that the leukemic clones were preexistent but were suppressed in the PNH and SAA stages, as development of symptomatic myeloid neoplasm through acquisition and accumulation of novel oncogenic mutations is unlikely in an interval of only 7 mo. Aggravation of inflammatory stressors due to disseminated tuberculosis likely contributed to the repression of normal and leukemic hematopoiesis, and the relief of inflammatory stressors due to anti-tuberculosis treatment contributed to penetration of neoplastic hematopoiesis. The concealed leukemic clones in the SAA and PNH stages raise the possibility of an inflammatory stress-fueled antileukemic mechanism.
Aggravated inflammatory stressors can repress normal and leukemic hematopoiesis, and relieved inflammatory stressors can facilitate penetration of neoplastic hematopoiesis.
Core Tip: A Chinese female had a 6-year history of nonsevere aplastic anemia and a 2-year history of paroxysmal nocturnal hemoglobinuria. With aggravation of systemic inflammatory symptoms, severe pancytopenia developed, and her hemoglobinuria disappeared. Laboratory findings met the diagnostic criteria for “severe aplastic anemia.” Anti-tuberculosis treatment resulted in leukemic transformation after a short duration of hematological remission. This case study revealed that aggravated inflammatory stressors can repress normal and leukemic hematopoiesis, and relieved inflammatory stressors can facilitate penetration of neoplastic hematopoiesis, suggesting an inflammatory stress-fueled antileukemic mechanism.
- Citation: Xiu NN, Yang XD, Xu J, Ju B, Sun XY, Zhao XC. Leukemic transformation during anti-tuberculosis treatment in aplastic anemia-paroxysmal nocturnal hemoglobinuria syndrome: A case report and review of literature. World J Clin Cases 2023; 11(28): 6908-6919
- URL: https://www.wjgnet.com/2307-8960/full/v11/i28/6908.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i28.6908
Acquired aplastic anemia (AA) is the paradigm of autoimmune hematopoietic failure (AHF). AA is generally considered a benign hematological disease resulting from autoimmune destructive impairment of hematopoietic progenitor cells[1,2]. Myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML) are well-known myeloid neoplasms resulting from somatic mutations that drive leukemic hematopoiesis[3-5]. In approximately 10%-15% of patients with severe AA (SAA), the disease phenotype is transformed into MDS or AML following antithymocyte globulin plus cyclosporine-based immunosuppressive therapy (IST)[6-8]. However, leukemic transformation in SAA patients during anti-tuberculosis treatment has not been reported. This case study reported a middle-aged Chinese female with a 6-year history of non-SAA and a 2-year history of paroxysmal nocturnal hemoglobinuria (PNH). With reactivation of tuberculosis infection, SAA developed, and hemoglobinuria disappeared. However, the disease phenotype was transformed into AML with mutated nucleophosmin-1 (NPM1) after a short duration of hematological remission during anti-tuberculosis treatment.
A 39-year-old Chinese female presented with aggravating fatigue that lasted for 3 mo.
Eight years prior, the patient experienced aggravating fatigue and was found to have pancytopenia. Diagnosis of non-SAA was made based on heavily reduced bone marrow (BM) cellularity and hematopoietic volume on aspirates and biopsy, decreased percentage of cluster of differentiation (CD) 34+ hematopoietic progenitors on immunological analysis of BM samples, and normal 46,XX karyotype on cytogenetic analysis of cultured BM cells. The patient was treated with cyclosporine (75 mg, three times daily) and stanozolol (2 mg, three times daily). With this immune suppressant treatment, complete hematological remission was achieved within 7 mo. Cyclosporine and stanozolol treatment was continued. Complete remission was maintained until hemoglobinuria occurred 2 years prior to presentation. Diagnosis of PNH was made based on an increase in BM cellularity and the percentage of erythroid progenitors (52.5% of all nucleated cells), a decrease in the percentages of CD55 and CD59 expression (7.85% and 11.98% on erythrocytes and 3.56% and 7.26% on granulocytes, respectively), normal 46,XX karyotype and negative Coombs test. Sodium bicarbonate (1.0 g, three times daily) was added. During the treatment of PNH, her complete blood cell (CBC) count results generally fluctuated within the following ranges: white blood cells (WBCs), 4.00-6.00 × 109/L; red blood cells (RBCs), 2.40-2.80 × 1012/L; hemoglobin levels (Hb), 100-110 g/L; platelets (Plts), 130-180 × 109/L; and absolute reticulocyte counts (Ret), 110-150 × 109/L.
Beginning 3 mo prior to this admission, the patient experienced aggravating fatigue that was far more severe than the degree of anemia. The patient experienced subjective fever, weight loss, night sweats, loss of appetite and abdominal distension. Several febrile episodes occurred during this period. Intravenous antibiotic treatments at another hospital relieved the febrile episodes, but elevated inflammatory indices (C-reactive protein and fibrinogen) persisted. With repeated febrile episodes, pancytopenia developed, and hemoglobinuria disappeared. The patient was sent to our hospital during this febrile episode.
The patient denied having diseases affecting the cardiovascular, endocrine, respiratory, gastrointestinal, hematological, urogenital or musculoskeletal systems before the diagnosis of non-SAA was made.
No family history of inherited, hematological, rheumatological or malignant diseases was recorded.
The patient was 157 cm tall and weighed 47.0 kg. Her vital signs were as follows: body temperature, 38.2 °C; respiratory rate, 20 breaths per minute; heart rate, 96 beats per minute; and blood pressure, 122/79 mmHg. Physical examination revealed the presence of mild tenderness of the right lower quadrant. There were no significant abnormalities in the nervous, respiratory, cardiovascular or musculoskeletal systems.
Routine laboratory examinations: On admission, the CBC showed the following results: WBCs, 2.10 × 109/L; absolute neutrophil count (ANC), 0.46 × 109/L; RBCs, 1.02 × 1012/L; Hb, 49 g/L; Plts, 25 × 109/L; Ret, 7.50 × 109/L; and C-reactive protein, 142.5 mg/L. Her coagulation profile showed an elevated serum fibrinogen concentration (4.070 g/L), with a D-dimer level of 0.02 mg/L. Biochemical tests showed a mildly decreased level of albumin (34.6 g/L) in the absence of abnormalities in markers of liver and renal function. Multiple pathogenic cultures of blood samples reported no growth of Gram-positive and Gram-negative bacteria. Negative serological test results for hepatitis virus A, B and C and HIV were obtained. Biological tests for Epstein-Barr virus and parvovirus B19 DNA were negative. Lymphocyte subgroup analysis revealed an increased percentage of the CD8+ population and decreased percentages of the CD4+ and CD19+ populations. Serum levels of interferon-gamma (IFN-γ), tumor necrosis factor-alpha (TNF-α) and interleukin-6 were elevated, indicating activation of T helper type 1 (Th1) immune responses. The IFN-γ release assay was positive. Aspirate from ascites was bloody and exudative with an increased number and percentage of mononuclear cells and an elevated level of adenosine deaminase.
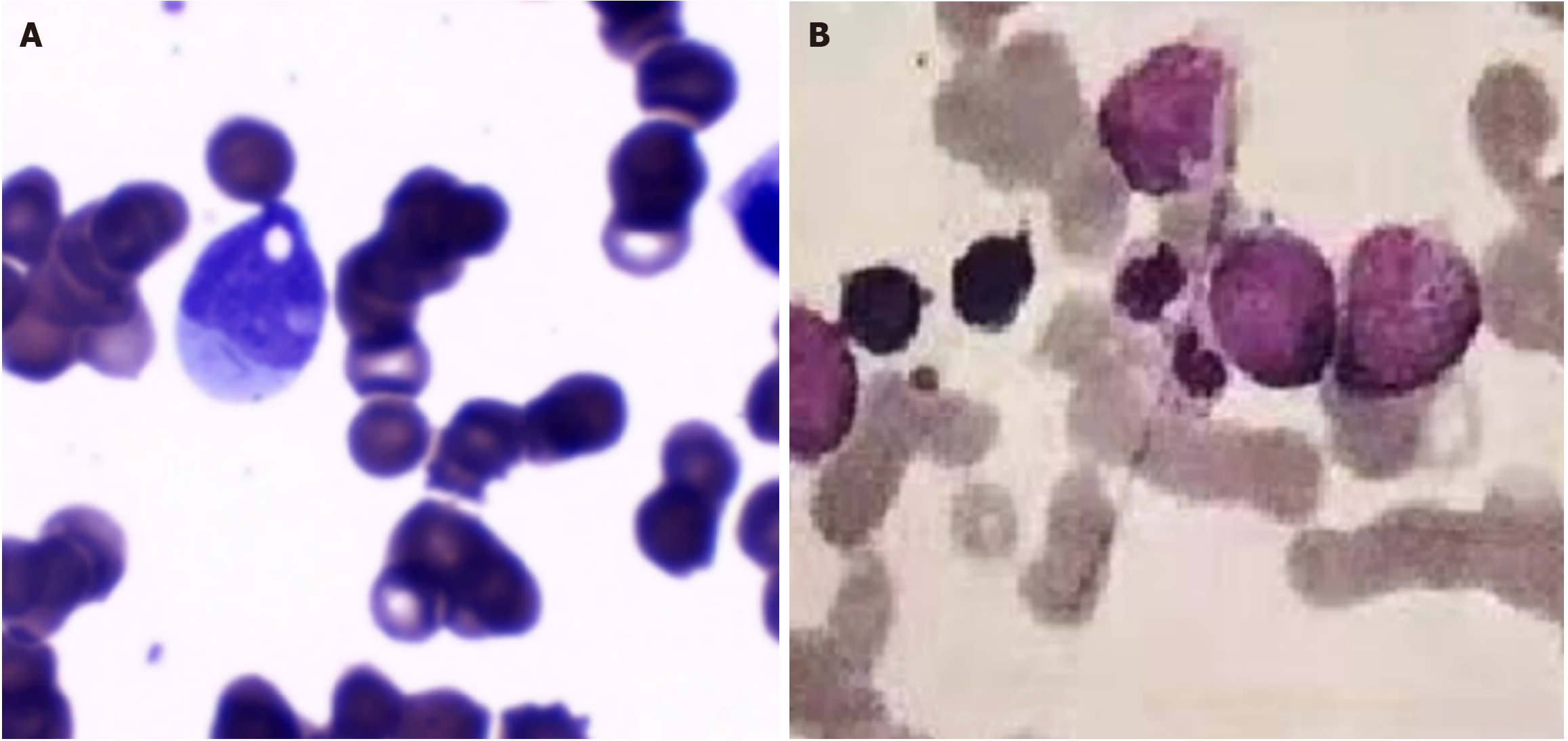
Specific laboratory examinations for blood diseases: Cytological evaluation of BM smears (Figure 1A) showed heavily decreased cellularity with a paucity of myeloblasts. Immunological analysis of BM samples revealed a significant decrease in percentages of CD34+ cells (0.27%), CD19+ cells (4.62%) and CD4+ cells (8.18%) and an increase in those of CD8+ cells (24.04%), CD5+ cells (8.22%) and CD57+ cells (15.77%), consistent with the immunological profile of Th1 immune responses in the BM environment. Normal blood expression levels of CD55 and CD59 (97.83% and 96.18% on erythrocytes and 99.25% and 98.63% on granulocytes, respectively) confirmed the absence of PNH clones. Cytogenetic analysis showed a normal 46,XX karyotype. Both direct and indirect Coombs tests were negative. These laboratory data met the Camitta diagnostic criteria for “SAA”[9] and indicated that the disease phenotype had been transformed from PNH to SAA.
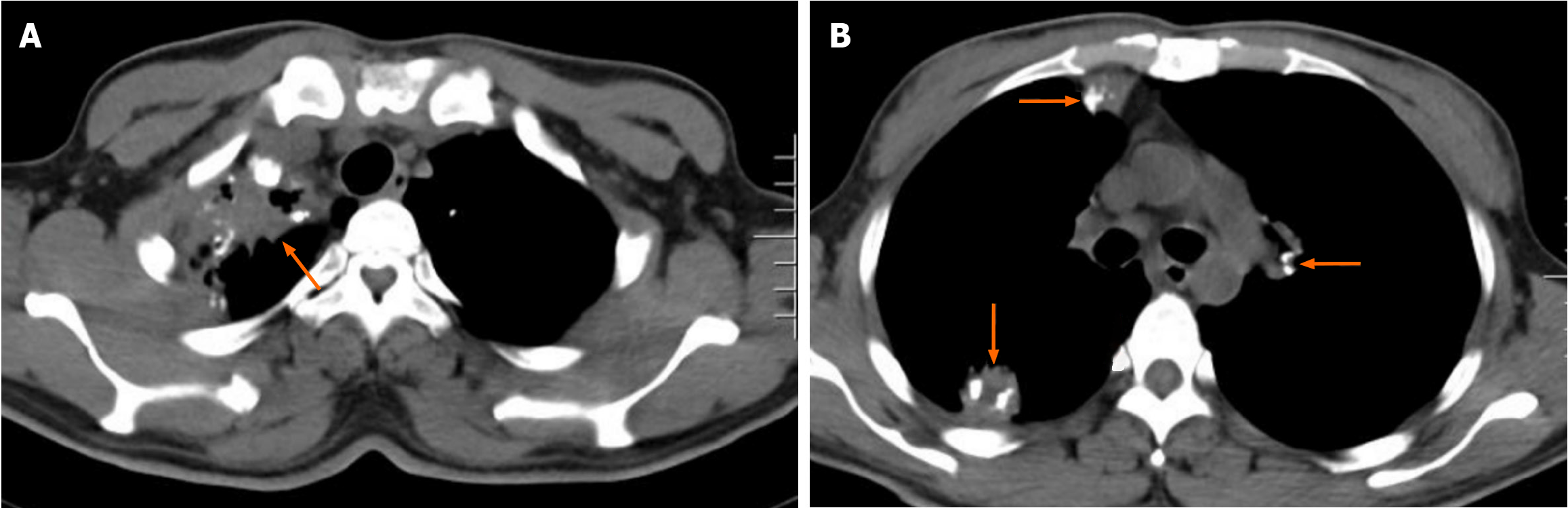
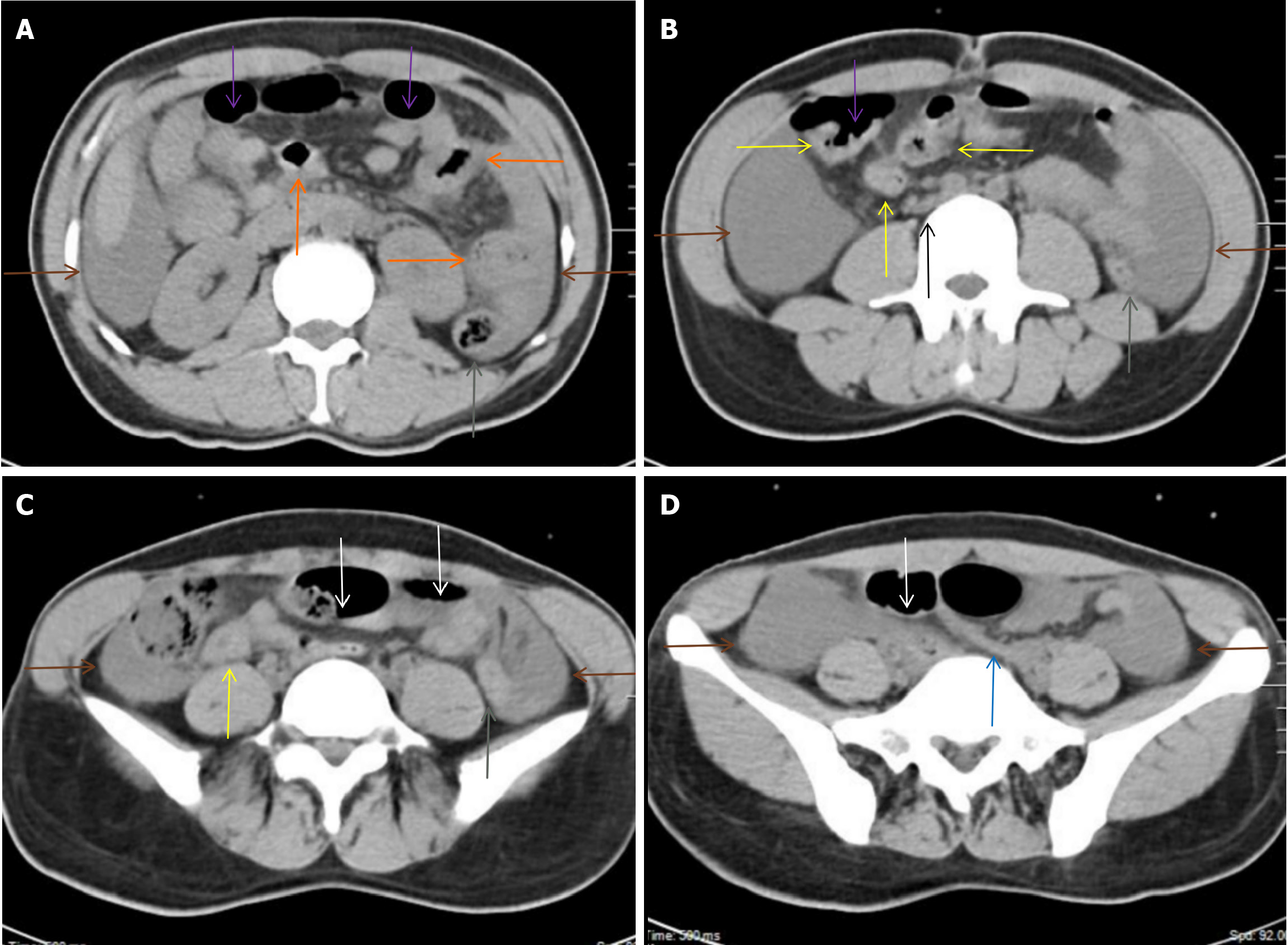
Because the patient presented with systemic inflammatory symptoms, computed tomography scans were performed to search for inflammatory niches. Radiological findings on chest (Figure 2) and abdominopelvic (Figure 3) computed tomography suggested reactivation of tuberculosis. Tuberculosis infected the lungs, pleura, mediastinum, intestines, celiac lymph nodes and peritoneum[10,11]. Definitive diagnosis of active tuberculosis was made due to identification of acid-fast bacilli in sputum.
The patient was diagnosed with SAA complicated by disseminated tuberculosis reactivation.
After disseminated tuberculosis was diagnosed, the patient was prescribed the standard anti-tuberculosis treatment modality, which included a combination of rifampicin (0.45 g/d), isoniazid (0.3 g/d), ethambutol (1.0 g/d) and pyrazinamide (1.0 g/d) for 2 mo and subsequently a combination of rifampicin and isoniazid for 6 mo. Other treatments included recombinant human granulocyte colony-stimulating factor for severe neutropenia and supportive care for anemia.
The patient’s systemic inflammatory symptoms quickly ameliorated, the pulmonary exudative lesions and ascites were gradually absorbed, and her performance status was significantly improved. One month later, the WBCs, ANC, Plts and Ret on CBC monitoring increased remarkably. Four months of anti-tuberculosis treatment led to normalization of hematological parameters. CBC results at the peak time showed WBCs at 7.45 × 109/L, ANC at 4.49 × 109/L, RBCs at 3.66 × 1012/L, Hb at 127 g/L, Plts at 274 × 109/L and Ret at 66.71 × 109/L.
Frustratingly, this hematological response lasted for only 3 mo, and pancytopenia reemerged during anti-tuberculosis treatment. At this time, morphological reevaluation of BM smears showed that the cellularity had become hyperplastic, with a remarkable increase in the percentage of myeloblasts, accounting for 16.0% of all nucleated cells (Figure 1B). Immunological analysis of the BM samples revealed an increased percentage of CD34+ cells, which accounted for 12.28% of nucleated cells. Molecular biological analysis identified myeloid neoplasm-associated gene mutations in NPM1 (with a variant allele frequency of 32.55%) and casitas B-lineage lymphoma (with a variant allele frequency of 38.26%). The laboratory data met the diagnostic criteria for AML with mutated NPM1[12,13]. One course of DA3+7 (daunorubicin, 60 mg/d, days 1-3; cytarabine, 200 mg/d, days 1-7) chemotherapy led to complete remission. After another course of DA3+7 chemotherapy, allogeneic hematopoietic stem cell transplantation (allo-HSCT) was performed. At the time this manuscript was finished, 11 mo had passed since allo-HSCT had been performed, and the patient remained in complete remission.
In this patient, aplastic cytopenia developed during an inflammatory episode due to disseminated tuberculosis reactivation. During active tuberculosis, BM cellularity became hypoplastic, with disappearance of PNH clones and absence of evident leukemic clones. The increased percentage of the CD8+ lymphocyte population and elevated serum levels of IFN-γ and TNF-α indicated activation of Th1 response-mediated autoimmunity. With effective anti-tuberculosis treatment, the disease phenotype was transformed from AHF into an advanced myeloid neoplasm. This case study highlighted the following intriguing points that are of great significance in theoretical research and clinical practice.
First, active tuberculosis can repress normal hematopoiesis in predisposed patients, inducing AHF. A few cases of aplastic cytopenia have been reported to be associated with disseminated tuberculosis[14-17] and even with Bacillus Calmette-Guerin (BCG) vaccination[18]. Th1 immune responses are the major defense mechanism against tuberculosis[19-21], and Mycobacterium tuberculosis antigens can directly activate Th1 responses[21,22]. Activated Th1 responses lead to production of a large amount of type I inflammatory cytokines[19-22] and thereby suppress host autologous hematopoiesis[23,24], which is the immunological signature of AA and hypoplastic MDS (hMDS)[1,25,26].
Currently, tuberculosis is still the commonest infectious disease[27,28], and its contribution to autoimmune diseases has been extensively investigated[29]. Despite great advances in recent decades, it is estimated that nearly a quarter of the world’s population is latently infected with M. tuberculosis[30,31]. When host immune function is compromised under certain conditions, such as aging, malnutrition, administration of immune suppressants due to treatment for autoimmune disorders, aggravation of psychological distresses, comorbidity of chronic organ dysfunction or coinfection with other pathogenic factors, latent tuberculosis can become reactivated. Active tuberculosis recalls specific and nonspecific responses due to the increased antigen load. Trained Th1 cells[32,33], cytotoxic T lymphocytes[34], natural killer/natural killer T cells[35,36], unconventional lymphocytes[37,38] and even CD5+ (B1) B cells[39] respond to antigen stimulation, secrete a large amount of IFN-γ, TNF-α and other proinflammatory factors and suppress granulopoiesis, erythropoiesis and megakaryocytopoiesis[23,24]. Immune dysregulation can occur not only in active disease but also in latent infection due to the high heterogeneity of bacterial toxicity and host immune competence[40,41].
Tuberculosis-associated aplastic cytopenia has been reported in disseminated tuberculosis instead of isolated pulmonary tuberculosis, which suggests that effective suppression of host hematopoiesis critically requires an additional inflammatory condition with an intensity that is maintained by sufficient activated immune cells and a large amount of proinflammatory mediators. In this patient, tuberculosis infected the lungs, pleura, mediastinum, intestines, celiac lymph nodes and peritoneum. Gut involvement of tuberculosis infection has a more potent influence on the systemic inflammatory state and thus likely plays a more important role in AHF development[42] because the gastrointestinal tract can provide sufficient activated immune cells and continuously supply intestine-derived antigens[43,44] from both pathogenic bacteria and commensal microbes[45,46].
In our investigation of inflammatory niches in SAA patients during inflammatory episodes, 5 of 17 recruited patients had imaging abnormalities suggestive of tuberculosis reactivation, all involving the gastrointestinal tract[47]. Gastrointestinal infections can induce inflammatory lesions in both infected and noninfected segments through induction of gut dysbiosis[48-50]. In gut dysbiosis and gut inflammatory disorders, impaired intestinal barrier functions allow close contact between intestine-derived antigens and host immune cells, thereby activating immune cells and creating an inflammatory milieu at an intensity sufficient to initiate and sustain autoimmunity in remote organ systems[50,51]. A gluten-free diet in celiac disease-associated aplastic cytopenia[52], resection of diseased colonic segments in neutropenic enterocolitis[53] and effective treatment of gut inflammatory disorders in aplastic crisis[54] can effectively relieve autoimmune responses and facilitate restoration of autologous hematopoiesis, reinforcing the role of inflammatory conditions in AHF pathogenesis[44]. In an animal model of AHF using allo-HSCT, it has been known for a long time that induction of aplastic cytopenia critically required engagement of the gut inflammatory milieu[55].
Second, aggravated inflammatory stressors due to active tuberculosis can suppress PNH clones, resulting in so-called “spontaneous remission.” Spontaneous remission in PNH has been reported, frequently following an infectious episode[56,57]. Disappearance of PNH clones during inflammatory episodes suggests that loss of glycosylphosphatidylinositol-anchored proteins likely enhances the tolerance of inflammatory cytokine-induced apoptosis rather than complete loss of the hematopoietic regulatory mechanisms in PNH clones[58,59]. In an intensive inflammatory milieu, PNH clones can be heavily suppressed. Spontaneous remission in PNH may be caused by an intensive inflammatory milieu due to fulminant inflammatory episodes through hematopoietic regulatory mechanisms.
Third, the most intriguing phenomenon is that active tuberculosis can repress leukemic hematopoiesis, leading to concealment of leukemic clones in SAA and PNH stages. This phenomenon raises the possibility that autoimmune responses in AHF may involve an antileukemic mechanism[60,61]. In this case, leukemic clones were concealed during active tuberculosis and penetrated during anti-tuberculosis treatment, suggesting that inflammatory stressors strengthened antileukemic activities and preferentially repressed leukemic clones[62,63].
Inflammatory stress-fueled antileukemic activities can also be inferred from spontaneous remission in AML[64-66]. To date, spontaneous remission has been reported in more than 200 AML patients. It occurs frequently following an infectious episode and aplastic cytopenia. The occurrence of spontaneous remission is usually ascribed to reversion of the immune exhaustion state and restoration of antileukemic activities due to secretion of a substantial amount of proinflammatory cytokines against invading pathogens[65-67]. In most cases, the remission duration is very short, and symptomatic AML frequently reemerges within 2-3 mo, indicating that the leukemic clones are not eradicated, even in inflammatory stress-fueled antileukemic activities[68].
Another phenomenon also suggests the existence of inflammatory stress-fueled antileukemic activities. A fraction of AML patients experience a period of prolonged hematopoietic suppression after intensive chemotherapy during which repeated or durable infectious episodes are the major complication. If patients survive prolonged hematopoietic suppression, they may experience deep remission, a longer remission duration and a lower probability of relapse[69,70]. Recombinant IFN-α[71,72], immune checkpoint inhibitors[73,74] and BCG vaccination[75,76] have been successfully used in the treatment of hematological malignancies, and the major adverse event is hematological toxicity. Much evidence supports the hypothesis that inflammatory stressors, induced either by infectious episodes or administration of immune-activating agents, can strengthen antileukemic activities. With relief of inflammatory stressors, the concealed leukemic clones expand, and the disease phenotype is transformed into symptomatic myeloid neoplasms.
Although disease phenotypic transformations occurred unexpectedly in this patient, it is not surprising that disseminated tuberculosis can repress leukemic hematopoiesis. Th1 immune responses are the major mechanism in defense against tuberculosis[19-22], and excessive Th1 immune responses can effectively repress granulopoiesis, erythropoiesis and megakaryocytopoiesis[23-25], including leukemic clones[61-63]. During active tuberculosis, our patient manifested aplastic pancytopenia. When antigen stimulation was removed due to effective treatment of tuberculosis, leukemic clones penetrated, suggesting that leukemic clones preexisted but were suppressed in the PNH and SAA stages. This is because development of a symptomatic myeloid neoplasm through acquisition and accumulation of novel oncogenic mutations is unlikely in an interval of only 7 mo. From this point of view, a chronic inflammatory milieu indeed serves as an antileukemic mechanism[17,61]. Leukemic evolution is the result of immune escape due to the elevated antileukemic threshold and immune exhaustion in the advanced stage[77,78].
With widespread application of the next-generation sequencing technique in diagnosis and risk stratification of hematological diseases[79], it has been found that approximately one-third of definitively diagnosed SAA patients harbor somatic mutations that are the well-known driver genetic abnormalities of myeloid neoplasms, although the number and clone size of mutant genes are smaller than those in MDS[7,8,26,80]. In approximately 10%-15% of SAA patients, the disease phenotype is transformed from SAA into myeloid neoplasms following antithymocyte globulin-based IST. In some of these patients, leukemic transformation appears during or shortly after IST[6-8]. Moreover, approximately 20%-30% of SAA patients fail to respond to IST, and these patients harbor a high frequency of unfavorable somatic mutations that are predictors of poor prognosis in myeloid neoplasms. Even in patients achieving a hematological response, the presence of unfavorable somatic mutations predicts a significantly increased risk of leukemic transformation[7,8].
Leukemic transformation in SAA patients following IST also suggests that autoimmunity in AHF operates as an antileukemic mechanism. hMDS is another acquired form of AHF. In hMDS patients, clonal expansion is a common dilemma with IST[81,82], providing alternative evidence for the contribution of autoimmune responses to suppressive activities against leukemic clones. Autoimmune responses in AHF target leukemic clones[60,61], whereas IST depletes autoimmune cytotoxic T lymphocytes[83], promoting expansion of leukemic clones and penetration of symptomatic neoplasms. The effect of IST may be similar to that of treatment for underlying infections on leukemic transformation, which is that while treatment of underlying infections removes immune-activating factors, IST intervenes in the immune attack pathology.
Accumulating evidence demonstrates that AHF and myeloid neoplasms have an intrinsic relationship regarding clonal hematopoiesis and disease evolution[77,78,80]. Although spontaneous transformation from SAA and PNH to advanced myeloid neoplasms has been reported[84,85] and is usually ascribed to a selective advantage over normal compartments under intensive immunological pressure due to acquisition and accumulation of novel oncogenic mutations and escape of immune surveillance due to immune exhaustion in chronic inflammatory milieu[77,78], the transformation process is very long, which is distinct from the process described for this patient.
AA, PNH, hMDS and hypoplastic AML are typical forms of AHF. Organ-specific autoimmunity is present mainly in the BM, suggesting that a primary immune-active environment exists[86-88]. In addition to pathogenic microbes that can survive in the BM in which exogenous antigens induce immune responses[89-91], neoplasm-associated antigens[81,92] or damage-associated molecular patterns[93,94], as the genetic or epigenetic products of genetically damaged hematopoietic progenitor cells, can initiate a primary immune-active BM environment and determine organ specificity. If the primary immune responses target neoplasm-associated antigens or damage-associated molecular patterns, they can represent an antileukemic mechanism. However, if the immune responses target antigens of less immunogenicity, the intensity of the primary immune-active BM environment may not be able to repress normal and leukemic hematopoiesis. In this situation, effective suppression of normal and leukemic hematopoiesis requires engagement of an additional inflammatory condition to strengthen antileukemic activities.
In a chronic inflammatory environment, upregulated expression of Toll-like receptors, the Nlrp3 inflammasome and human leucocyte antigen-DR increases sensitivity to antigen stimulation[94-96]. Even in the presence of inflammatory stress-fueled antileukemic activities, leukemic clones may not be eradicated[68], resulting in disease chronicity in the presence of additional inflammatory stressors and leukemic transformation after removal of inflammatory stressors through treatment of underlying inflammatory disorders[61,63] or IST[6-8], which can reasonably explain the high frequency of leukemic evolution following IST.
This finding suggests that patients with myeloid neoplasms who are ineligible for intensive treatments or receive maintenance therapy can be treated with immune-modifying agents, such as recombinant IFN-α, some types of endotoxins, immune checkpoint inhibitors, poly I:C, BCG vaccination or a combination modality, to artificially create an appropriate chronic or intermittent inflammatory milieu.
Limitations of this case study include the following: (1) The precise mechanism of the role of tuberculosis in the initiation of AHF and antileukemic activities was not elucidated; (2) The difference in suppressive activities between normal and leukemic hematopoiesis was not elucidated; and (3) More cases are needed to validate the exact role of tuberculosis in strengthening antileukemic activities.
Disseminated tuberculosis can cause AHF, suppressing both normal and leukemic hematopoiesis. Inflammatory stressors due to active tuberculosis may strengthen antileukemic activities of immune surveillance against malignant proliferation. Removal of inflammatory stressors due to anti-tuberculosis treatment may facilitate expansion of leukemic clones and penetration of symptomatic myeloid neoplasms. This finding suggests that patients with myeloid neoplasms who are ineligible for intensive treatments or receive maintenance therapy can be treated with immune-activating agents to artificially create an appropriate chronic or intermittent inflammatory condition, which may favor patient survival.
The authors thank Fan-Jun Meng (Department of Hematology, The Affiliated Hospital of Qingdao University) for his assistance in the analysis, diagnosis and treatment of the patient and in the revision of this manuscript.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Diamantidis MD, Greece S-Editor: Fan JR L-Editor: Filipodia P-Editor: Zhang XD
| 1. | Giudice V, Selleri C. Aplastic anemia: Pathophysiology. Semin Hematol. 2022;59:13-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 47] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 2. | Shallis RM, Ahmad R, Zeidan AM. Aplastic anemia: Etiology, molecular pathogenesis, and emerging concepts. Eur J Haematol. 2018;101:711-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 69] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 3. | Li H, Hu F, Gale RP, Sekeres MA, Liang Y. Myelodysplastic syndromes. Nat Rev Dis Primers. 2022;8:74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 41] [Reference Citation Analysis (0)] |
| 4. | Cook MR, Karp JE, Lai C. The spectrum of genetic mutations in myelodysplastic syndrome: Should we update prognostication? EJHaem. 2022;3:301-313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 5. | Bouligny IM, Maher KR, Grant S. Mechanisms of myeloid leukemogenesis: Current perspectives and therapeutic objectives. Blood Rev. 2023;57:100996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 23] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 6. | Patel BA, Groarke EM, Lotter J, Shalhoub R, Gutierrez-Rodrigues F, Rios O, Quinones Raffo D, Wu CO, Young NS. Long-term outcomes in patients with severe aplastic anemia treated with immunosuppression and eltrombopag: a phase 2 study. Blood. 2022;139:34-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 79] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 7. | Groarke EM, Patel BA, Shalhoub R, Gutierrez-Rodrigues F, Desai P, Leuva H, Zaimoku Y, Paton C, Spitofsky N, Lotter J, Rios O, Childs RW, Young DJ, Dulau-Florea A, Dunbar CE, Calvo KR, Wu CO, Young NS. Predictors of clonal evolution and myeloid neoplasia following immunosuppressive therapy in severe aplastic anemia. Leukemia. 2022;36:2328-2337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 28] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 8. | Peffault de Latour R, Kulasekararaj A, Iacobelli S, Terwel SR, Cook R, Griffin M, Halkes CJM, Recher C, Barraco F, Forcade E, Vallejo JC, Drexler B, Mear JB, Smith AE, Angelucci E, Raymakers RAP, de Groot MR, Daguindau E, Nur E, Barcellini W, Russell NH, Terriou L, Iori AP, La Rocca U, Sureda A, Sánchez-Ortega I, Xicoy B, Jarque I, Cavenagh J, Sicre de Fontbrune F, Marotta S, Munir T, Tjon JML, Tavitian S, Praire A, Clement L, Rabian F, Marano L, Hill A, Palmisani E, Muus P, Cacace F, Frieri C, van Lint MT, Passweg JR, Marsh JCW, Socié G, Mufti GJ, Dufour C, Risitano AM; Severe Aplastic Anemia Working Party of the European Society for Blood and Marrow Transplantation. Eltrombopag Added to Immunosuppression in Severe Aplastic Anemia. N Engl J Med. 2022;386:11-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 161] [Article Influence: 53.7] [Reference Citation Analysis (0)] |
| 9. | Killick SB, Bown N, Cavenagh J, Dokal I, Foukaneli T, Hill A, Hillmen P, Ireland R, Kulasekararaj A, Mufti G, Snowden JA, Samarasinghe S, Wood A, Marsh JC; British Society for Standards in Haematology. Guidelines for the diagnosis and management of adult aplastic anaemia. Br J Haematol. 2016;172:187-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 525] [Article Influence: 52.5] [Reference Citation Analysis (0)] |
| 10. | Im JG, Itoh H, Han MC. CT of pulmonary tuberculosis. Semin Ultrasound CT MR. 1995;16:420-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Gupta P, Kumar S, Sharma V, Mandavdhare H, Dhaka N, Sinha SK, Dutta U, Kochhar R. Common and uncommon imaging features of abdominal tuberculosis. J Med Imaging Radiat Oncol. 2019;63:329-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 12. | Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, Bloomfield CD, Cazzola M, Vardiman JW. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391-2405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5219] [Cited by in RCA: 6755] [Article Influence: 750.6] [Reference Citation Analysis (0)] |
| 13. | Khoury JD, Solary E, Abla O, Akkari Y, Alaggio R, Apperley JF, Bejar R, Berti E, Busque L, Chan JKC, Chen W, Chen X, Chng WJ, Choi JK, Colmenero I, Coupland SE, Cross NCP, De Jong D, Elghetany MT, Takahashi E, Emile JF, Ferry J, Fogelstrand L, Fontenay M, Germing U, Gujral S, Haferlach T, Harrison C, Hodge JC, Hu S, Jansen JH, Kanagal-Shamanna R, Kantarjian HM, Kratz CP, Li XQ, Lim MS, Loeb K, Loghavi S, Marcogliese A, Meshinchi S, Michaels P, Naresh KN, Natkunam Y, Nejati R, Ott G, Padron E, Patel KP, Patkar N, Picarsic J, Platzbecker U, Roberts I, Schuh A, Sewell W, Siebert R, Tembhare P, Tyner J, Verstovsek S, Wang W, Wood B, Xiao W, Yeung C, Hochhaus A. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Myeloid and Histiocytic/Dendritic Neoplasms. Leukemia. 2022;36:1703-1719. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 2163] [Article Influence: 721.0] [Reference Citation Analysis (0)] |
| 14. | Demiroğlu H, Ozcebe OI, Ozdemir L, Sungur A, Dündar S. Pancytopenia with hypocellular bone marrow due to miliary tuberculosis: an unusual presentation. Acta Haematol. 1994;91:49-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 15. | Rutovitz JJ. Miliary tuberculosis causing pancytopenia. A report of 2 cases. S Afr Med J. 1986;69:451-452. [PubMed] |
| 16. | Zubair AB, Razzaq MT, Hashmi AW, Ali SMY, Israr MM, Sadiq SM, Khan MF, Haider Z, Sabir M, Kaneez M. Clinical Characteristics and Etiological Spectrum of Pancytopenia in Pediatric Age Group: A Cross-Sectional Outlook From a Developing Country. Cureus. 2022;14:e27842. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 17. | Sun XY, Yang XD, Xu J, Xiu NN, Ju B, Zhao XC. Tuberculosis-induced aplastic crisis and atypical lymphocyte expansion in advanced myelodysplastic syndrome: A case report and review of literature. World J Clin Cases. 2023;11:4713-4722. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 18. | Long HJ. Aplastic anemia, a rare complication of disseminated BCG infection: case report. Mil Med. 1982;147:1067-1070. [PubMed] |
| 19. | Carabalí-Isajar ML, Rodríguez-Bejarano OH, Amado T, Patarroyo MA, Izquierdo MA, Lutz JR, Ocampo M. Clinical manifestations and immune response to tuberculosis. World J Microbiol Biotechnol. 2023;39:206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 42] [Reference Citation Analysis (0)] |
| 20. | Prezzemolo T, Guggino G, La Manna MP, Di Liberto D, Dieli F, Caccamo N. Functional Signatures of Human CD4 and CD8 T Cell Responses to Mycobacterium tuberculosis. Front Immunol. 2014;5:180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 156] [Cited by in RCA: 200] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 21. | Akter S, Chauhan KS, Dunlap MD, Choreño-Parra JA, Lu L, Esaulova E, Zúñiga J, Artyomov MN, Kaushal D, Khader SA. Mycobacterium tuberculosis infection drives a type I IFN signature in lung lymphocytes. Cell Rep. 2022;39:110983. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 22. | Kathamuthu GR, Sridhar R, Baskaran D, Babu S. Dominant expansion of CD4+, CD8+ T and NK cells expressing Th1/Tc1/Type 1 cytokines in culture-positive lymph node tuberculosis. PLoS One. 2022;17:e0269109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 23. | Li F, Liu X, Niu H, Lv W, Han X, Zhang Y, Zhu B. Persistent stimulation with Mycobacterium tuberculosis antigen impairs the proliferation and transcriptional program of hematopoietic cells in bone marrow. Mol Immunol. 2019;112:115-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 24. | Khan N, Downey J, Sanz J, Kaufmann E, Blankenhaus B, Pacis A, Pernet E, Ahmed E, Cardoso S, Nijnik A, Mazer B, Sassetti C, Behr MA, Soares MP, Barreiro LB, Divangahi M. M. tuberculosis Reprograms Hematopoietic Stem Cells to Limit Myelopoiesis and Impair Trained Immunity. Cell. 2020;183:752-770.e22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 184] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 25. | Patel BA, Giudice V, Young NS. Immunologic effects on the haematopoietic stem cell in marrow failure. Best Pract Res Clin Haematol. 2021;34:101276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 26. | Votavova H, Belickova M. Hypoplastic myelodysplastic syndrome and acquired aplastic anemia: Immunemediated bone marrow failure syndromes (Review). Int J Oncol. 2022;60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 27. | Raviglione MC, Snider DE Jr, Kochi A. Global epidemiology of tuberculosis. Morbidity and mortality of a worldwide epidemic. JAMA. 1995;273:220-226. [PubMed] |
| 28. | Menzies NA, Quaife M, Allwood BW, Byrne AL, Coussens AK, Harries AD, Marx FM, Meghji J, Pedrazzoli D, Salomon JA, Sweeney S, van Kampen SC, Wallis RS, Houben RMGJ, Cohen T. Lifetime burden of disease due to incident tuberculosis: a global reappraisal including post-tuberculosis sequelae. Lancet Glob Health. 2021;9:e1679-e1687. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 113] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 29. | Belyaeva IV, Kosova AN, Vasiliev AG. Tuberculosis and Autoimmunity. Pathophysiology. 2022;29:298-318. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 30. | Dye C, Scheele S, Dolin P, Pathania V, Raviglione MC. Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. WHO Global Surveillance and Monitoring Project. JAMA. 1999;282:677-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1956] [Cited by in RCA: 1886] [Article Influence: 72.5] [Reference Citation Analysis (0)] |
| 31. | Houben RM, Dodd PJ. The Global Burden of Latent Tuberculosis Infection: A Re-estimation Using Mathematical Modelling. PLoS Med. 2016;13:e1002152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 967] [Cited by in RCA: 1280] [Article Influence: 142.2] [Reference Citation Analysis (0)] |
| 32. | Arrigucci R, Lakehal K, Vir P, Handler D, Davidow AL, Herrera R, Estrada-Guzmán JD, Bushkin Y, Tyagi S, Lardizabal AA, Gennaro ML. Active Tuberculosis Is Characterized by Highly Differentiated Effector Memory Th1 Cells. Front Immunol. 2018;9:2127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 33. | Adekambi T, Ibegbu CC, Kalokhe AS, Yu T, Ray SM, Rengarajan J. Distinct effector memory CD4+ T cell signatures in latent Mycobacterium tuberculosis infection, BCG vaccination and clinically resolved tuberculosis. PLoS One. 2012;7:e36046. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 34. | Rozot V, Vigano S, Mazza-Stalder J, Idrizi E, Day CL, Perreau M, Lazor-Blanchet C, Petruccioli E, Hanekom W, Goletti D, Bart PA, Nicod L, Pantaleo G, Harari A. Mycobacterium tuberculosis-specific CD8+ T cells are functionally and phenotypically different between latent infection and active disease. Eur J Immunol. 2013;43:1568-1577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 152] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 35. | Venkatasubramanian S, Cheekatla S, Paidipally P, Tripathi D, Welch E, Tvinnereim AR, Nurieva R, Vankayalapati R. IL-21-dependent expansion of memory-like NK cells enhances protective immune responses against Mycobacterium tuberculosis. Mucosal Immunol. 2017;10:1031-1042. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 87] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 36. | Garand M, Goodier M, Owolabi O, Donkor S, Kampmann B, Sutherland JS. Functional and Phenotypic Changes of Natural Killer Cells in Whole Blood during Mycobacterium tuberculosis Infection and Disease. Front Immunol. 2018;9:257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 37. | Comeau K, Paradis P, Schiffrin EL. Human and murine memory γδ T cells: Evidence for acquired immune memory in bacterial and viral infections and autoimmunity. Cell Immunol. 2020;357:104217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 38. | Zufferey C, Germano S, Dutta B, Ritz N, Curtis N. The contribution of non-conventional T cells and NK cells in the mycobacterial-specific IFNγ response in Bacille Calmette-Guérin (BCG)-immunized infants. PLoS One. 2013;8:e77334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 39. | Flores-Gonzalez J, Ramón-Luing LA, Romero-Tendilla J, Urbán-Solano A, Cruz-Lagunas A, Chavez-Galan L. Latent Tuberculosis Patients Have an Increased Frequency of IFN-γ-Producing CD5+ B Cells, Which Respond Efficiently to Mycobacterial Proteins. Pathogens. 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 40. | Esmail H, Barry CE 3rd, Young DB, Wilkinson RJ. The ongoing challenge of latent tuberculosis. Philos Trans R Soc Lond B Biol Sci. 2014;369:20130437. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 226] [Cited by in RCA: 222] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 41. | Cadena AM, Fortune SM, Flynn JL. Heterogeneity in tuberculosis. Nat Rev Immunol. 2017;17:691-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 327] [Article Influence: 40.9] [Reference Citation Analysis (0)] |
| 42. | Wipperman MF, Bhattarai SK, Vorkas CK, Maringati VS, Taur Y, Mathurin L, McAulay K, Vilbrun SC, Francois D, Bean J, Walsh KF, Nathan C, Fitzgerald DW, Glickman MS, Bucci V. Gastrointestinal microbiota composition predicts peripheral inflammatory state during treatment of human tuberculosis. Nat Commun. 2021;12:1141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 43. | Shen L. Functional morphology of the gastrointestinal tract. Curr Top Microbiol Immunol. 2009;337:1-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 44. | Zhao XC, Sun XY, Zhao L, Meng FJ. Gut inflammation in the pathogenesis of acquired aplastic anemia. Chin Med J (Engl). 2020;133:1878-1881. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (1)] |
| 45. | Littman DR, Pamer EG. Role of the commensal microbiota in normal and pathogenic host immune responses. Cell Host Microbe. 2011;10:311-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 402] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 46. | Eloe-Fadrosh EA, Rasko DA. The human microbiome: from symbiosis to pathogenesis. Annu Rev Med. 2013;64:145-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 134] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 47. | Zhao XC, Xue CJ, Song H, Gao BH, Han FS, Xiao SX. Bowel inflammatory presentations on computed tomography in adult patients with severe aplastic anemia during flared inflammatory episodes. World J Clin Cases. 2023;11:576-597. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
| 48. | Baral T, Kurian SJ, Thomas L, Udyavara Kudru C, Mukhopadhyay C, Saravu K, Manu MK, Singh J, Munisamy M, Kumar A, Khandelwal B, Rao M, Sekhar Miraj S. Impact of tuberculosis disease on human gut microbiota: a systematic review. Expert Rev Anti Infect Ther. 2023;21:175-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 49. | Dehghani T, Gholizadeh O, Daneshvar M, Nemati MM, Akbarzadeh S, Amini P, Afkhami H, Kohansal M, Javanmard Z, Poortahmasebi V. Association Between Inflammatory Bowel Disease and Viral Infections. Curr Microbiol. 2023;80:195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 50. | Haneishi Y, Furuya Y, Hasegawa M, Picarelli A, Rossi M, Miyamoto J. Inflammatory Bowel Diseases and Gut Microbiota. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 99] [Reference Citation Analysis (0)] |
| 51. | Mu Q, Kirby J, Reilly CM, Luo XM. Leaky Gut As a Danger Signal for Autoimmune Diseases. Front Immunol. 2017;8:598. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 266] [Cited by in RCA: 381] [Article Influence: 47.6] [Reference Citation Analysis (0)] |
| 52. | Salmeron G, Patey N, de Latour RP, Raffoux E, Gluckman E, Brousse N, Socié G, Robin M. Coeliac disease and aplastic anaemia: a specific entity? Br J Haematol. 2009;146:122-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (1)] |
| 53. | Tokar B, Aydoğdu S, Paşaoğlu O, Ilhan H, Kasapoğlu E. Neutropenic enterocolitis: is it possible to break vicious circle between neutropenia and the bowel wall inflammation by surgery? Int J Colorectal Dis. 2003;18:455-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 54. | Zhao XC, Zhao L, Sun XY, Xu ZS, Ju B, Meng FJ, Zhao HG. Excellent response of severe aplastic anemia to treatment of gut inflammation: A case report and review of the literature. World J Clin Cases. 2020;8:425-435. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 55. | Heidt PJ, Vossen JM. Experimental and clinical gnotobiotics: influence of the microflora on graft-versus-host disease after allogeneic bone marrow transplantation. J Med. 1992;23:161-173. [PubMed] |
| 56. | Korkama ES, Armstrong AE, Jarva H, Meri S. Spontaneous Remission in Paroxysmal Nocturnal Hemoglobinuria-Return to Health or Transition Into Malignancy? Front Immunol. 2018;9:1749. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 57. | Hillmen P, Lewis SM, Bessler M, Luzzatto L, Dacie JV. Natural history of paroxysmal nocturnal hemoglobinuria. N Engl J Med. 1995;333:1253-1258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 591] [Cited by in RCA: 592] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 58. | Barcellini W, Fermo E, Guia Imperiali F, Zaninoni A, Bianchi P, Boschetti C, Zanella A. Increased resistance of PIG-A- bone marrow progenitors to tumor necrosis factor a and interferon gamma: possible implications for the in vivo dominance of paroxysmal nocturnal hemoglobinuria clones. Haematologica. 2004;89:651-656. [PubMed] |
| 59. | Karadimitris A, Notaro R, Koehne G, Roberts IA, Luzzatto L. PNH cells are as sensitive to T-cell-mediated lysis as their normal counterparts: implications for the pathogenesis of paroxysmal nocturnal haemoglobinuria. Br J Haematol. 2000;111:1158-1163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 60. | Nissen C, Stern M. Acquired immune mediated aplastic anemia: is it antineoplastic? Autoimmun Rev. 2009;9:11-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 61. | Zhao XC, Sun XY, Ju B, Meng FJ, Zhao HG. Acquired aplastic anemia: Is bystander insult to autologous hematopoiesis driven by immune surveillance against malignant cells? World J Stem Cells. 2020;12:1429-1438. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 62. | Kiladjian JJ, Giraudier S, Cassinat B. Interferon-alpha for the therapy of myeloproliferative neoplasms: targeting the malignant clone. Leukemia. 2016;30:776-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 101] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 63. | Li X, Yang Y, Yuan J, Hong P, Freie B, Orazi A, Haneline LS, Clapp DW. Continuous in vivo infusion of interferon-gamma (IFN-gamma) preferentially reduces myeloid progenitor numbers and enhances engraftment of syngeneic wild-type cells in Fancc-/- mice. Blood. 2004;104:1204-1209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 64. | Imataki O, Ishida T, Kida JI, Uemura M, Fujita H, Kadowaki N. Repeated spontaneous remission of acute myeloid leukemia in response to various infections: a case report. BMC Infect Dis. 2023;23:215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 65. | Jimemez C, Ribera JM, Abad E, Pintos G, Milla F, Junca J, Feliu E. Increased serum tumour necrosis factor during transient remission in acute leukaemia. Lancet. 1993;341:1600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 66. | Musto P, D'Arena G, Melillo L, Cascavilla N, La Sala A, Ladogana S, Carotenuto M. Spontaneous remission in acute myeloid leukaemia: a role for endogenous production of tumour necrosis factor and interleukin-2? Br J Haematol. 1994;87:879-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 67. | Müller-Schmah C, Solari L, Weis R, Pfeifer D, Scheibenbogen C, Trepel M, May AM, Engelhardt R, Lübbert M. Immune response as a possible mechanism of long-lasting disease control in spontaneous remission of MLL/AF9-positive acute myeloid leukemia. Ann Hematol. 2012;91:27-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 68. | Grunwald VV, Hentrich M, Schiel X, Dufour A, Schneider S, Neusser M, Subklewe M, Fiegl M, Hiddemann W, Spiekermann K, Rothenberg-Thurley M, Metzeler KH. Patients with spontaneous remission of high-risk MDS and AML show persistent preleukemic clonal hematopoiesis. Blood Adv. 2019;3:2696-2699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 69. | Sung L, Aplenc R, Alonzo TA, Gerbing RB, Wang YC, Meshinchi S, Gamis AS. Association between prolonged neutropenia and reduced relapse risk in pediatric AML: A report from the children's oncology group. Int J Cancer. 2016;139:1930-1935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 70. | Aoki T, Takahashi H, Tanaka S, Shiba N, Hasegawa D, Iwamoto S, Terui K, Moritake H, Nakayama H, Shimada A, Koh K, Goto H, Kosaka Y, Saito AM, Horibe K, Kinoshita A, Tawa A, Taga T, Adachi S, Tomizawa D. Predisposition to prolonged neutropenia after chemotherapy for paediatric acute myeloid leukaemia is associated with better prognosis in the Japanese Paediatric Leukaemia/Lymphoma Study Group AML-05 study. Br J Haematol. 2021;193:176-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 71. | Healy FM, Dahal LN, Jones JRE, Floisand Y, Woolley JF. Recent Progress in Interferon Therapy for Myeloid Malignancies. Front Oncol. 2021;11:769628. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 72. | Fan S, Pan TZ, Dou LP, Zhao YM, Zhang XH, Xu LP, Wang Y, Huang XJ, Mo XD. Preemptive interferon-α therapy could prevent relapse of acute myeloid leukemia following allogeneic hematopoietic stem cell transplantation: A real-world analysis. Front Immunol. 2023;14:1091014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 73. | Abaza Y, Zeidan AM. Immune Checkpoint Inhibition in Acute Myeloid Leukemia and Myelodysplastic Syndromes. Cells. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 47] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 74. | Greiner J, Götz M, Hofmann S, Schrezenmeier H, Wiesneth M, Bullinger L, Döhner H, Schneider V. Specific T-cell immune responses against colony-forming cells including leukemic progenitor cells of AML patients were increased by immune checkpoint inhibition. Cancer Immunol Immunother. 2020;69:629-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 75. | Kennedy A, Sahu KK, Cerny J. Role of Immunomodulation of BCG Therapy on AML Remission. Int Med Case Rep J. 2021;14:115-119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 76. | Reizenstein P. Adjuvant immunotherapy with BCG of acute myeloid leukemia: a 15-year follow-up. Br J Haematol. 1990;75:288-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 77. | Teague RM, Kline J. Immune evasion in acute myeloid leukemia: current concepts and future directions. J Immunother Cancer. 2013;1:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 84] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 78. | Bowman RL, Busque L, Levine RL. Clonal Hematopoiesis and Evolution to Hematopoietic Malignancies. Cell Stem Cell. 2018;22:157-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 337] [Article Influence: 56.2] [Reference Citation Analysis (0)] |
| 79. | Skibenes ST, Clausen I, Raaschou-Jensen K. Next-generation sequencing in hypoplastic bone marrow failure: What difference does it make? Eur J Haematol. 2021;106:3-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 80. | Mufti GJ, Marsh JCW. Somatic Mutations in Aplastic Anemia. Hematol Oncol Clin North Am. 2018;32:595-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 81. | Fattizzo B, Levati GV, Giannotta JA, Cassanello G, Cro LM, Zaninoni A, Barbieri M, Croci GA, Revelli N, Barcellini W. Low-Risk Myelodysplastic Syndrome Revisited: Morphological, Autoimmune, and Molecular Features as Predictors of Outcome in a Single Center Experience. Front Oncol. 2022;12:795955. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 82. | Stahl M, DeVeaux M, de Witte T, Neukirchen J, Sekeres MA, Brunner AM, Roboz GJ, Steensma DP, Bhatt VR, Platzbecker U, Cluzeau T, Prata PH, Itzykson R, Fenaux P, Fathi AT, Smith A, Germing U, Ritchie EK, Verma V, Nazha A, Maciejewski JP, Podoltsev NA, Prebet T, Santini V, Gore SD, Komrokji RS, Zeidan AM. The use of immunosuppressive therapy in MDS: clinical outcomes and their predictors in a large international patient cohort. Blood Adv. 2018;2:1765-1772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 104] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 83. | Patel BA, Townsley DM, Scheinberg P. Immunosuppressive therapy in severe aplastic anemia. Semin Hematol. 2022;59:21-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 84. | Sun L, Babushok DV. Secondary myelodysplastic syndrome and leukemia in acquired aplastic anemia and paroxysmal nocturnal hemoglobinuria. Blood. 2020;136:36-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 69] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 85. | Li Y, Li X, Ge M, Shi J, Qian L, Zheng Y, Wang J. Long-term follow-up of clonal evolutions in 802 aplastic anemia patients: a single-center experience. Ann Hematol. 2011;90:529-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 86. | Kouroukli O, Symeonidis A, Foukas P, Maragkou MK, Kourea EP. Bone Marrow Immune Microenvironment in Myelodysplastic Syndromes. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 87. | Zhong FM, Yao FY, Liu J, Zhang HB, Li MY, Jiang JY, Xu YM, Yang WM, Li SQ, Zhang J, Cheng Y, Xu S, Huang B, Wang XZ. Inflammatory response mediates cross-talk with immune function and reveals clinical features in acute myeloid leukemia. Biosci Rep. 2022;42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 88. | Liu B, Shao Y, Liu Z, Liu C, Zhang T, Fu R. Bone Marrow Plasma Cytokine Signature Profiles in Severe Aplastic Anemia. Biomed Res Int. 2020;2020:8789275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 89. | Behzadi Fard M, Kaviani S, Atashi A. Parvovirus B19 Infection in Human Bone Marrow Mesenchymal Stem Cells Affects Gene Expression of IL-6 and TNF-α and also Affects Hematopoietic Stem Cells Differentiation. Indian J Hematol Blood Transfus. 2019;35:765-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 90. | Zhang T, Liu C, Liu H, Li L, Wang T, Fu R. Epstein Barr Virus Infection Affects Function of Cytotoxic T Lymphocytes in Patients with Severe Aplastic Anemia. Biomed Res Int. 2018;2018:6413815. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 91. | Li G, Zhao J, Cheng L, Jiang Q, Kan S, Qin E, Tu B, Zhang X, Zhang L, Su L, Zhang Z. HIV-1 infection depletes human CD34+CD38- hematopoietic progenitor cells via pDC-dependent mechanisms. PLoS Pathog. 2017;13:e1006505. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 92. | Fraison JB, Grignano E, Braun T, Adès L, Chollet-Martin S, Roland-Nicaise P, Fenaux P, Fain O, Mekinian A. Autoantibodies in myelodysplastic syndromes and chronic myelomonocytic leukemia. Leuk Lymphoma. 2019;60:2594-2596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 93. | Xin J, Breslin P, Wei W, Li J, Gutierrez R, Cannova J, Ni A, Ng G, Schmidt R, Chen H, Parini V, Kuo PC, Kini AR, Stiff P, Zhu J, Zhang J. Necroptosis in spontaneously-mutated hematopoietic cells induces autoimmune bone marrow failure in mice. Haematologica. 2017;102:295-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 94. | Ratajczak MZ, Bujko K, Cymer M, Thapa A, Adamiak M, Ratajczak J, Abdel-Latif AK, Kucia M. The Nlrp3 inflammasome as a "rising star" in studies of normal and malignant hematopoiesis. Leukemia. 2020;34:1512-1523. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 85] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 95. | Maratheftis CI, Andreakos E, Moutsopoulos HM, Voulgarelis M. Toll-like receptor-4 is up-regulated in hematopoietic progenitor cells and contributes to increased apoptosis in myelodysplastic syndromes. Clin Cancer Res. 2007;13:1154-1160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 127] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 96. | Giudice V, Feng X, Lin Z, Hu W, Zhang F, Qiao W, Ibanez MDPF, Rios O, Young NS. Deep sequencing and flow cytometric characterization of expanded effector memory CD8(+)CD57(+) T cells frequently reveals T-cell receptor Vβ oligoclonality and CDR3 homology in acquired aplastic anemia. Haematologica. 2018;103:759-769. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 73] [Article Influence: 10.4] [Reference Citation Analysis (0)] |