Published online Oct 6, 2023. doi: 10.12998/wjcc.v11.i28.6841
Peer-review started: June 22, 2023
First decision: August 8, 2023
Revised: August 22, 2023
Accepted: September 4, 2023
Article in press: September 4, 2023
Published online: October 6, 2023
Processing time: 95 Days and 6 Hours
Immune checkpoint inhibitors, including programmed death-ligand 1 (PD-L1) and programmed death-1 (PD-1) have recently been approved to treat locally advanced and metastatic urothelial carcinoma (UC). However, some patients experience rapid tumor progression rather than any clinical benefit from anti-PD-L1/PD-1 therapy.
A 73-year-old woman with bladder UC showed the progression of multiple metastases after surgery and chemotherapy for over 12 mo. The patient could not tolerate further chemotherapy. Next-generation sequencing was performed, and the results indicated that the tumor mutational burden was 6.4 mutations/Mb. The patient received the anti-PD-L1 agent toripalimab combined with albumin-bound paclitaxel. Compared with the baseline staging before immunotherapy, the patient had a treatment failure time of < 2 mo, an increase in tumor burden of > 50%, and a > 2-fold increase in progression, indicating hyperprogression.
Selecting patients most likely to respond to treatment with immunotherapeutic agents remains challenging. For older patients with advanced UC who have already exhausted multi-line chemotherapy options, immunotherapy should be used prudently if no effective biomarker is available. Further studies are required to clarify the causes and mechanisms of hyperprogression.
Core Tip: We report a patient with bladder urothelial carcinoma (UC) who received anti- programmed death-ligand 1 agent toripalimab after surgery and chemotherapy over 12 mo had a treatment failure time less than 2 mo and showed a hyperprogression. Currently, it is still a challenge to select the patients most likely to respond to treatment with immunotherapeutic agents. For elderly patients with advanced UC, immunotherapy should be used prudently if there is no clear effective biomarker. In this case presentation, we include information on genetic alterations. With continued clinical trials and basic research, the risk factors for immunotherapy-related hyperprogressive disease will become clearer.
- Citation: Yang HY, Du YX, Hou YJ, Lu DR, Xue P. Hyperprogression after anti-programmed death-1 therapy in a patient with urothelial bladder carcinoma: A case report. World J Clin Cases 2023; 11(28): 6841-6849
- URL: https://www.wjgnet.com/2307-8960/full/v11/i28/6841.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i28.6841
Over 80500 new bladder cancer cases and 32900 deaths were estimated to have occurred in 2016, making it the 12th most frequent cancer and the leading cause of cancer-related deaths in China[1]. More than 90% of bladder cancers are urothelial carcinomas (UC). Cisplatin-based chemotherapy is the standard first-line treatment that prolongs the survival of patients with advanced UC; however, up to two-thirds of the patients are ineligible because of impaired performance status or comorbidities[2]. Immune checkpoint inhibitors (ICIs) have become the standard of care for patients with multiple cancer types[3-5]. ICIs have been shown to produce durable objective responses and are well tolerated in patients with metastatic UC. New immunotherapies also result in novel tumor response patterns, such as delayed tumor response and pseudoprogression[5-7]. However, immunotherapy can produce opposing effects in a subset of patients, as described by Champiat et al[8]. Approximately 4%-29% of patients receiving immunotherapy develop immune hyperprogression[8-10], which is defined as a time-to-treatment failure (TTF) of < 2 mo, > 50% increase in tumor burden compared with pre-immunotherapy imaging, and > 2-fold increase in progression rate[9]. Several studies have suggested that specific alterations may be associated with hyperprogression[9,11]. We recently identified a patient whose disease accelerated paradoxically after immunotherapy. Here, we describe a case of hyperprogression and its corresponding genomic profile.
A 73-year-old woman with UC showed the progression of multiple metastases.
Tumor showed the progression after surgery and chemotherapy over 12 mo.
No previous complaints of discomfort.
She denied any family history of malignant tumours.
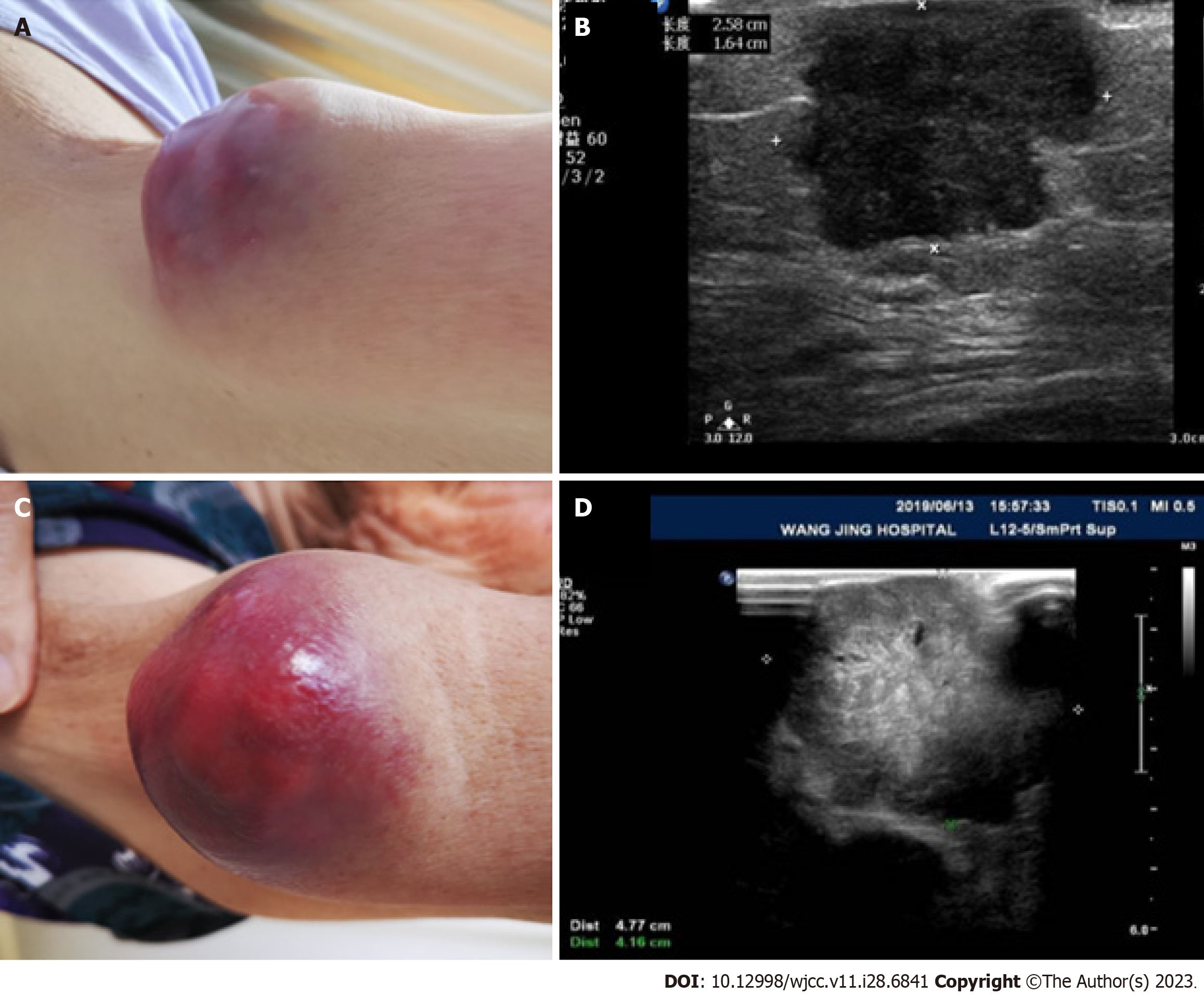
On physical examination, the vital signs were as follows: Body temperature, 36.5 °C; blood pressure, 125/79 mmHg; heart rate, 85 beats per min; respiratory rate, 18 breaths per min. Flat abdomen, no abdominal varicose veins, no abdominal muscle tension, no tenderness, no rebound pain, no liquid wave tremor, no water vibration sound, some abdominal subcutaneous mass.
No abnormality was found in routine blood, urine analyses.
In November 2018, positron emission tomography/computed tomography showed multiple subcutaneous metastases in the whole body.
Combined with the patient’s medical history, the final diagnosis was urothelial bladder carcinoma.
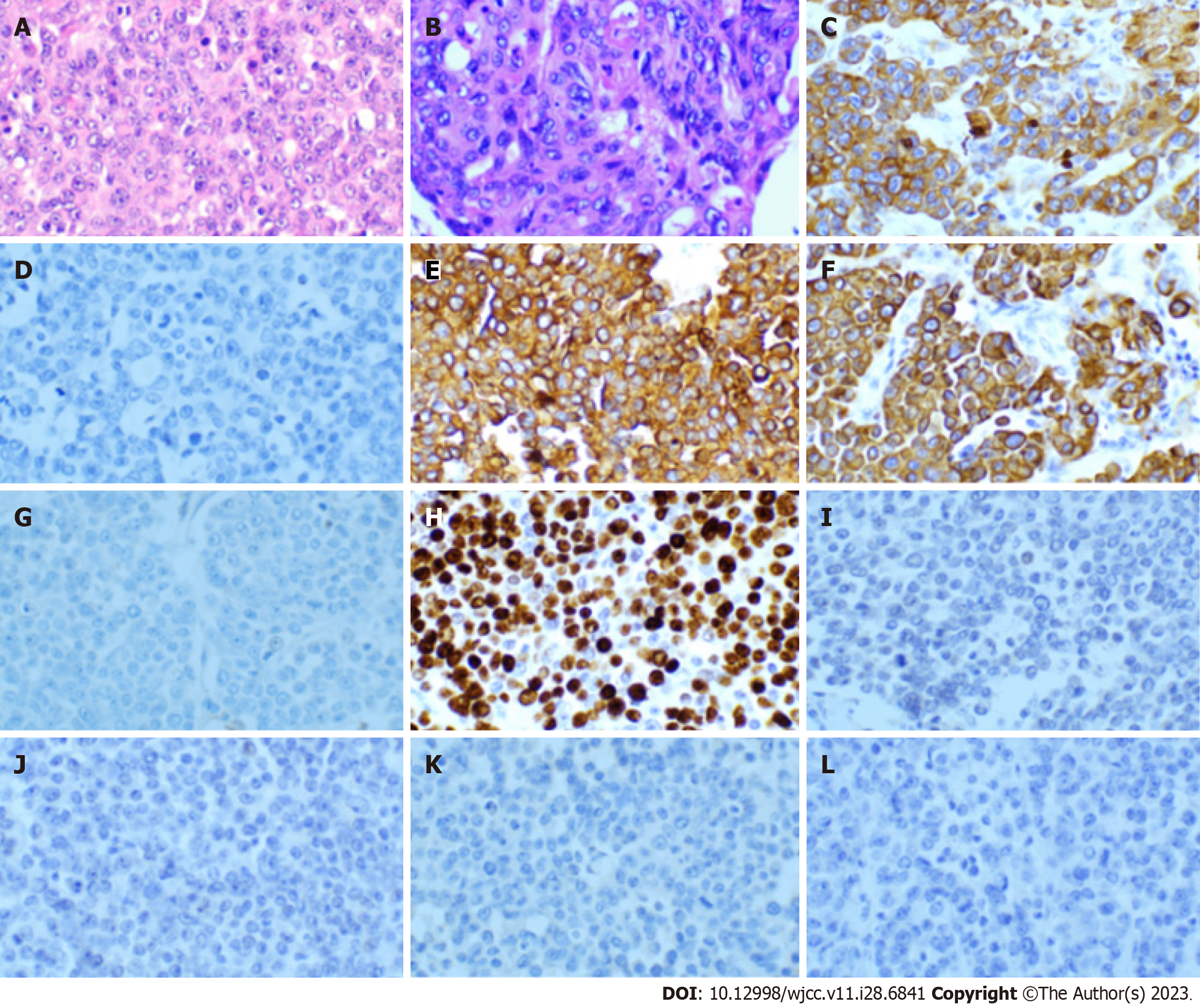
We performed a fine-needle biopsy of the abdominal subcutaneous mass, and the immunohistochemistry results suggested that the lesion was indeed a metastatic UC (Figure 1). Immunohistochemistry is a widely used diagnostic technique in histopathology. This technique utilizes the principle of specific binding of an antigen to an antibody to identify intracellular antigens in tissues via a color-producing chemical reaction[12]. At the end of 2018, the patient began receiving gemcitabine (1.6 g, d1.8, q3w) in combination with cisplatin (30.0 mg, d1-3, q3w) chemotherapy at our department. However, after two cycles of chemotherapy, the volume of the subcutaneous nodules increased. The patient was then switched to pemetrexed for four cycles of monotherapy, and the efficacy was evaluated as a partial response. However, because of 4th-degree myelosuppression, the patient could not tolerate chemotherapy any further. Pre-immunotherapy and next-generation sequencing (NGS)[13] were performed (mean coverage depth > 1000 for up to 576 genes), and the results showed multiple genetic alterations (Table 1). NGS is a significant step forward in Personalized Medicine, as it enables the detection of somatic driver mutations, resistance mechanisms, quantification of mutational burden, and germline mutations. Moreover, the NGS results indicated that the microsatellites were stable, and the tumor mutational burden was 6.4 mutations/Mb. These results suggest that ICI therapy may be effective in this patient. Therefore, in early May 2019, the patient started receiving the anti-programmed death-ligand 1 (PD-L1) agent toripalimab (240 mg, d1, q3w) in combination with albumin-bound paclitaxel (200 mg, d1, q3w). However, at the end of one treatment cycle, the subcutaneous nodules had not shrunk. At the beginning of June, the original immunotherapy combined with a chemotherapy regimen was continued until the subsequent treatment.
| Gene | Genetic variation information | Gene mutation abundance |
| TP53 | p.R273H 8 Exon | 31.56% |
| MST1R | p.A1301G 19 Exon | 8.82% |
| WWTR1 | p.Q233del 4 Exon | 3.54% |
| KMT2D | p.P4949fs 48 Exon | 17.82% |
| p.P4055fs 39 Exon | 8.00% | |
| p.T698A 10 Exon | 9.48% | |
| CDK8 | p.Q377del 12 Exon | 2.91% |
| PTPRT | p.V769E 15 Exon | 0.99% |
| GNAS | p.R844H 8 Exon | 1.69% |
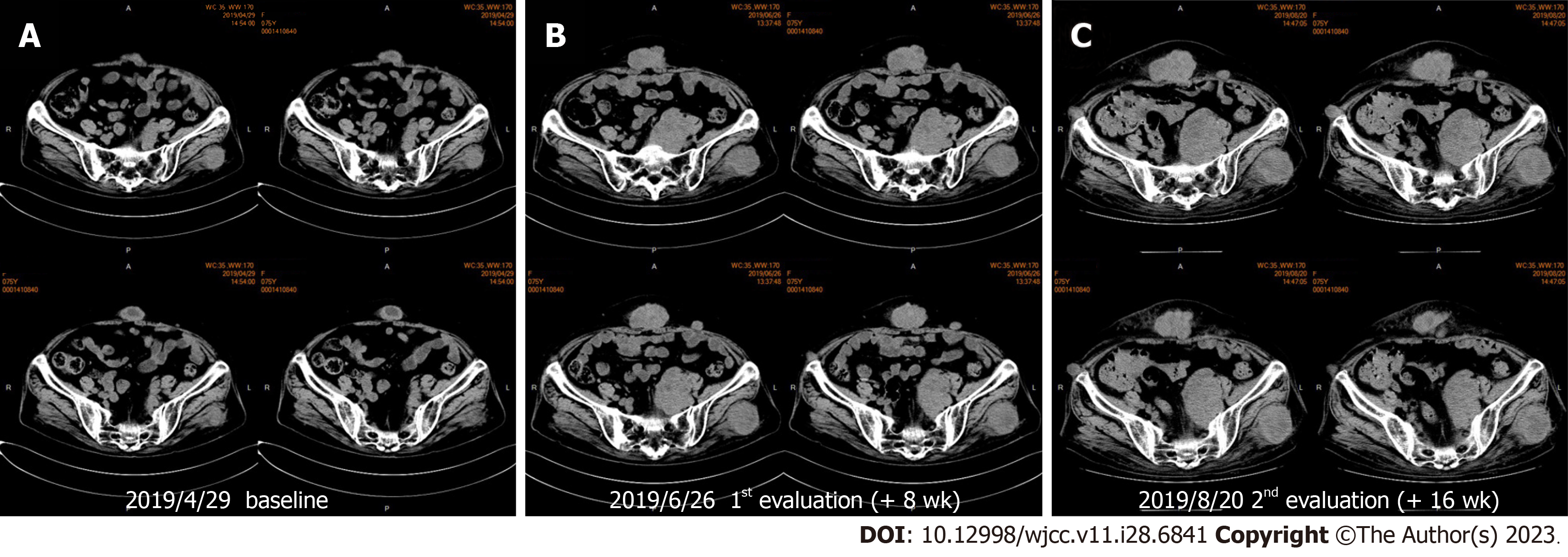
Unfortunately, after two cycles of immunotherapy combined with chemotherapy, repeat imaging performed half a month later confirmed progression. Some subcutaneous nodules and intrapelvic metastases increased significantly, indicating that the patient possibly experienced hyperprogression after immunotherapy (Figures 2 and 3). Subcutaneous nodular metastases in multiple parts of the body increased by > 2 times compared with those before immunotherapy. Compared with the baseline staging before immunotherapy, the patient had a treatment failure time of < 2 mo, an increase in tumor burden of > 50%, and a 2-fold increase in progression, which indicated hyperprogression. As the bone marrow suppression of the patient improved and the blood cell count returned to normal, pemetrexed chemotherapy was resumed.
Advanced UC has a poor prognosis, especially in patients who undergo ineffective or poorly tolerated chemotherapy[14]. First-line chemotherapy is often not well-tolerated and is associated with a short response duration. Data from clinical trials suggest that the median overall survival of patients with advanced UC receiving platinum-based chemotherapy is approximately 12-16 mo[15]. Moreover, after the failure of first-line chemotherapy, metastatic UC is a universally fatal malignancy, with a median survival of 7 mo. In 2014, a phase Ia clinical trial of atezolizumab revealed noteworthy activity in metastatic UC, establishing a new era of ICIs for treating UC[16]. ICIs, such as programmed death-1 (PD-1) and PD-L1 antibodies, have demonstrated superiority over chemotherapy for treating advanced UC in second-line settings. Since 2016, five checkpoint inhibitors, including PD-1 and PD-L1 antibodies, have been approved by the Food and Drug Administration to treat advanced UC[17].
IMvigor 210, a phase II study of the PD-L1 antibody atezolizumab (1200 mg every 3 wk) for the treatment of advanced UC, showed that higher levels of PD-L1 protein expression in immune cells were associated with a higher response rate to atezolizumab. Additionally, longer overall survival with atezolizumab was strongly correlated with an increased tumor mutation load. Subsequently, a phase III randomized controlled trial was conducted to compare atezolizumab with chemotherapy in patients with locally advanced or metastatic UC who had progressed to platinum-containing regimens. The results suggested that the overall survival did not differ significantly between patients overexpressing PD-L1 in the atezolizumab group and those in the chemotherapy group (median 11.1 mo vs 10.6 mo, respectively; hazard ratio = 0.87, P = 0.41)[18,19]. Another PD-L1 antibody used for treating platinum-refractory metastatic UC, durvalumab (10 mg/kg every 2 wk), demonstrated an objective response rate of 31% in the UC expansion cohort. Patients with high PD-L1 expression achieved a response rate of 46% and overall survival of 20 mo compared with 8 mo in the PD-L1 Low-expression subgroup[20]. A third PD-L1 antibody, avelumab, was also approved for treating metastatic UC after progression to platinum-based chemotherapy, based on the findings of a bladder cancer cohort in the JAVELIN phase I program. The objective response rate (ORR) was 17%, with high PD-L1 expression enriching the response (24% compared with only 14% in the PD-L1 Low-expression subgroup)[21].
The CheckMate 275 is a single-arm phase II study similar to the IMvigor 210 cohort 2. In this phase II study, treatment with nivolumab (3 mg/kg every 2 wk) resulted in substantial tumor reduction and an ORR of 19.6% in patients with platinum-refractory metastatic UC. However, the median duration of response was not attained at a median follow-up of 7 mo. Although investigators found a statistically significant improvement in overall survival among subgroups with PD-L1 expression levels > 1%, responses were observed after ignoring PD-L1 expression[22].
Following KEYNOTE-052[23] and KEYNOTE-012[24] clinical trials characterizing the safety and efficacy of pembrolizumab (KEYNOTE-045), KEYNOTE-045 was designed to detect differences in overall survival and progression-free survival, primarily in the entire cohort. The pembrolizumab group achieved a median survival of 10.3 mo, whereas the chemotherapy group only reached 7.4 mo. Interestingly, the PD-L1 high-expression subgroups of KEYNOTE-045 did not exhibit longer survival in either treatment arm, which was different from that of IMvigor211, in which the chemotherapy arm fared better in the PD-L1 high-expression subgroup[25].
Notably, first-line chemotherapy is often not well-tolerated and is associated with a short response duration. Furthermore, more than two-thirds of patients are unfit for cisplatin-based chemotherapy, and alternative carboplatin-based regimens are associated with overall survival of < 1 year[2,26]. Given the breakthroughs in second-line immunotherapy for metastatic UC, immunotherapy is being used as the first-line treatment for UC, and relevant clinical trials have achieved satisfactory results.
In the IMvigor210 study, 119 patients with advanced UC in cohort 1 were ineligible for cisplatin treatment because of renal impairment (70%), Eastern Cooperative Oncology Group performance status (20%), hearing loss, and neuropathy. These patients exhibited poor prognoses in the absence of cisplatin-based chemotherapy. Significantly, the ORR of the first-line atezolizumab treatment was 23% (9% complete response and 13% partial response), which was higher than that observed in the second-line setting.
A phase II trial of pembrolizumab for cisplatin-ineligible patients with metastatic UC enrolled 370 patients treated with first-line pembrolizumab. The primary endpoint was the response rate for the overall cohort, which was stratified by PD-L1 expression status using the combined positive score. The ORR was 24% for the overall cohort, and the ORR for patients with a combined positive score > 10% was 38%[25]. These data facilitated the approval of pembrolizumab as a first-line treatment for cisplatin-ineligible patients with advanced UC.
Considering the good ORR of ICIs in the treatment of metastatic UC, subsequent trials were initiated to broadly assess the efficacy of PD-1/L1 blockade combined with cytotoxic chemotherapy, including gemcitabine/carboplatin, nanoparticle albumin-bound paclitaxel, carboplatin, and oxaliplatin, in a first-line setting. Clinical trials are currently underway. However, in May 2018, the Food and Drug Administration issued a safety alert for atezolizumab and pembrolizumab owing to decreased survival relative to chemotherapy among PD-L1 Low-expression subgroups[27]. Therefore, the PD-1/L1 blockade is not the first-line treatment of choice for patients with advanced UC who cannot tolerate carboplatin treatment because of advanced age or comorbidities with low PD-1 expression.
Although immunotherapy has made great progress as the first- and second-line treatment for advanced UC, its ORR is not very high. However, selecting patients who are most likely to respond to treatment with immunotherapeutic agents remains challenging. Our patient did not respond to the ICI. Immunotherapy may also result in a unique response pattern known as pseudoprogression, where tumors initially appear larger on imaging, but the lesions subsequently regress[28]. Moreover, pseudoprogression differs from hyperprogression. Pseudoprogression is defined as ≤ 50% increase in tumor burden compared with pre-immunotherapy imaging within 2 mo, and the symptoms of patients should not be more severe than that of pre-immunotherapy. In this case, an older patient with metastatic UC and several gene amplifications or mutations, who did not receive radiation therapy, progressed after immunotherapy for less than 2 mo. This indicated that the ICI-associated progression was not pseudoprogression but true hyperprogression.
Immunotherapy-induced hyperprogression is a well-known phenomenon that has been recently described. Champiat et al[8] demonstrated that 9% of patients (12/131) showed hyperprogression compared with baseline imaging. In addition, older age (> 70 years) was associated with hyperprogression. Champiat et al[8] analyzed 218 patients treated with anti-PD-1 or anti-PD-L1 monotherapy. They found 131 patients (60%) with a clinically meaningful tumor growth rate and eventually identified 12 patients with hyperprogressive disease (HPD), representing 9% of the evaluable patients. Furthermore, they analyzed the patient characteristics and their associations with HPD using continuous variables. However, they only observed an association between the HPD status and mean age (66 years vs 55 years, P = 0.007). Patients aged > 65 years had a 19% (7/36) rate of HPD compared with only 5% (5 of 95) of patients aged < 64 years (P = 0.018). More than three independent phase III trials have indicated that older patients benefit less from immunotherapy than younger patients[3,29]. The median overall survival of patients with HPD was 4.6 mo less than that of patients without HPD. Champiat et al[8] also observed a significant inverse correlation between the tumor growth rate at baseline and response to anti-PD-1/PD-L1 (P = 0.0039). Other studies have shown that slow-growing tumors are less likely to respond, in contrast to what was previously observed with targeted therapy[30,31]. A total of 49 patients had HPD at the first evaluation according to the Response Evaluation Criteria in Solid Tumors, and patients with HPD exhibited a lower rate of new lesions than those without HPD (33% vs 84%, P = 0.0019). Furthermore, patients who exhibited a rapid growth rate only in the new lesions were not considered to have HPD.
Kato et al[9] investigated potential genomic markers associated with hyperprogression after immunotherapy and analyzed 155 patients who underwent immunotherapy and molecular profiling. The results showed that the epidermal growth factor receptor (EGFR) [odds ratio (OR): 10.2; P = 0.002], mouse double-minute 2 homolog (MDM2)/4 (OR > 11.9; P = 0.001), and DNA methyltransferase 3 alpha (DNMT3A) (OR 9.33; P = 0.03) were significantly associated with poor clinical outcomes and a TTF of < 2 mo. Four of the six patients with MDM2 family amplifications had a TTF of < 2 mo and demonstrated hyperprogression with an increase in lesions compared with pre-immunotherapy. Another two MDM2/MDM4-amplified patients also demonstrated rapid clinical deterioration. Similarly, 80% of the patients with EGFR alterations had a TTF of < 2 mo. Two (20%) patients showed rapid hyperprogression. Among the five patients with DNMT3A alterations, four had a TTF of < 2 mo, but it was impossible to determine whether the patient had a clear HPD because of incomplete data. This study suggested that patients for whom anti-PD1/PDL1 monotherapy is planned may require genomic testing to determine whether they harbor specific alterations associated with hyperprogression. However, the relationship between age and HPD was not determined in this study. Therefore, the precise risk factors of HPD remain unknown.
TP53, the center of tumor suppression, was discovered during viral infections and has been shown to play a significant role in immunity and inflammation. However, mutant p53 not only cripples immune functions but also subverts them through its neomorphic gain-of-functions, which promote tumorigenesis, metastasis, and invasion, indicating that the guardian of immunity becomes its saboteur through mutation[32].
NGS of our patient revealed a TP53 mutation abundance of up to 31.56%, which may indicate disruption of the innate tumor immune function and alteration of the tumor microenvironment. We speculate that using PD-L1 on this basis may be an intra-cohort cause of accelerated cancer progression. However, because of the lack of reported cases of NGS-related immune hyperprogression, we could not draw any conclusions.
In this case presentation, we include information on genetic alterations. With continued clinical trials and basic research, the risk factors for immunotherapy-related HPD will become clearer. Genetic changes in this patient, which may or may not be related to immune hyperprogression, have not been previously reported. However, we could not obtain sufficient tissue samples to perform immunohistochemical staining for PD-L1. Therefore, it was not possible to determine whether the patient was suitable for ICI treatment.
In summary, older patients with metastatic UC who have undergone multiline chemotherapy should be closely monitored when treated with anti-PD1/PD-L1 agents. Although it is clear that there are no risk-related genetic alterations that promote immune hyperprogression. Further translational studies with more patients and larger validation cohorts are urgently required.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Al-Emam AMA, Saudi Arabia S-Editor: Chen YL L-Editor: A P-Editor: Chen YL
| 1. | Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11444] [Cited by in RCA: 13190] [Article Influence: 1465.6] [Reference Citation Analysis (3)] |
| 2. | Galsky MD, Hahn NM, Rosenberg J, Sonpavde G, Hutson T, Oh WK, Dreicer R, Vogelzang N, Sternberg CN, Bajorin DF, Bellmunt J. Treatment of patients with metastatic urothelial cancer "unfit" for Cisplatin-based chemotherapy. J Clin Oncol. 2011;29:2432-2438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 368] [Cited by in RCA: 499] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 3. | Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE, Holgado E, Waterhouse D, Ready N, Gainor J, Arén Frontera O, Havel L, Steins M, Garassino MC, Aerts JG, Domine M, Paz-Ares L, Reck M, Baudelet C, Harbison CT, Lestini B, Spigel DR. Nivolumab vs Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373:123-135. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5686] [Cited by in RCA: 6720] [Article Influence: 672.0] [Reference Citation Analysis (0)] |
| 4. | Horn L, Mansfield AS, Szczęsna A, Havel L, Krzakowski M, Hochmair MJ, Huemer F, Losonczy G, Johnson ML, Nishio M, Reck M, Mok T, Lam S, Shames DS, Liu J, Ding B, Lopez-Chavez A, Kabbinavar F, Lin W, Sandler A, Liu SV; IMpower133 Study Group. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N Engl J Med. 2018;379:2220-2229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1567] [Cited by in RCA: 2350] [Article Influence: 335.7] [Reference Citation Analysis (0)] |
| 5. | Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe S, O'Brien M, Rao S, Hotta K, Leiby MA, Lubiniecki GM, Shentu Y, Rangwala R, Brahmer JR; KEYNOTE-024 Investigators. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med. 2016;375:1823-1833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5948] [Cited by in RCA: 7472] [Article Influence: 830.2] [Reference Citation Analysis (0)] |
| 6. | Wang Q, Gao J, Wu X. Pseudoprogression and hyperprogression after checkpoint blockade. Int Immunopharmacol. 2018;58:125-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 123] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 7. | Soria F, Beleni AI, D'Andrea D, Resch I, Gust KM, Gontero P, Shariat SF. Pseudoprogression and hyperprogression during immune checkpoint inhibitor therapy for urothelial and kidney cancer. World J Urol. 2018;36:1703-1709. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 69] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 8. | Champiat S, Dercle L, Ammari S, Massard C, Hollebecque A, Postel-Vinay S, Chaput N, Eggermont A, Marabelle A, Soria JC, Ferté C. Hyperprogressive Disease Is a New Pattern of Progression in Cancer Patients Treated by Anti-PD-1/PD-L1. Clin Cancer Res. 2017;23:1920-1928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 703] [Cited by in RCA: 902] [Article Influence: 100.2] [Reference Citation Analysis (0)] |
| 9. | Kato S, Goodman A, Walavalkar V, Barkauskas DA, Sharabi A, Kurzrock R. Hyperprogressors after Immunotherapy: Analysis of Genomic Alterations Associated with Accelerated Growth Rate. Clin Cancer Res. 2017;23:4242-4250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 538] [Cited by in RCA: 690] [Article Influence: 86.3] [Reference Citation Analysis (0)] |
| 10. | Saâda-Bouzid E, Defaucheux C, Karabajakian A, Coloma VP, Servois V, Paoletti X, Even C, Fayette J, Guigay J, Loirat D, Peyrade F, Alt M, Gal J, Le Tourneau C. Hyperprogression during anti-PD-1/PD-L1 therapy in patients with recurrent and/or metastatic head and neck squamous cell carcinoma. Ann Oncol. 2017;28:1605-1611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 451] [Article Influence: 64.4] [Reference Citation Analysis (0)] |
| 11. | Mao S, Zhang J, Guo Y, Zhang Z, Wu Y, Zhang W, Wang L, Geng J, Yan Y, Yao X. Hyperprogression after anti-programmed cell death ligand-1 therapy in a patient with recurrent metastatic urothelial bladder carcinoma following first-line cisplatin-based chemotherapy: a case report. Drug Des Devel Ther. 2019;13:291-300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Tan WCC, Nerurkar SN, Cai HY, Ng HHM, Wu D, Wee YTF, Lim JCT, Yeong J, Lim TKH. Overview of multiplex immunohistochemistry/immunofluorescence techniques in the era of cancer immunotherapy. Cancer Commun (Lond). 2020;40:135-153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 285] [Cited by in RCA: 392] [Article Influence: 78.4] [Reference Citation Analysis (0)] |
| 13. | Morganti S, Tarantino P, Ferraro E, D'Amico P, Duso BA, Curigliano G. Next Generation Sequencing (NGS): A Revolutionary Technology in Pharmacogenomics and Personalized Medicine in Cancer. Adv Exp Med Biol. 2019;1168:9-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 130] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 14. | Choueiri TK, Ross RW, Jacobus S, Vaishampayan U, Yu EY, Quinn DI, Hahn NM, Hutson TE, Sonpavde G, Morrissey SC, Buckle GC, Kim WY, Petrylak DP, Ryan CW, Eisenberger MA, Mortazavi A, Bubley GJ, Taplin ME, Rosenberg JE, Kantoff PW. Double-blind, randomized trial of docetaxel plus vandetanib versus docetaxel plus placebo in platinum-pretreated metastatic urothelial cancer. J Clin Oncol. 2012;30:507-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 152] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 15. | von der Maase H, Sengelov L, Roberts JT, Ricci S, Dogliotti L, Oliver T, Moore MJ, Zimmermann A, Arning M. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol. 2005;23:4602-4608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1220] [Cited by in RCA: 1396] [Article Influence: 69.8] [Reference Citation Analysis (0)] |
| 16. | Powles T, Eder JP, Fine GD, Braiteh FS, Loriot Y, Cruz C, Bellmunt J, Burris HA, Petrylak DP, Teng SL, Shen X, Boyd Z, Hegde PS, Chen DS, Vogelzang NJ. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515:558-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1718] [Cited by in RCA: 1912] [Article Influence: 191.2] [Reference Citation Analysis (0)] |
| 17. | Lattanzi M, Balar AV. Current Status and Future Direction of Immunotherapy in Urothelial Carcinoma. Curr Oncol Rep. 2019;21:24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Rosenberg JE, Hoffman-Censits J, Powles T, van der Heijden MS, Balar AV, Necchi A, Dawson N, O'Donnell PH, Balmanoukian A, Loriot Y, Srinivas S, Retz MM, Grivas P, Joseph RW, Galsky MD, Fleming MT, Petrylak DP, Perez-Gracia JL, Burris HA, Castellano D, Canil C, Bellmunt J, Bajorin D, Nickles D, Bourgon R, Frampton GM, Cui N, Mariathasan S, Abidoye O, Fine GD, Dreicer R. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet. 2016;387:1909-1920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2517] [Cited by in RCA: 2886] [Article Influence: 320.7] [Reference Citation Analysis (0)] |
| 19. | Powles T, Durán I, van der Heijden MS, Loriot Y, Vogelzang NJ, De Giorgi U, Oudard S, Retz MM, Castellano D, Bamias A, Fléchon A, Gravis G, Hussain S, Takano T, Leng N, Kadel EE 3rd, Banchereau R, Hegde PS, Mariathasan S, Cui N, Shen X, Derleth CL, Green MC, Ravaud A. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2018;391:748-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 880] [Cited by in RCA: 1050] [Article Influence: 150.0] [Reference Citation Analysis (0)] |
| 20. | Powles T, O'Donnell PH, Massard C, Arkenau HT, Friedlander TW, Hoimes CJ, Lee JL, Ong M, Sridhar SS, Vogelzang NJ, Fishman MN, Zhang J, Srinivas S, Parikh J, Antal J, Jin X, Gupta AK, Ben Y, Hahn NM. Efficacy and Safety of Durvalumab in Locally Advanced or Metastatic Urothelial Carcinoma: Updated Results From a Phase 1/2 Open-label Study. JAMA Oncol. 2017;3:e172411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 559] [Cited by in RCA: 699] [Article Influence: 87.4] [Reference Citation Analysis (0)] |
| 21. | Patel MR, Ellerton J, Infante JR, Agrawal M, Gordon M, Aljumaily R, Britten CD, Dirix L, Lee KW, Taylor M, Schöffski P, Wang D, Ravaud A, Gelb AB, Xiong J, Rosen G, Gulley JL, Apolo AB. Avelumab in metastatic urothelial carcinoma after platinum failure (JAVELIN Solid Tumor): pooled results from two expansion cohorts of an open-label, phase 1 trial. Lancet Oncol. 2018;19:51-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 461] [Article Influence: 57.6] [Reference Citation Analysis (0)] |
| 22. | Sharma P, Retz M, Siefker-Radtke A, Baron A, Necchi A, Bedke J, Plimack ER, Vaena D, Grimm MO, Bracarda S, Arranz JÁ, Pal S, Ohyama C, Saci A, Qu X, Lambert A, Krishnan S, Azrilevich A, Galsky MD. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2017;18:312-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 997] [Cited by in RCA: 1268] [Article Influence: 158.5] [Reference Citation Analysis (0)] |
| 23. | Balar AV, Castellano D, O'Donnell PH, Grivas P, Vuky J, Powles T, Plimack ER, Hahn NM, de Wit R, Pang L, Savage MJ, Perini RF, Keefe SM, Bajorin D, Bellmunt J. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, phase 2 study. Lancet Oncol. 2017;18:1483-1492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 726] [Cited by in RCA: 1002] [Article Influence: 125.3] [Reference Citation Analysis (0)] |
| 24. | Plimack ER, Bellmunt J, Gupta S, Berger R, Chow LQ, Juco J, Lunceford J, Saraf S, Perini RF, O'Donnell PH. Safety and activity of pembrolizumab in patients with locally advanced or metastatic urothelial cancer (KEYNOTE-012): a non-randomised, open-label, phase 1b study. Lancet Oncol. 2017;18:212-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 277] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 25. | Bellmunt J, de Wit R, Vaughn DJ, Fradet Y, Lee JL, Fong L, Vogelzang NJ, Climent MA, Petrylak DP, Choueiri TK, Necchi A, Gerritsen W, Gurney H, Quinn DI, Culine S, Sternberg CN, Mai Y, Poehlein CH, Perini RF, Bajorin DF; KEYNOTE-045 Investigators. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N Engl J Med. 2017;376:1015-1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2051] [Cited by in RCA: 2613] [Article Influence: 326.6] [Reference Citation Analysis (0)] |
| 26. | De Santis M, Bellmunt J, Mead G, Kerst JM, Leahy M, Maroto P, Gil T, Marreaud S, Daugaard G, Skoneczna I, Collette S, Lorent J, de Wit R, Sylvester R. Randomized phase II/III trial assessing gemcitabine/carboplatin and methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer who are unfit for cisplatin-based chemotherapy: EORTC study 30986. J Clin Oncol. 2012;30:191-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 427] [Cited by in RCA: 536] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 27. | Eckstein M, Erben P, Kriegmair MC, Worst TS, Weiß CA, Wirtz RM, Wach S, Stoehr R, Sikic D, Geppert CI, Weyerer V, Bertz S, Breyer J, Otto W, Keck B, Burger M, Taubert H, Weichert W, Wullich B, Bolenz C, Hartmann A, Erlmeier F. Performance of the Food and Drug Administration/EMA-approved programmed cell death ligand-1 assays in urothelial carcinoma with emphasis on therapy stratification for first-line use of atezolizumab and pembrolizumab. Eur J Cancer. 2019;106:234-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 71] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 28. | Chiou VL, Burotto M. Pseudoprogression and Immune-Related Response in Solid Tumors. J Clin Oncol. 2015;33:3541-3543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 557] [Cited by in RCA: 675] [Article Influence: 67.5] [Reference Citation Analysis (0)] |
| 29. | Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, Barlesi F, Kohlhäufl M, Arrieta O, Burgio MA, Fayette J, Lena H, Poddubskaya E, Gerber DE, Gettinger SN, Rudin CM, Rizvi N, Crinò L, Blumenschein GR Jr, Antonia SJ, Dorange C, Harbison CT, Graf Finckenstein F, Brahmer JR. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373:1627-1639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6945] [Cited by in RCA: 7499] [Article Influence: 749.9] [Reference Citation Analysis (0)] |
| 30. | Ferté C, Fernandez M, Hollebecque A, Koscielny S, Levy A, Massard C, Balheda R, Bot B, Gomez-Roca C, Dromain C, Ammari S, Soria JC. Tumor growth rate is an early indicator of antitumor drug activity in phase I clinical trials. Clin Cancer Res. 2014;20:246-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 144] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 31. | Ferté C, Koscielny S, Albiges L, Rocher L, Soria JC, Iacovelli R, Loriot Y, Fizazi K, Escudier B. Tumor growth rate provides useful information to evaluate sorafenib and everolimus treatment in metastatic renal cell carcinoma patients: an integrated analysis of the TARGET and RECORD phase 3 trial data. Eur Urol. 2014;65:713-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 32. | Agupitan AD, Neeson P, Williams S, Howitt J, Haupt S, Haupt Y. P53: A Guardian of Immunity Becomes Its Saboteur through Mutation. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 65] [Article Influence: 13.0] [Reference Citation Analysis (0)] |