Published online Oct 6, 2023. doi: 10.12998/wjcc.v11.i28.6831
Peer-review started: July 11, 2023
First decision: August 4, 2023
Revised: August 21, 2023
Accepted: September 11, 2023
Article in press: September 11, 2023
Published online: October 6, 2023
Processing time: 75 Days and 12.6 Hours
Type 2 hereditary hemorrhagic telangiectasia (HHT) is a rare autosomal dominant disease and is associated with ALK1 gene mutations. Type 2 HHT patients primarily suffer from recurrent bleeding. There is currently no promising treatment.
A 5-year-old Chinese patient (III23) was admitted to Zhongshan Hospital for recurrent melena occurring over 2 mo. She had been experiencing epistaxis for years and had been diagnosed with idiopathic pulmonary hypertension 4 mo before presentation. Abdominal computed tomography examination showed hepatic arteriovenous malformation. Gene testing revealed a c.1121G>A mutation on the ALK1 gene. According to the international diagnostic criteria, this patient was diagnosed with HHT. In addition, 8 more family members exhibited HHT symptoms to varying degrees. Gene testing in 5 family members (2 with HHT symptoms and 3 without HHT symptoms) revealed the ALK1 c.1121G>A mutation in the 2 family members with HHT symptoms. This missense mutation results in the substitution of arginine for glutamine at amino acid position 374 (R374Q) in the conserved functional kinase domain of ALK1. Biological studies revealed that this mutation decreased the kinase activity of ALK1 and impeded the phosphorylation of its substrate Smad1. Moreover, the R374Q mutant downregulated the protein level of collagen-1, a fibrogenic factor, indicating abnormal fiber generation during vascular formation.
The R374Q mutant of ALK1 and its subsequent influence on fiber generation highly indicated its pathogenic role in this family with type 2 HHT. Detection of this gene mutation will facilitate early diagnosis of suspected type 2 HHT patients, and mechanistic studies will provide insights for future therapy.
Core Tip: Hereditary hemorrhagic telangiectasia (HHT) is a rare autosomal dominant hereditary disease characterized by varying degrees of bleeding symptoms. The diagnosis of HHT is difficult when relying on bleeding symptoms, while gene testing is robust and reliable for identifying HHT patients. Here, we report a patient with type 2 HHT who was undiagnosed for HHT for years due to varied symptoms involving multiple organs. We found an ALK1 gene mutation in the patient and her family members. We further verified the pathogenic role of this mutation in inducing vascular malformation by basic research studies.
- Citation: Chen YL, Jiang HY, Li DP, Lin J, Chen Y, Xu LL, Gao H. Multi-organ hereditary hemorrhagic telangiectasia: A case report. World J Clin Cases 2023; 11(28): 6831-6840
- URL: https://www.wjgnet.com/2307-8960/full/v11/i28/6831.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i28.6831
Hereditary hemorrhagic telangiectasia (HHT), a rare autosomal dominant hereditary disorder, is characterized by abnormal bleeding and telangiectasia occurring predominantly in the mucosa of the nose, oral cavity, and the skin of the face and hands[1]. The underlying pathology of HHT patients is due to the lack of normal capillaries that link the artery and vein. Therefore, blood from the arteries flows directly into the veins at high pressure and ruptures the fragile sites in the shallow mucosa or deep visceral organs[2]. The common epistaxis and telangiectasia are easily discovered and treated, while the atypically silent lesions in the lung, liver, stomach, and bowel are often neglected, leading to delayed diagnosis and severe damage[1]. Constant silent bleeding can contribute to severe anemia, and abrupt massive bleeding in major organs can lead to death.
In these circumstances, early diagnosis of HHT is of extreme importance to educate patients about precautions against aberrant bleeding and prevention of detrimental outcomes. Since HHT is a dominant hereditary disease that is primarily caused by aberrant mutation of the ENG gene (type 1 HHT)[3] or the ALK1 gene (type 2 HHT)[4], gene sequencing of ENG and ALK1 is a useful method for HHT diagnosis. However, hundreds of mutations on ENG and ALK1 have been discovered[5], and mutations that are crucial for HHT occurrence have not been fully examined. Therefore, it is imperative to determine the pathogenic mutations that could be used for HHT diagnosis.
In this study, we analyzed a Chinese family with type 2 HHT and discovered a mutation, c.1121G>A (p.R374Q), in ALK1. Biological studies revealed that this mutation decreased the kinase activity of ALK1 and reduced expression of collagen-1, indicating its pathogenic role in dysregulating fiber generation during vascular formation. Hence, our study provided a potential genetic marker for type 2 HHT diagnosis, and the underlying mechanistic exploration provided the foundation of better treatments.
A 56-year-old Chinese female patient (III23) was admitted to Zhongshan Hospital for constant black stools occurring for 2 mo.
The patient had recurrent epistaxis for years and noticed black stool for the prior 2 mo without abdominal pain or other gastrointestinal (GI) symptoms.
The patient also suffered from fatigue and shortness of breath and was diagnosed with idiopathic pulmonary hypertension and right heart failure in a local hospital 4 mo prior to presentation.
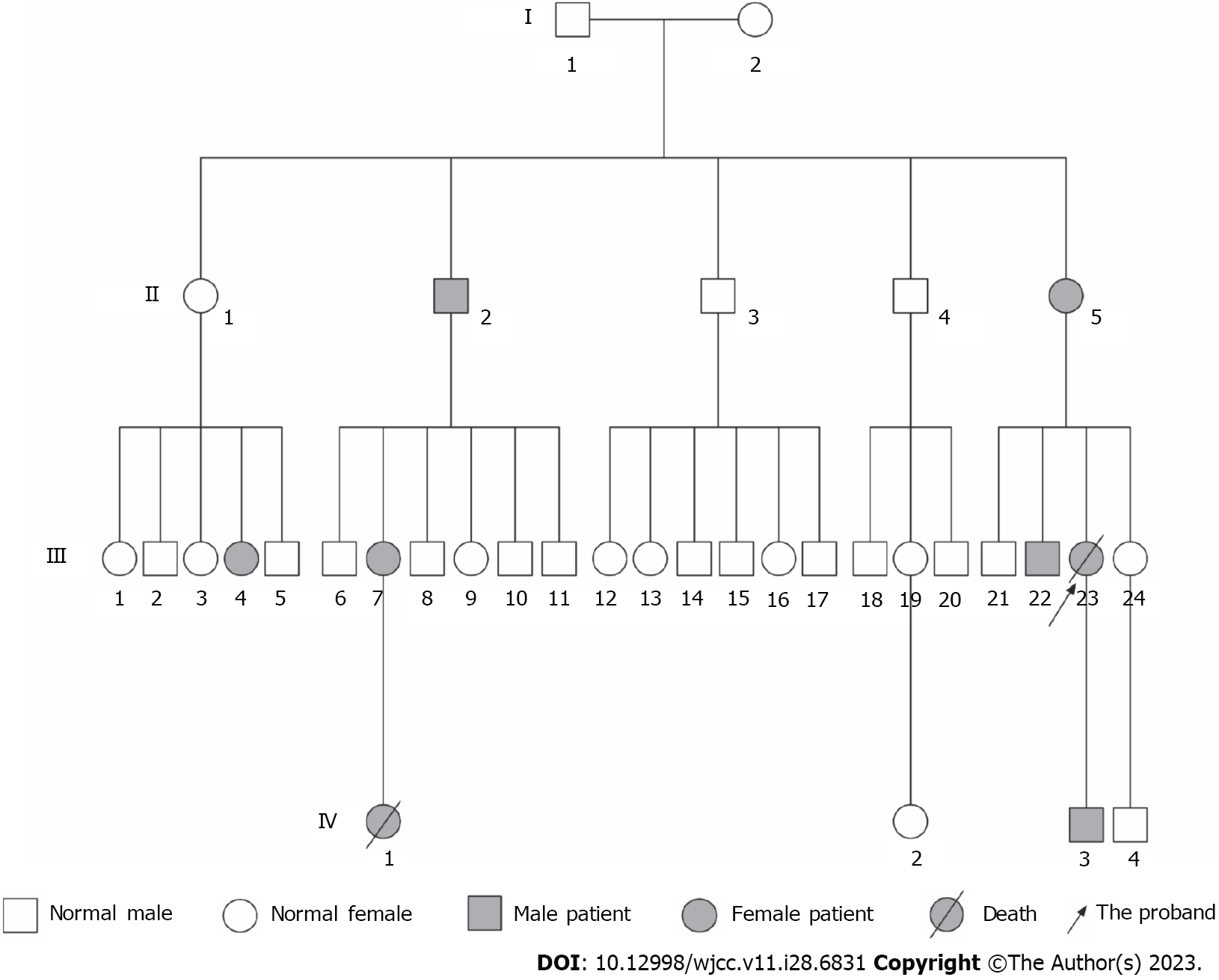
Several individuals in the patient’s family had symptoms of epistaxis and GI bleeding as shown in Figure 1. The patient’s elder brother (III22) experienced GI bleeding. Her son (IV3) experienced only epistaxis. Another 5 members in this family experienced symptoms of epistaxis (II2, II5, III4, III7, and IV1). During the follow-up, a niece (IV1) was found to experience pulmonary hypertension in addition to epistaxis and died 2 years after being diagnosed with pulmonary hypertension.
Physical examination upon admission revealed a body temperature of 37 °C, heart rate of 82 beats/min, respiratory rate of 20 breaths/min, and blood pressure of 100/70 mmHg. The skin examination showed a mild cyanosis. Cardiovascular examination revealed an expanded heart border to the right, and systolic murmur was detected at the tricuspid valve area. The abdomen was bilateral and soft. Percussion pain presented in the liver and spleen area.
A routine blood test identified hypochromic and microcytic erythrocytes with a low level of hemoglobin (58 g/L; normal range: 110-150 g/L). It also revealed abnormalities in total protein (53 g/L; normal range: 61-83 g/L), albumin (33 g/L; normal range: 35-50 g/L), and transglutaminase (63 U/L; normal range: < 47 U/L). Stool occult blood test was positive.
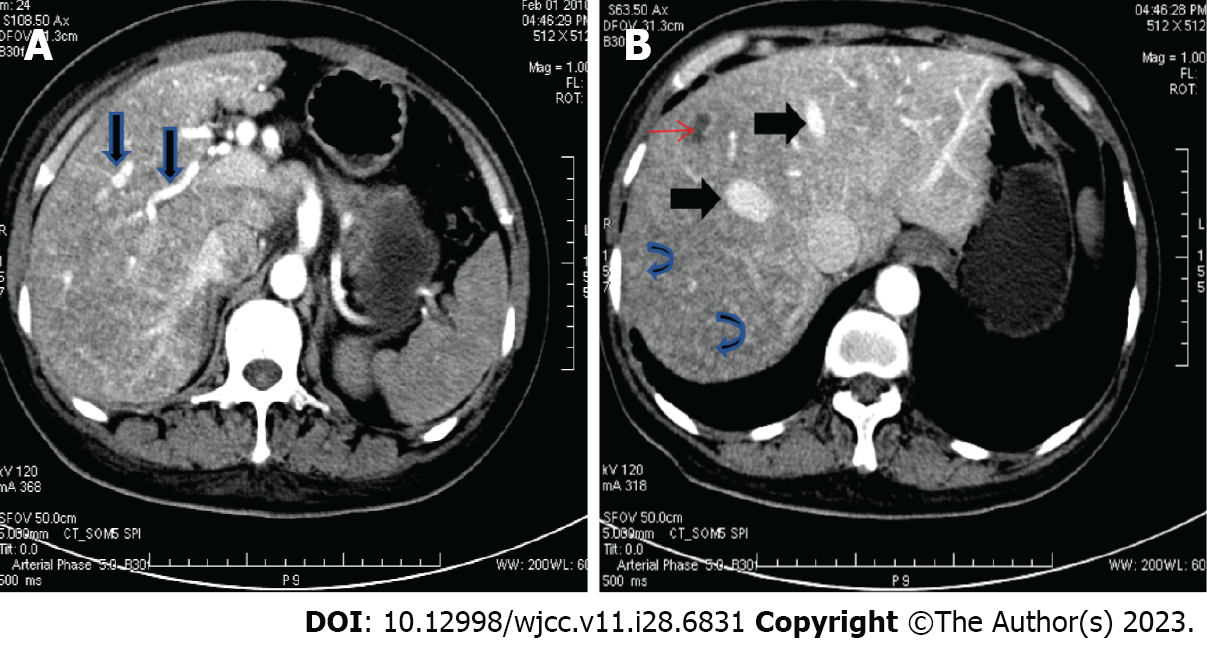
Abdominal contrast-enhanced computed tomography (CT) scan showed dilated hepatic arteries (Figure 2A), diffuse parenchymal telangiectasia, and early filling of hepatic veins on the arterial phase (Figure 2B), which suggested a diagnosis of HHT. Barium X-ray examination for the upper GI tract indicated antral gastritis and duodenitis.
Since the patient experienced epistaxis and hepatic arteriovenous malformations (AVMs) were revealed on the abdominal CT scan, HHT was highly suspected. HHT is a dominant hereditary disease and is primarily caused by mutations in ENG (type 1 HHT)[3], ALK1 (type 2 HHT)[4], and SMAD4[6]. Therefore, we subsequently performed gene testing in several individuals in this family, including the patient (III23), her elder brother (III22), a sister (III24), her son (IV3), a nephew (IV4), and a niece (IV2).
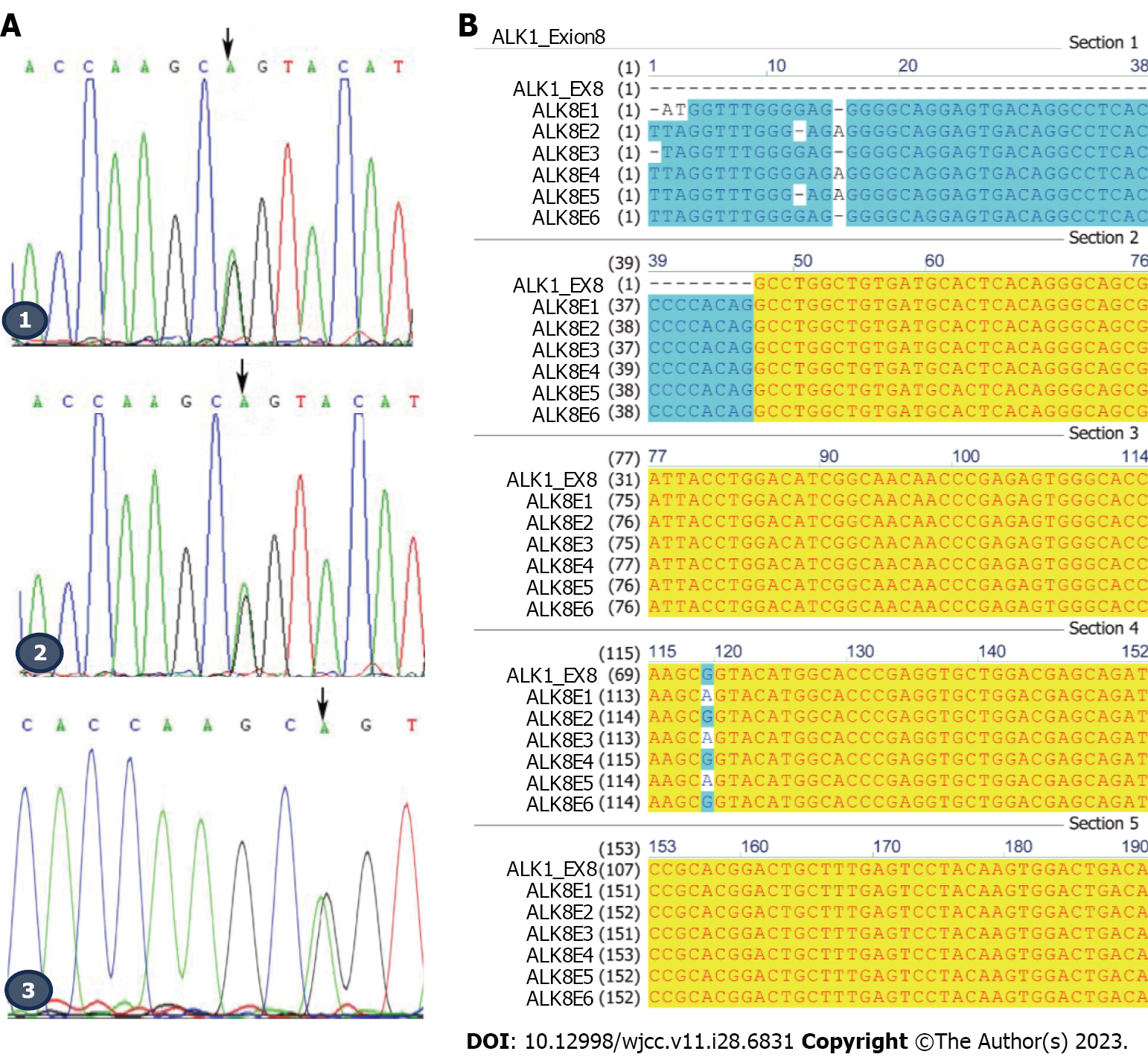
We amplified the ALK1 gene using the primers as shown in Table 1 and sent the PCR DNA products for Sanger sequencing. The DNA sequencing showed that a missense mutation, c.1121G>A, in exon 8 of ALK1 occurred in the patient (III23), her son (IV3), and elder brother (III22). They all experienced symptoms of HHT, indicating type 2 HHT in this family (Figure 3A and B). This mutation did not occur in the other 3 family members (III24, IV2, and IV4) who did not experience HHT symptoms (Figure 3B). The c.1121G>A mutation contributed to a translational change from arginine to glutamine at amino acid position 374 (R374Q) of the ALK1 protein. These results indicated that the c.1121G>A mutation might contribute to the pathogenesis of type 2 HHT in this family.
| Gene | Primer, 5’-3’ |
| ALK1-exon3-F | AGCTGGGACCACAGTGGCTGA |
| ALK1-exon3-R | GAGGGCAGGGGCCAAGAAGAT |
| ALK1-exon7-F | GACGACTCCAGCCTCCCTTAG |
| ALK1-exon7-R | CAAGCTCCGCCCACCTGTGAA |
| ALK1-exon8-F | AGGTTTGGGAGAGGGGCAGGA |
| ALK1-exon8-R | GGCTCCACAGGCTGATTCCCCTT |
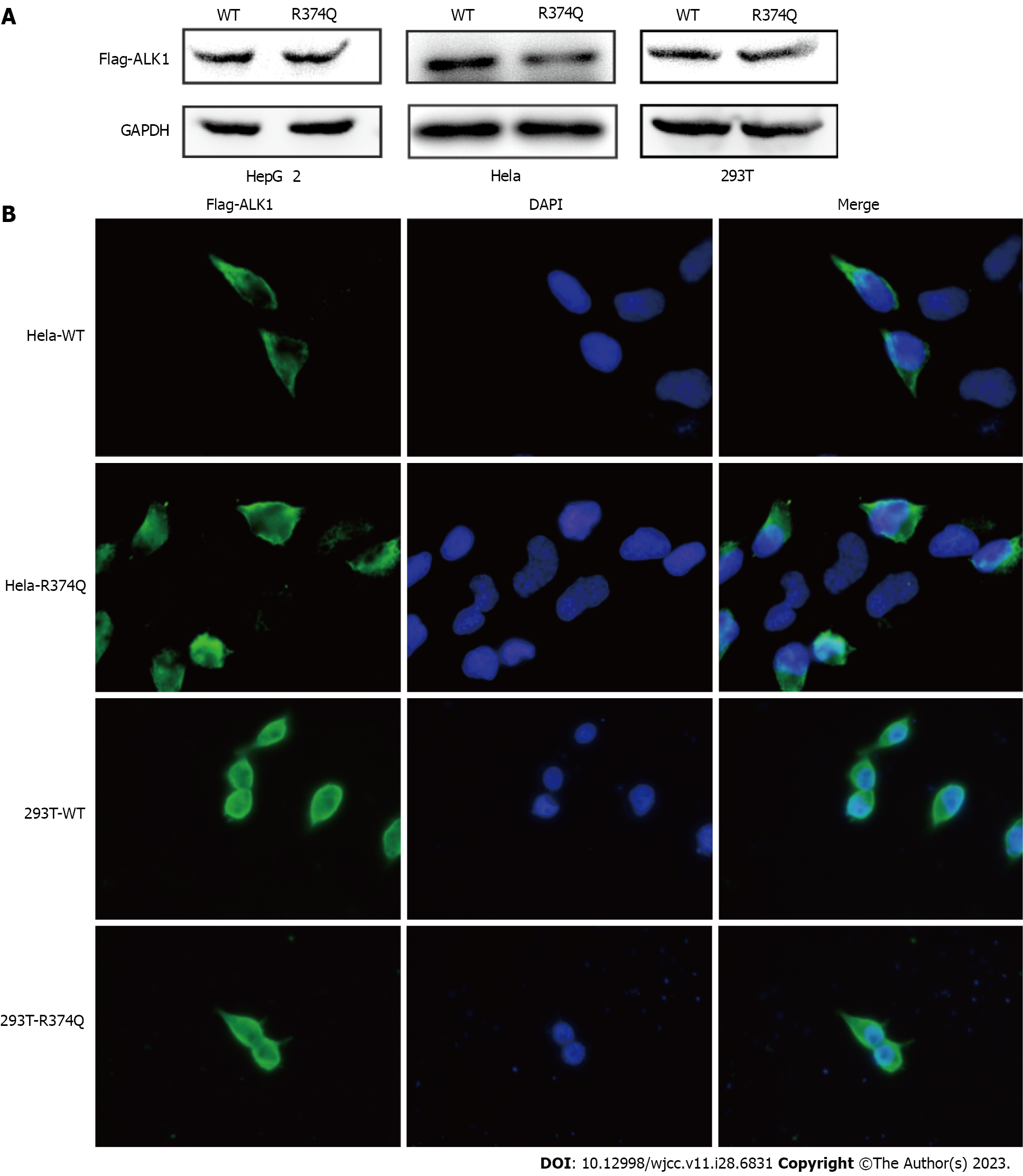
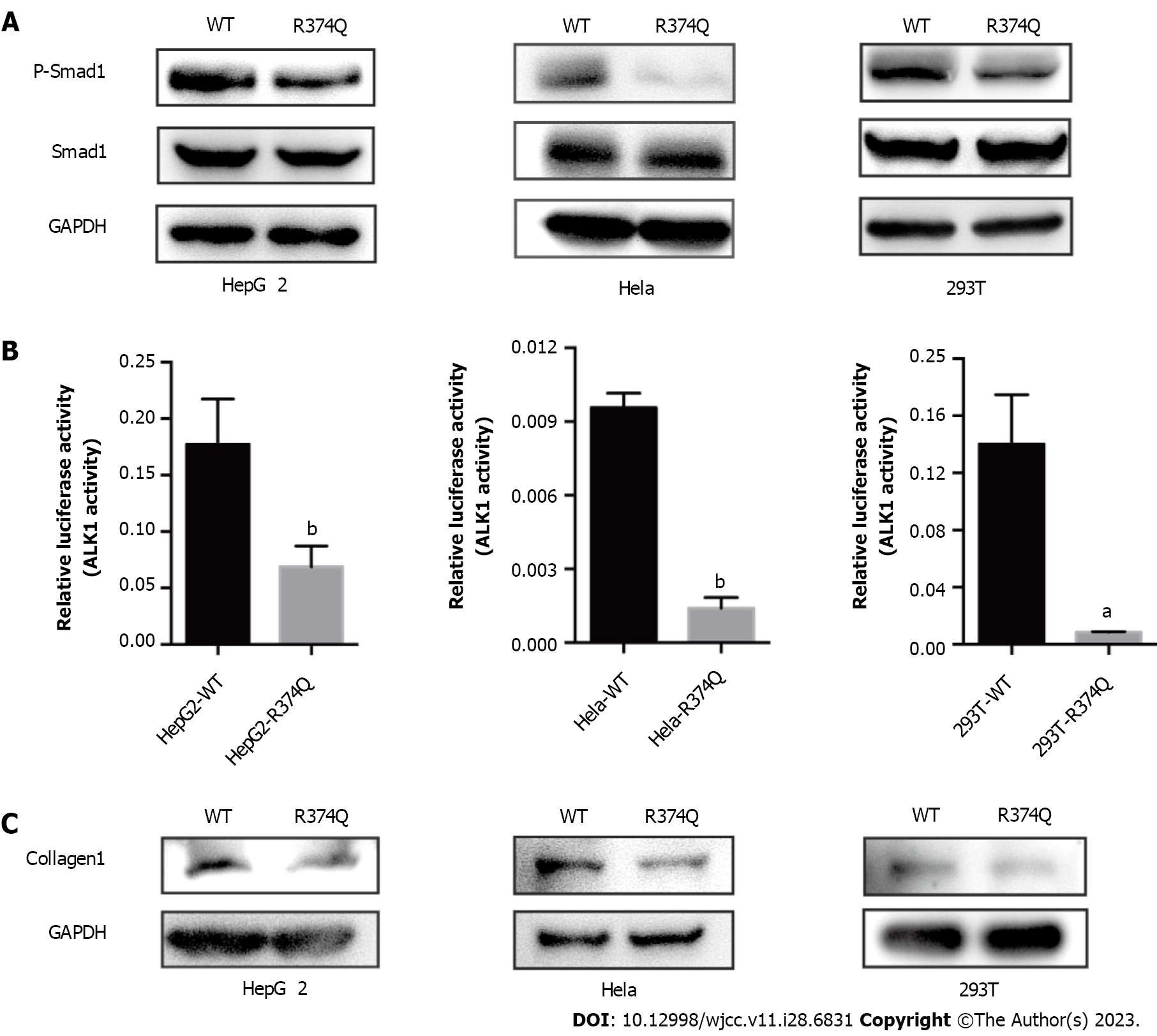
To address this question, we performed biochemical studies to investigate the effect of the c.1121G>A mutation on the biological function of ALK1. First, we detected the mutant protein expression in cultured cells. The western blot showed no significant difference in ALK1 protein levels between the wildtype (WT) and R374Q groups (Figure 4A). The immunofluorescence assay also showed that the mutant ALK1 protein could be detected in cells, and it was mainly located at cell membranes similar to the WT ALK1 protein (Figure 4B). These results indicated that the c.1121G>A mutation exerted no influence on protein expression or subcellular distribution.
Previous computer modeling studies demonstrated that the substitution of arginine to glutamine at amino acid position 374 (p.R374Q) could disturb H-bonding schemes of the intracellular kinase domain of ALK1[7]. Therefore, we assumed that the R374Q mutation could affect the kinase activity of ALK1. ALK1 is one of the components of TGF-β receptor that can phosphorylate Smad1 once activated and mediate signal reactions associated with vascular angiogenesis[8]. We detected the phosphorylation level of Smad1 in 293T, HepG2, and Hela cell lines. The results showed that the phosphorylation of Smad1 was significantly reduced in the mutant group compared to the WT group (Figure 5A). To further verify the inhibitory effect of the R374Q mutation on the kinase activity of ALK1, we introduced a Smad1-responsive transcriptional reporter, BRE4-luciferase reporter plasmid, to 293T, HepG2, and Hela cell lines. When transfecting WT ALK1 into those cells, we observed robust gene expression of the BRE4-luciferase. When the R374Q mutant was overexpressed, the expression of the BRE4-luciferase was significantly reduced compared to the WT group (Figure 5B). Hence, the R374Q mutation impeded the kinase activity of ALK1.
Telangiectasis is a pathologic change in HHT that is characterized by abnormal vascular dilation. It may occur when fiber generation in vessel walls is decreased. Previous studies suggested that ALK1/Smad1 played an important role in organ fibrosis such as in the liver and kidney[8]. Hence, we investigated whether the R374Q mutation affected profibrotic activity. The result showed that the R374Q mutant significantly abrogated the protein expression of a fibrogenic factor, collagen-1 (Figure 5C), indicating that the R374Q mutant could inhibit fiber generation during vascular formation.
The patient was diagnosed with type 2 HHT according to the Curaçao criteria[9]. In addition, the patient was also diagnosed with pulmonary arterial hypertension, right heart failure, and severe iron anemia.
Omeprazole and hydrotalcite were administrated to prevent upper GI bleeding. The patient did not experience new active bleeding and was discharged.
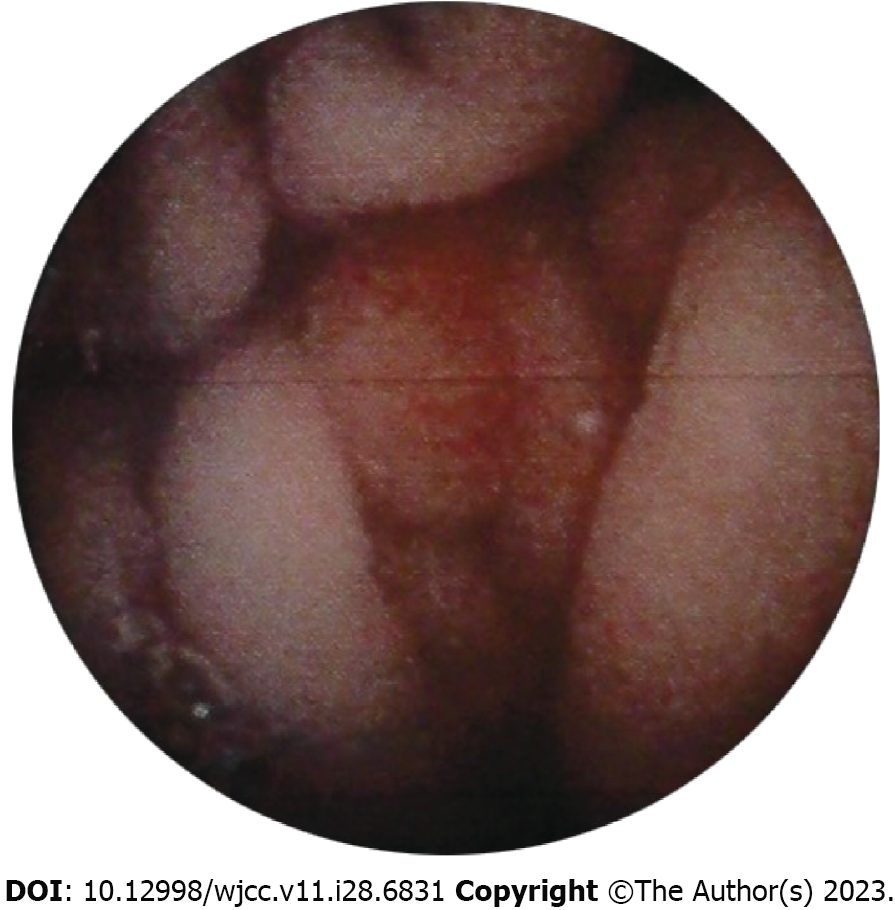
The patient constantly experienced melena after discharge. One month later, she went to a local pulmonary hospital because of pulmonary arterial hypertension-associated shortness of breath. She experienced acute vomiting of blood as well as melena while receiving treatment at the pulmonary hospital. Scattered erythematous mucosa was identified in the stomach without typical ulcers (Figure 6). After hemostatic therapy, she was discharged. However, the patient consistently had symptoms of GI bleeding and died of heart failure 1 year later.
HHT is an autosomal dominant hereditary disease that occurs in 1 out of 5000 people[10]. However, the real incidence might be higher because many potential HHT patients are not diagnosed due to their varied presentation of symptoms. The HHT-related vascular malformation can occur in any organ, thereby contributing to the diversity of symptoms that vary from superficial skin telangiectasia to epistaxis to deeper organ issues, like pulmonary hypertension, GI bleeding, cerebral bleeding, etc. In addition, patients can have symptoms involving one organ or multiple organs concurrently. Patients with common epistaxis and telangiectasia are easily recognized, while those with atypically silent lesions in the lung, liver, and stomach are often neglected. In this case, the patient who was diagnosed with pulmonary hypertension was not screened for HHT until she presented with more indicative symptoms like GI bleeding.
The delayed diagnosis can be avoided by utilizing gene testing. HHT is an autosomal dominant hereditary disease[1] that is caused by gene mutations in ENG (type 1 HHT)[3], ALK1 (type 2 HHT)[4], SMAD4 (juvenile polyposis JP-HHT)[11], and BMP9 (HHT5)[12]. Over a thousand mutations have been reported within the ENG and ALK1 genes (http://arup.utah.edu/database/HHT/index.php). However, whether all the reported mutations are involved in causing HHT symptoms is unknown. Therefore, it is crucial to determine the pathogenic mutations of HHT. Identifying the diagnostic HHT pathogenic gene pool is of great value to enable patients with limited symptoms to be diagnosed in a timely manner.
Herein, we identified the mutation c.1121G>A in exon 8 of ALK1 in 3 patients in this family, which supported a diagnosis of type 2 HHT. The mutation, c.1121G>A, contributes to a substitution at amino acid position 374 (R374Q) that is located in the conserved functional kinase domain of ALK1. Typically the active loop (between amino acids 371 and 374) has a conformational change when activated and then facilitates its binding with substrates or ATP[13]. We investigated the influence of the R374Q mutation on ALK1 activity. According to our studies, the R374Q mutation did not affect protein expression or cellular localization, but it decreased the kinase activity of ALK1. It induced a lower gene product in the BRE4 luciferase reporter assay and impeded the phosphorylation of Smad1. Since the Smad1/Smad5 pathway is involved in TGF-β-mediated angiogenesis[8], the decreased level of phosphorylated Smad1 indicates that the R374Q mutant inhibited the kinase activity of ALK1 as well as downstream reactions, leading to abnormal angiogenesis. Moreover, telangiectasia is a pathological manifestation in type 2 HHT. The lack of protective fibers in vessel walls causes the abnormal dilatation. Exploration of the effect of the R374Q mutant on fibrogenesis revealed that the R374Q mutant reduced the protein level of collagen-1, a fibrogenic factor that can enhance fiber generation[14]. Hence, the R374Q mutant played a role in downregulating fibrogenesis and eventually causing vascular malformation.
According to these biomolecular studies, the R374Q mutant of ALK1 played a pathological role in HHT development in this family. The R374Q mutation was also reported in other HHT families[2,7,15-18], further demonstrating its prevalent occurrence and pathological effect in HHT. Intriguingly, most of the HHT patients with the R374Q mutation were reported experiencing hepatic AVMs and GI bleeding, suggesting a possible correlation between genotype to phenotype[2,7,15]. Besides, independent studies in different populations found that hepatic AVMs and GI bleeding were more common in patients with ALK1 mutations[19,20]. In this family, the proband suffered from GI bleeding and hepatic AVMs. Her elder brother experienced GI bleeding, while her son only experienced epistaxis. The diversity of phenotype in the son might be attributed to incomplete penetrance. Given the poor prognosis of the HHT patients carrying the R374Q mutation, this son is most likely to develop hepatic AVMs and GI bleeding when he gets older.
Unfortunately, there is no effective treatment to stop the progression of HHT. The son of the proband can only be educated on precautions against aberrant bleeding and to stop bleeding quickly to prevent detrimental outcomes. To develop novel therapeutic strategies, a transgenic mouse model is recommended to introduce specific mutations to HHT-associated genes, like c.1121G>A on the ALK1 gene, and monitor the phenotype of the HHT-mimic mice. Effective drug treatments that impede HHT progression and improve survival could be developed in this type of animal model.
Overall, we discovered a gene mutation c.1121G>A (p.R374Q) in ALK1 in a Chinese family with type 2 HHT. Detection of this mutation may facilitate early diagnosis of HHT, which would enable medical care to be implemented in a timely manner to prevent fatal damage. In addition, the study of the pathogenic mechanism of the R374Q mutant might provide insights for impeding HHT pathogenic progress and developing treatments.
We thank Ronggui Hu, Xingxing Xu, and Yonghui Tao (Institute of Biochemistry and Cell Biology, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences) for providing technical support. We thank the Yueguang Chen laboratory (Department of Biological Sciences and Bio-technology, Tsinghua University, Beijing, China) for providing the BRE4 reporter plasmid as a gift.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Genetics and heredity
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Baysal M, Turkey; Kumar S, India S-Editor: Chen YL L-Editor: A P-Editor: Chen YL
| 1. | Ragsdale JA. Hereditary hemorrhagic telangiectasia: from epistaxis to life-threatening GI bleeding. Gastroenterol Nurs. 2007;30:293-9; quiz 300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 2. | Abdalla SA, Geisthoff UW, Bonneau D, Plauchu H, McDonald J, Kennedy S, Faughnan ME, Letarte M. Visceral manifestations in hereditary haemorrhagic telangiectasia type 2. J Med Genet. 2003;40:494-502. [PubMed] |
| 3. | McAllister KA, Grogg KM, Johnson DW, Gallione CJ, Baldwin MA, Jackson CE, Helmbold EA, Markel DS, McKinnon WC, Murrell J. Endoglin, a TGF-beta binding protein of endothelial cells, is the gene for hereditary haemorrhagic telangiectasia type 1. Nat Genet. 1994;8:345-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1084] [Cited by in RCA: 1057] [Article Influence: 34.1] [Reference Citation Analysis (1)] |
| 4. | Johnson DW, Berg JN, Baldwin MA, Gallione CJ, Marondel I, Yoon SJ, Stenzel TT, Speer M, Pericak-Vance MA, Diamond A, Guttmacher AE, Jackson CE, Attisano L, Kucherlapati R, Porteous ME, Marchuk DA. Mutations in the activin receptor-like kinase 1 gene in hereditary haemorrhagic telangiectasia type 2. Nat Genet. 1996;13:189-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 791] [Cited by in RCA: 793] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 5. | Albiñana V, Zafra MP, Colau J, Zarrabeitia R, Recio-Poveda L, Olavarrieta L, Pérez-Pérez J, Botella LM. Mutation affecting the proximal promoter of Endoglin as the origin of hereditary hemorrhagic telangiectasia type 1. BMC Med Genet. 2017;18:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 6. | Gallione CJ, Richards JA, Letteboer TG, Rushlow D, Prigoda NL, Leedom TP, Ganguly A, Castells A, Ploos van Amstel JK, Westermann CJ, Pyeritz RE, Marchuk DA. SMAD4 mutations found in unselected HHT patients. J Med Genet. 2006;43:793-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 179] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 7. | Abdalla SA, Cymerman U, Johnson RM, Deber CM, Letarte M. Disease-associated mutations in conserved residues of ALK-1 kinase domain. Eur J Hum Genet. 2003;11:279-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 50] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Lafyatis R. Transforming growth factor β--at the centre of systemic sclerosis. Nat Rev Rheumatol. 2014;10:706-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 259] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 9. | Shovlin CL, Guttmacher AE, Buscarini E, Faughnan ME, Hyland RH, Westermann CJ, Kjeldsen AD, Plauchu H. Diagnostic criteria for hereditary hemorrhagic telangiectasia (Rendu-Osler-Weber syndrome). Am J Med Genet. 2000;91:66-67. [PubMed] |
| 10. | Kjeldsen AD, Vase P, Green A. Hereditary haemorrhagic telangiectasia: a population-based study of prevalence and mortality in Danish patients. J Intern Med. 1999;245:31-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 284] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 11. | Gallione C, Aylsworth AS, Beis J, Berk T, Bernhardt B, Clark RD, Clericuzio C, Danesino C, Drautz J, Fahl J, Fan Z, Faughnan ME, Ganguly A, Garvie J, Henderson K, Kini U, Leedom T, Ludman M, Lux A, Maisenbacher M, Mazzucco S, Olivieri C, Ploos van Amstel JK, Prigoda-Lee N, Pyeritz RE, Reardon W, Vandezande K, Waldman JD, White RI Jr, Williams CA, Marchuk DA. Overlapping spectra of SMAD4 mutations in juvenile polyposis (JP) and JP-HHT syndrome. Am J Med Genet A. 2010;152A:333-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 114] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 12. | Wooderchak-Donahue WL, McDonald J, O'Fallon B, Upton PD, Li W, Roman BL, Young S, Plant P, Fülöp GT, Langa C, Morrell NW, Botella LM, Bernabeu C, Stevenson DA, Runo JR, Bayrak-Toydemir P. BMP9 mutations cause a vascular-anomaly syndrome with phenotypic overlap with hereditary hemorrhagic telangiectasia. Am J Hum Genet. 2013;93:530-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 228] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 13. | Gu Y, Jin P, Zhang L, Zhao X, Gao X, Ning Y, Meng A, Chen YG. Functional analysis of mutations in the kinase domain of the TGF-beta receptor ALK1 reveals different mechanisms for induction of hereditary hemorrhagic telangiectasia. Blood. 2006;107:1951-1954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Twardowski T, Fertala A, Orgel JP, San Antonio JD. Type I collagen and collagen mimetics as angiogenesis promoting superpolymers. Curr Pharm Des. 2007;13:3608-3621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 92] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 15. | Chen YJ, Yang QH, Liu D, Liu QQ, Eyries M, Wen L, Wu WH, Jiang X, Yuan P, Zhang R, Soubrier F, Jing ZC. Clinical and genetic characteristics of Chinese patients with hereditary haemorrhagic telangiectasia-associated pulmonary hypertension. Eur J Clin Invest. 2013;43:1016-1024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | Fontalba A, Fernandez-L A, García-Alegria E, Albiñana V, Garrido-Martin EM, Blanco FJ, Zarrabeitia R, Perez-Molino A, Bernabeu-Herrero ME, Ojeda ML, Fernandez-Luna JL, Bernabeu C, Botella LM. Mutation study of Spanish patients with hereditary hemorrhagic telangiectasia. BMC Med Genet. 2008;9:75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Heimdal K, Dalhus B, Rødningen OK, Kroken M, Eiklid K, Dheyauldeen S, Røysland T, Andersen R, Kulseth MA. Mutation analysis in Norwegian families with hereditary hemorrhagic telangiectasia: founder mutations in ACVRL1. Clin Genet. 2016;89:182-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Letteboer TG, Zewald RA, Kamping EJ, de Haas G, Mager JJ, Snijder RJ, Lindhout D, Hennekam FA, Westermann CJ, Ploos van Amstel JK. Hereditary hemorrhagic telangiectasia: ENG and ALK-1 mutations in Dutch patients. Hum Genet. 2005;116:8-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 75] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 19. | Karlsson T, Cherif H. Mutations in the ENG, ACVRL1, and SMAD4 genes and clinical manifestations of hereditary haemorrhagic telangiectasia: experience from the Center for Osler's Disease, Uppsala University Hospital. Ups J Med Sci. 2018;123:153-157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 20. | Lux A, Salway F, Dressman HK, Kröner-Lux G, Hafner M, Day PJ, Marchuk DA, Garland J. ALK1 signalling analysis identifies angiogenesis related genes and reveals disparity between TGF-beta and constitutively active receptor induced gene expression. BMC Cardiovasc Disord. 2006;6:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 1.8] [Reference Citation Analysis (0)] |