Published online Oct 6, 2023. doi: 10.12998/wjcc.v11.i28.6823
Peer-review started: June 28, 2023
First decision: August 10, 2023
Revised: August 22, 2023
Accepted: September 7, 2023
Article in press: September 7, 2023
Published online: October 6, 2023
Processing time: 88 Days and 19.9 Hours
Rhabdomyolysis is a life-threatening condition, often leading to progressive renal failure and death. It is caused by destruction of skeletal muscle and the release of myoglobin and other intracellular contents into the circulation. The most frequent cause of this condition is “crush syndrome”, although several others have been described and paraneoplastic inflammatory myopathies associated with various types of cancer are repeatedly reported.
We describe a rare case of a patient with pancreatic cancer who developed rhabdomyolysis early on, possibly due to paraneoplastic myositis leading to acute renal failure and eventually to rapid death. A 78-year-old Caucasian woman was referred to our hospital for obstructive jaundice and weight loss due to a lesion in the pancreatic head. She presented increasingly severe renal insufficiency with anuria, a dramatic increase in creatine phosphokinase (36000 U/L, n.v. 20-180 U/L) and myoglobin (> 120000 μg/L, n.v. 12-70 μg/L). On clinical examination, the patient showed increasing pain in the lower limbs associated with muscle weakness which was severe enough to immobilize her. Paraneoplastic myopathy linked to the malignant lesion of the pancreatic head was suspected. The patient was treated with hemodialysis and intravenous methylprednisolone. Despite all the efforts to prepare the patient for surgery, her general condition rapidly deteriorated and she eventually died 30 d after hospital admission.
The possible causes of rhabdomyolysis in this patient with pancreatic cancer are discussed, the development of paraneoplastic myopathy being the most likely. Clinicians should bear in mind that these syndromes may become clinically manifest at any stage of the cancer course and their early diagnosis and treatment could improve the patient’s prognosis.
Core Tip: Rhabdomyolysis is a life-threatening condition often leading to progressive renal failure and death. It is caused by destruction of skeletal muscle and the release of myoglobin and other intracellular contents into the circulation. There are several causes of this condition and paraneoplastic inflammatory myopathies associated with various types of cancer are reported. We describe the case of a patient with pancreatic cancer who developed rhabdomyolysis early on, possibly due to paraneoplastic myositis. The possible causes of rhabdomyolysis in patients with pancreatic cancer are discussed, paraneoplastic myopathy being the most likely.
- Citation: Costantini A, Moletta L, Pierobon ES, Serafini S, Valmasoni M, Sperti C. Paraneoplastic myopathy-related rhabdomyolysis and pancreatic cancer: A case report and review of the literature. World J Clin Cases 2023; 11(28): 6823-6830
- URL: https://www.wjgnet.com/2307-8960/full/v11/i28/6823.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i28.6823
Rhabdomyolysis is a potentially life-threatening syndrome characterized by the breakdown of skeletal muscle fibers with the release of intracellular products into the extracellular fluid and systemic circulation[1]. Rhabdomyolysis is usually caused by crush injury, alcohol abuse, use of certain medications (statins) or illicit drugs, infections (recently some cases of coronavirus disease 2019 were described)[2], electrolyte abnormalities such as hyponatremia or hypokalemia among others[1]. Several other causes are described, however, and paraneoplastic inflammatory myopathies (IIMs) associated with various types of cancer are repeatedly reported[3]. Paraneoplastic IIMs leading to rhabdomyolysis, namely dermatomyositis (DM) and polymyositis (PM), are rare conditions[4]; they typically feature symmetrical proximal weakness, but they can also involve the respiratory and esophageal muscles, causing dyspnea and dysphagia. Rhabdomyolysis is a rare but possible complication of these uncommon diseases: Its prevalence in cancer-related myopathies in not known, nor are the possible risk factors. Rhabdomyolysis must always be feared in these patients and aggressively treated. At times, it may represent the first manifestation of the disease. On the other hand, the association between these myopathies and cancer is well established, with malignancies occurring in up to 60% of patients with these diseases (higher rates in DM than in PM). The types of cancer most often associated with inflammatory myositis include non-Hodgkin lymphoma, and breast, lung, bladder, colorectal and pancreatic cancer; adenocarcinoma is the most frequent cancer type, accounting for 70% of all associated tumors in both DM and PM[3]. The prognosis of myositis largely depends on the prognosis of the underlying malignancy and how it is treated, but it is known to be poor when significant muscle weakness is present at diagnosis[4]. We report here the case of a patient with pancreatic cancer who developed rhabdomyolysis with related acute kidney disease, possibly caused by paraneoplastic myositis.
A 78-year-old Caucasian woman developed obstructive jaundice and recent significant weight loss (12 kg in 6 mo).
Jaundice started 2 wk before presentation.
The patient’s medical history included a metabolic disease with hypertension, type 2 diabetes mellitus and hypercholesterolemia. She suffered a transient ischemic attack in 2016. She was a non-smoker, and her alcohol intake was estimated to be 8 g per day (one 100 mL glass). Her body mass index was 21.9 kg/m2. Her ongoing therapies included: Anti-platelets (clopidogrel), short- and long-acting insulin, angiotensin converting enzyme-inhibitors and statins (atorvastatin 40 mg/die). She was admitted to our surgical ward to palliate the jaundice, complete the diagnostic work-up and consider a possible surgical or oncological treatment.
Not significant.
On clinical examination, the patient showed diffuse jaundice and increasing pain in the lower limbs associated with muscle weakness which was severe enough to immobilize her. She also rapidly developed anuria with acute kidney disease caused by severe rhabdomyolysis.
On admission, the blood tests revealed: High levels of hepatic enzymes [i.e. total bilirubin 301.9 μmol/L (n.v. 1.7-17.0 μmol/L), alanine transaminase (ALT) 529 U/L (n.v. 7-35 U/L), aspartate aminotransferase (AST) 1177 U/L (n.v. 10-35 U/L), alkaline phophatase 1677 U/L (n.v. 33-98 U/L) and γ-glutamiltransferase 1930 U/L (n.v. 3- 45 U/L)]; a dramatic increase in [creatine phosphokinase (CK), 36000 U/L, n.v. 20-180 U/L) and myoglobin (> 120000 μg/L, n.v. 12-70 μg/L)]; and well above normal levels of creatinine (181 μmol/L, n.v. 45-84 μmol/L) and urea (11.4 mmol/L, n.v. 2.5-7.5 mmol/L). Cancer markers were also elevated: Carbohydrate antigen 19-9 1664 kU/L (n.v. 0-30 kU/L), carcinoembryonic antigen 8.5 μg/L (n.v. 0-4 μg/L) and alpha-fetoprotein 2.2 μg/L (n.v. 0.0-7.4 μg/L).
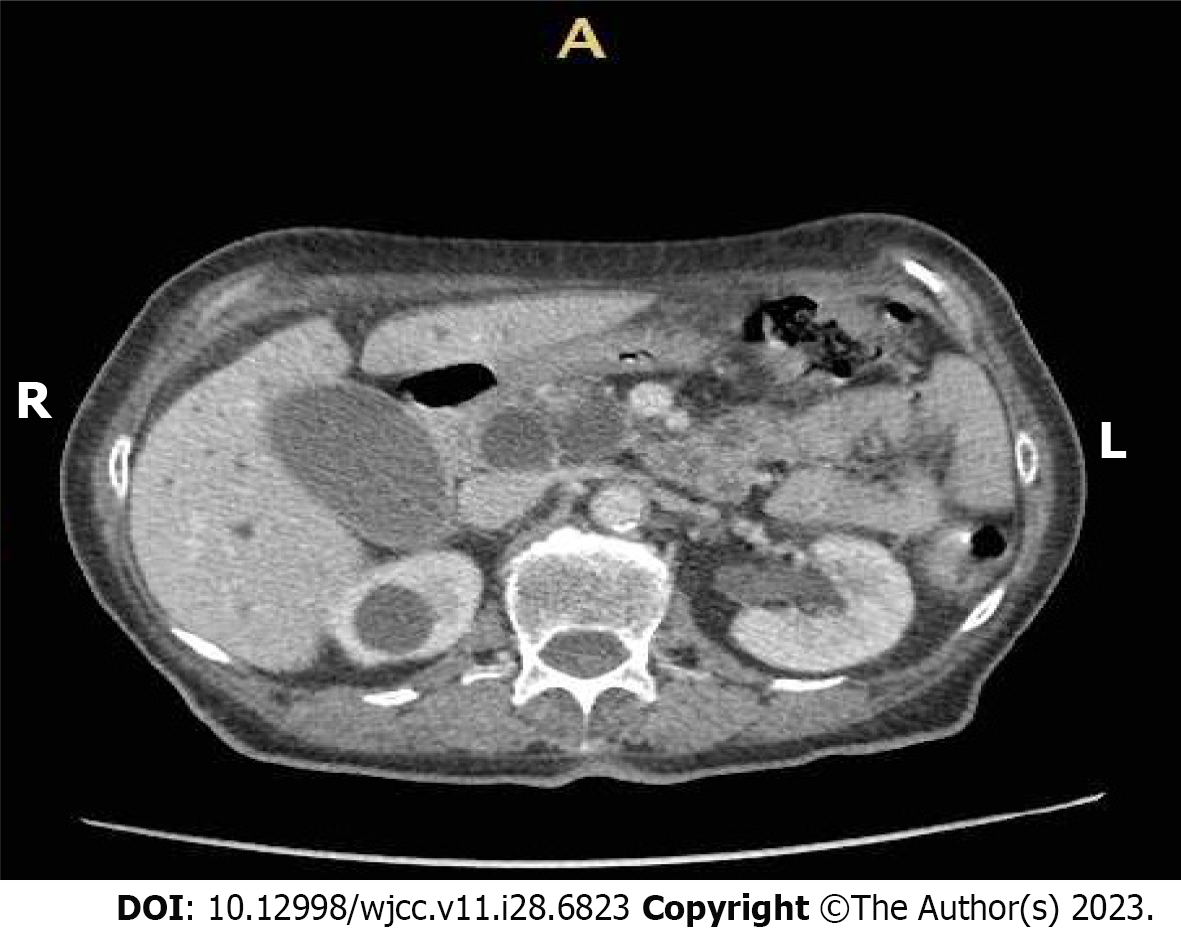
Before admission, the patient had undergone radiological imaging, ultrasound and contrast-enhanced computed tomography (CT), which showed a marked dilation of the biliary and pancreatic ducts caused by a tumor of the pancreatic head (Figure 1).
The patient underwent a complete autoimmune investigation [i.e. anti-liver-kidney-microsomal (LKM) antibodies, anti-mitochondrial antibodies, anti-neutrophil cytoplasmic antibodies, anti-cDNA, anti-cardiolipin antibodies, anti-endomysial antibodies, anti-ribosome antibodies, anti-Jo-1 antibodies, anti-smooth muscle antibody, human epithelial-2 antibodies, anti-Scl70 antibodies, anti-HMGCoA-R, anti-signal recognition particle, anti-LKM1, anti-liver cytosolic antigen], the outcome of which was negative. Electromyography (EMG) revealed signs of a myogenic impairment in both the lower and upper limbs, with reduced motor conduction speed along the right common peroneal nerve.
The diagnosis of paraneoplastic myopathy with rhabdomyolysis caused by pancreatic cancer was hypothesized.
Given the increasingly severe renal insufficiency with anuria, the patient underwent urgent central venous catheter placement and started replacement therapy (hemodialysis) with specific filters to remove myoglobin; this treatment was required throughout her hospital stay. A percutaneous external biliary drainage was then positioned to palliate the jaundice, achieving a gradual improvement in the patient’s liver and blood test findings. Intravenous methylprednisolone was also started at a dose of 500 mg a day for 5 d, then reduced to 80 mg per day and gradually tapered down to 20 mg per day, resulting in a slow improvement in CK and myoglobin levels. Statin therapy (a well-known causal factor for rhabdomyolysis) was suspended immediately. Based on recent literature, the only way to treat the patient’s paraneoplastic myopathy was to remove the cancer. We made every effort to prepare her for surgery, but her critical conditions, continuous need for replacement therapy (hemodialysis), hemodynamic instability and declining platelet count made the procedure impossible. No chemo- or radiotherapies could be attempted for the same reasons.
The patient died 30 d after the initial hospital admission.
The rhabdomyolysis in the described patient with pancreatic cancer may have arisen from different causes. Based on the patient’s clinical and EMG data, paraneoplastic inflammatory myopathy was suspected. Idiopathic IIMs form a heterogeneous group of acquired connective tissue diseases that mainly affect the skeletal muscles[5]. The old criteria summarized by Bohan and Peter[6] nearly 50 years ago and still widely used have undergone subsequent modifications. Although universally accepted classification criteria for IIMs have not yet been established, the five most recognized types are DM, immune-mediated necrotizing myopathy, overlap myositis (including antisynthetase syndrome), sporadic inclusion-body myositis and PM[7]. The European League Against Rheumatism and American College of Rheumatology IIM classification criteria were recently published[8], showing better sensitivity, specificity and classification accuracy when compared to previous criteria. They are still based essentially on clinical data, although some histological data obtained with muscle biopsy have increased the probability of a correct classification. This classification, which is to be considered provisional, has raised some criticism[9] especially because results on myositis-specific antibodies (MSAs) were not considered (except for anti-Jo1 antibody), apparently for the lack of available data. Moreover, some caveats concerning MSA results were recently raised[10], and individual assessment of MSAs have low sensitivity for IIM and thus their absence does not exclude an IIM diagnosis[11]. This may apply to our patient in whom the assessment of MSAs was negative.
We believe that our patient developed rhabdomyolysis caused by paraneoplastic myositis due to a tumor of the pancreatic head. There are several reports of an association between cancer and IIMs in adults[3] although the pathogenesis of the latter remains unclear. In an attempt to explain the development of myositis in cancer, some studies have focused on the common autoantigen expression and immune targeting between cancer tissue and muscle tissue in myositis. For example, Casciola-Rosen et al[12] showed that Mi-2 and histidyl transfer-RNA synthetase (Jo-1), ubiquitously expressed in normal tissue, were increased many times over in myositis muscle tissue, with the greatest antigen expression in the most damaged and regenerating muscle fibers. They further showed that myositis-specific antigen expression was increased in random muscle biopsies of several cancer types (breast, lung) known to be associated with the development of myositis, but not in biopsies of the corresponding non-muscle tissues. By extension, these findings suggest that certain tumors and diseased/regenerating muscle are antigenic mimickers. A potential mechanism for the development of cancer-associated myositis (CAM) can thus be hypothesized: The increased myositis autoantigen expression in a nascent tumor leads to the generation of T and B cells directed against those antigens with subsequent successful tumor immunity. This hypothesis, though plausible, raises additional questions as to why crossover immunity only occurs in the minority of patients developing CAM, whereas most patients feature either a malignant or autoimmune phenotype.
The pathophysiology of rhabdomyolysis and the exact pathways by which the various insults can lead to muscle injury are also poorly understood. Several mechanisms have been proposed to explain the association between myositis-related rhabdomyolysis and cancer[1]: The presence of tumor-produced mediators, which may induce immunoreaction against muscle fibers and skin; a common carcinogenic factor, which may trigger concomitant immunoreactions; or a cross-reactivity between tumor and skin/muscle antigens. All these mechanisms occur with a compromised immune system. The final steps of the process leading to rhabdomyolysis are direct muscle cell injury or failure of their energy supply[1].
From the clinical point of view, the concomitant presence of paraneoplastic myopathy may be a negative prognostic factor. The prognosis is also poor when patients report significant weakness at presentation[5]. On admission to our surgical ward, the patient had jaundice, severe weakness that rapidly immobilized her, lower limb myalgias, oliguria (with dark urine) and anuria caused by severe rhabdomyolysis. This triad of myalgia, weakness and tea-colored urine has already been described in such patients, but only in less than 10% of cases[1]. Hill et al[13] found that 137 of 914 cases of PM were associated with cancer and reported a slightly higher standardized incidence ratio in men than in women (1.4 vs 1.2). The mean age at diagnosis is 50-60 years[5]. Muscle weakness is the most common clinical feature of IIMs and CAM: It is commonly localized proximally and symmetrically, is rapidly progressive and can be severe enough to lead to immobility[5,14]. Myalgias are reported in less than 30% of patients with IIMs and CAM. Respiratory muscles may sometimes be affected, with fatal consequences[5,14]. Other common features of these disorders are elevated serum muscle enzymes, myopathic changes in EMG, characteristic muscle biopsy abnormalities, and no histopathological signs of other types of myopathies. Laboratory investigations usually reveal high serum muscle enzyme levels (such as CK, myoglobin, lactate dehydrogenase, aldolase, AST and ALT) in patients suffering from these autoimmune diseases, though patients in some reported series had normal CK levels at presentation[15]. Our patient’s muscle enzyme levels (e.g. CK and myoglobin) were elevated on admission. They were caused by rhabdomyolysis, which led to acute kidney failure and the need for replacement therapy.
Muscle biopsy is essential to establish a diagnosis, improving the accuracy of the clinical data[8]. The cellular infiltrate is typically seen mainly within the fascicle with inflammatory cells invading individual muscle fibers. Abnormal muscle fibers are scattered throughout the fascicle. In addition, there is evidence of cell-mediated immune mechanisms, with cytotoxic CD8+ T cells (which recognize antigens on the muscle fiber surface), and enhanced expression of major histocompatibility complex antigens by the muscle fibers[16]. Unfortunately, in our case a muscle biopsy was not possible due to the patient’s rapidly deteriorating condition. At the moment, there are no diagnostic markers and methods to identify paraneoplastic myopathies in cancer patients, in particular in those with pancreatic cancer. In the latter, early detection of paraneoplastic myopathy does not generally change the treatment and course of the disease, due to the poor prognosis of this type of cancer. However, the detection of some myositis-specific autoantibodies [i.e. anti-transcriptional intermediary factor-1 γ (TIF1-γ), anti-nuclear matrix protein (NXP) and others] in patients with pancreatic cancer[17] may suggest the presence of cancer-associated myositis, thus warning against the use of some agents (especially gemcitabine) and limiting iodine-contrast studies in the management of these patients. In fact, both can cause or worsen the CAM and the overall course of the disease.
Myopathy associated with pancreatic cancer remains a rare syndrome. When Yang et al[18] conducted a meta-analysis on 20 studies of cancer-associated myopathies, only 3 involved a patient with pancreatic cancer. Hill et al[13] reviewed 95 PM patients who developed cancer and found only one case of pancreatic cancer. Another two studies, on 33 and 43 patients respectively, reported one case of pancreatic cancer each[19,20]. As a result, only a handful of isolated cases of pancreatic adenocarcinoma associated with PM are reported in the literature[15,19-24]. Table 1 summarizes these reports. Three of the six patients reported had been treated with gemcitabine, a known cause of drug-induced myopathy[25], which could have played a role. Chemotherapeutic agents typically cause myopathies at sites previously exposed to radiation, but gemcitabine is unique in that it can also cause myopathies in radiation-naïve patients. In most cases, gemcitabine induces “radiation recall”[25], consisting in inflammatory reactions triggered by cytotoxic drugs at previously irradiated sites. No chemo- or radiotherapies were initiated in our patient, so this mechanism can be ruled out.
| Ref. | Sex/age | Statin use | Clinical presentation | Cancer type | Diagnosis | Treatment | Gemcitabine use | Additional findings | Follow-up and outcome |
| Kida et al[19], 2007 | M, 53 | NR | Arthralgia | AK | CT | Steroids | No | Late-stage | Died 4 yr |
| Myalgias muscolar weakness | Biopsy | Fluorouracil radiotherapy | Carcinoma | After diagnosis | |||||
| Syrios et al[15], 2011 | M, 52 | NR | Weight loss | AK | CT | Steroids | Yes | - | Remission |
| Recurrent pancreatitis | EMG biopsy | Gemcitabine erlotinib | Six months (then relapse) | ||||||
| Siddiqui et al[20], 2011 | F, 86 | NR | Muscolar weakness | AK | CT | Steroids | No | - | Remission |
| Biopsy | Surgery | Six months | |||||||
| Amroun et al[21], 2012 | M, 47 | NR | Abdominal pain | AK | CT | Surgery | Yes | Myopathy | Remission |
| Jaundice weight loss asthenia | MRI Biopsy | Gemcitabine | Developed during 4th cycle of chemotherapy | Nine months, then death due to pneumonia | |||||
| Padniewski et al[22], 2020 | M, 66 | Yes | Generalized fatigue | AK | CT | Steroids | Yes, later in | Myopathy | Gemcitabine |
| Muscolar weakness loose stools weight loss | MRI | FOLFOX followed by gemcitabine | Course | Developed prior to gemcitabine | Trial died 1 mo later | ||||
| Decraene et al[23], 2021 | M, 69 | NR | Jaundice | NET | MRI | Steroids | No | - | Remission |
| Diarrhea weight loss | EMG biopsy | Surgery carboplatinum-etoposide | Six months (no recurrence) | ||||||
| Ozcan et al[24], 2022 | M, 86 | Yes | Myalgias | AK | MRI | Steroids | Yes, later in | Metastatic | Third line |
| Muscolar weakness | MRCP ERCP biopsy | Surgery FOLFOX followed by FOLFIRI | Course | Carcinoma | Palliative treatment (gemcitabine and abraxane) | ||||
| Present case, 2022 | F, 78 | Yes | Jaundice | Unknown | CT | Steroids | No | AKI treated with | Died during |
| Weight loss myalgias muscolar weakness | EMG | Replacement therapy | Hospitalization |
A significant increase in plasma concentrations of cell-free DNA and evidence of Kirsten rat sarcoma mutations on EUS-FNAC have been reported as a marker of tumoremia[26]. It would be interesting to see if these markers are associated with an increase in CK levels or specific anti-tumor autoantibodies in patients with paraneoplastic syndromes. As mentioned earlier, our patient’s condition prevented any invasive procedures such as EUS-guided FNAC or biopsy. A cohort study on patients with IIMs found some MSAs (i.e. anti-TIF1-γ), anti-NXP 2 and anti-SUMO activating enzyme 1) associated with an increased risk of cancer[18]. The pathophysiological link between these specific autoantibodies and tumors still needs to be clarified.
Paraneoplastic myopathies are typically treated in much the same way as idiopathic myopathies with high-dose corticosteroids followed by a transition to a steroid-sparing agent such as methotrexate or azathioprine[27]. The response to this type of therapy usually persists from weeks to months. It has been reported that paraneoplastic myopathy is less likely to respond to steroid therapy, so underlying malignancies should be investigated in patients who fail to respond to steroids. As for the other causes of rhabdomyolysis, the removal of causal factors (drugs-statins, alcohol abuse, trauma, metabolic disorders, intense muscular exercise, infections, etc.) can improve or even resolve the condition. Therefore, treatment of the associated cancer usually results in improvement in a patient’s myositis[3,21]. Unfortunately, this was impossible in our patient because her condition rapidly deteriorated and was soon fatal. As far as the treatment of rhabdomyolysis is concerned, its most important therapeutic goal is to avoid acute kidney injury caused by hypovolemia. The correction of hyperkalemia and acidosis are also mandatory. Acute kidney injury develops in one-third of patients[28] with rhabdomyolysis and represents its most serious complication as the result of accumulation of myoglobin. Hemodialysis clears myoglobin from the bloodstream, thereby potentially decreasing the amount of renal damage. However, a Cochrane review on the potential benefit of this approach showed that the mortality rate remains unchanged despite the improvement in myoglobin, creatinine and electrolyte levels[29]. Therefore, hemodialysis should be considered only when life-threatening electrolyte abnormalities emerge. This applied to our patient, although she did not benefit from this treatment.
Other causes of rhabdomyolysis could have been considered in our patient. The presence of “crush” syndrome, one of the most frequent causes of rhabdomyolysis[28], was easily excluded, as trauma, burns or high-voltage current injury were absent in the patient’s history. A more plausible causal factor was the use of statins[1] and thus statin was immediately discontinued in our patient. Statins are safe, well tolerated and the most efficient drugs in the treatment of hypercholesterolemia. The most severe adverse effect of statins is myotoxicity, rhabdomyolysis being the worst, possibly resulting in acute renal failure, disseminated intravascular coagulation and death[30]. This caused the withdrawal in 2001 of one of the first drugs of this class (cerivastatin) after more than 100 rhabdomyolysis-related deaths had been directly linked to its use[30]. Statins induce necrosis of skeletal muscle, and the muscle contents (myoglobin and others) are released into the blood flow causing acute renal failure. The exact pathophysiology ofstatin-induced myopathyis not fully known yet, and multiple pathophysiological mechanisms may contribute to statin myotoxicity. It seems to be dose-related and is increased when statins are used in combination with agents sharing common metabolic pathways[1]. Rhabdomyolysis secondary to statin use has proved to be extremely rare. An analysis of 30 randomized controlled trials (n = 83,858) identified 7 cases of rhabdomyolysis in patients who received statin therapy vs 5 cases in placebo-treated patients[1]. In an Food and Drug Administration study over a period of 29 mo the occurrence of rhabdomyolysis was most common with simvastatin (36%) and cerivastatin (32%); fewer occurrences were seen with atorvastatin (12%), pravastatin (12%), lovastatin (6%) and fluvastatin (2%)[31]. Although our patient was on a statin less frequently associated with rhabdomyolysis (atorvastatin), the presence of a biliary tract obstruction may have led to increased concentrations of the drug leading to increased myotoxicity. However, withdrawal of the statin therapy and the resolution of biliary obstruction with an external drainage did not improve her clinical conditions, as instead reported in other cases[32,33]. Therefore, even if statin assumption cannot be ruled out with certainty as a cause of the clinical picture, it seems unlikely in our case.
Another possible cause of rhabdomyolysis in our patient was the recent infusion of iodine contrast during a diagnostic CT performed a week before hospital admission. Only two case reports are available in the literature of rhabdomyolysis after iodine contrast administration, one after CT scan[34] and the other after endoscopic retrograde cholangiopancreatography[35]. However, severe symptoms arose in those cases within hours from the procedure, and thus this etiology is also unlikely in our patient. Hence, paraneoplastic IIM remains the most probable cause of the rhabdomyolysis in her case, even though a definitive diagnosis could not be made.
Paraneoplastic myopathies may occur, albeit very rarely, in patients with pancreatic cancer. They can eventually progress to muscular necrosis and rhabdomyolysis. Even if other causes of rhabdomyolysis may be more frequent and must be ruled out, clinicians should bear in mind that these syndromes may become clinically manifest at any stage of the cancer course and their early diagnosis and treatment could improve the patient’s prognosis. In the case of a recent onset of myopathy (and/or renal insufficiency due to rhabdomyolysis), an underlying malignancy must be sought. Increased awareness among clinicians (general practitioners or specialists) may allow an early diagnosis and treatment. Surgical removal of the underlying tumor, if possible, remains the treatment of choice and seems to lead to a better outcome, although as yet there are not enough data in the literature to confirm this hypothesis.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Jeong KY, South Korea; Zhao CF, China S-Editor: Qu XL L-Editor: A P-Editor: Zhao S
| 1. | Torres PA, Helmstetter JA, Kaye AM, Kaye AD. Rhabdomyolysis: pathogenesis, diagnosis, and treatment. Ochsner J. 2015;15:58-69. [PubMed] |
| 2. | Chedid NR, Udit S, Solhjou Z, Patanwala MY, Sheridan AM, Barkoudah E. COVID-19 and Rhabdomyolysis. J Gen Intern Med. 2020;35:3087-3090. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 3. | Zahr ZA, Baer AN. Malignancy in myositis. Curr Rheumatol Rep. 2011;13:208-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 112] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 4. | Maugars YM, Berthelot JM, Abbas AA, Mussini JM, Nguyen JM, Prost AM. Long-term prognosis of 69 patients with dermatomyositis or polymyositis. Clin Exp Rheumatol. 1996;14:263-274. [PubMed] |
| 5. | Aggarwal R, Oddis CV. Paraneoplastic myalgias and myositis. Rheum Dis Clin North Am. 2011;37:607-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 6. | Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts). N Engl J Med. 1975;292:344-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3355] [Cited by in RCA: 3478] [Article Influence: 69.6] [Reference Citation Analysis (0)] |
| 7. | Selva-O'Callaghan A, Pinal-Fernandez I, Trallero-Araguás E, Milisenda JC, Grau-Junyent JM, Mammen AL. Classification and management of adult inflammatory myopathies. Lancet Neurol. 2018;17:816-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 257] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 8. | Lundberg IE, Tjärnlund A, Bottai M, Werth VP, Pilkington C, Visser M, Alfredsson L, Amato AA, Barohn RJ, Liang MH, Singh JA, Aggarwal R, Arnardottir S, Chinoy H, Cooper RG, Dankó K, Dimachkie MM, Feldman BM, Torre IG, Gordon P, Hayashi T, Katz JD, Kohsaka H, Lachenbruch PA, Lang BA, Li Y, Oddis CV, Olesinska M, Reed AM, Rutkowska-Sak L, Sanner H, Selva-O'Callaghan A, Song YW, Vencovsky J, Ytterberg SR, Miller FW, Rider LG; International Myositis Classification Criteria Project consortium, The Euromyositis register and The Juvenile Dermatomyositis Cohort Biomarker Study and Repository (JDRG) (UK and Ireland). 2017 European League Against Rheumatism/American College of Rheumatology classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups. Ann Rheum Dis. 2017;76:1955-1964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 824] [Cited by in RCA: 838] [Article Influence: 104.8] [Reference Citation Analysis (0)] |
| 9. | Malaviya AN. 2017 EULAR/ACR classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups: little emphasis on autoantibodies, why? Ann Rheum Dis. 2018;77:e77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 10. | Schepens N, Herroelen PH, Decavele AS, Vanacker A. Severe rhabdomyolysis due to idiopathic inflammatory myopathies, a wary manifestation of a heterogenous pathology. Acta Clin Belg. 2023;78:160-164. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 11. | Cavazzana I, Fredi M, Ceribelli A, Mordenti C, Ferrari F, Carabellese N, Tincani A, Satoh M, Franceschini F. Testing for myositis specific autoantibodies: Comparison between line blot and immunoprecipitation assays in 57 myositis sera. J Immunol Methods. 2016;433:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 112] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 12. | Casciola-Rosen L, Nagaraju K, Plotz P, Wang K, Levine S, Gabrielson E, Corse A, Rosen A. Enhanced autoantigen expression in regenerating muscle cells in idiopathic inflammatory myopathy. J Exp Med. 2005;201:591-601. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 276] [Cited by in RCA: 278] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 13. | Hill CL, Zhang Y, Sigurgeirsson B, Pukkala E, Mellemkjaer L, Airio A, Evans SR, Felson DT. Frequency of specific cancer types in dermatomyositis and polymyositis: a population-based study. Lancet. 2001;357:96-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 757] [Cited by in RCA: 675] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 14. | András C, Ponyi A, Constantin T, Csiki Z, Szekanecz E, Szodoray P, Dankó K. Dermatomyositis and polymyositis associated with malignancy: a 21-year retrospective study. J Rheumatol. 2008;35:438-444. [PubMed] |
| 15. | Syrios J, Kechagias G, Xynos ID, Gamaletsou MN, Papageorgiou A, Agrogiannis G, Tsavaris N. Pancreatic adenocarcinoma-associated polymyositis treated with corticosteroids along with cancer specific treatment: case report. BMC Gastroenterol. 2011;11:33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Emslie-Smith AM, Arahata K, Engel AG. Major histocompatibility complex class I antigen expression, immunolocalization of interferon subtypes, and T cell-mediated cytotoxicity in myopathies. Hum Pathol. 1989;20:224-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 243] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 17. | Lu X, Peng Q, Wang G. The role of cancer-associated autoantibodies as biomarkers in paraneoplastic myositis syndrome. Curr Opin Rheumatol. 2019;31:643-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 18. | Yang Z, Lin F, Qin B, Liang Y, Zhong R. Polymyositis/dermatomyositis and malignancy risk: a metaanalysis study. J Rheumatol. 2015;42:282-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 121] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 19. | Kida Y, Maeshima E, Furukawa K, Ichikawa T, Goda M, Ichinose M. A case of polymyositis with a significantly high level of KL-6 associated with pancreatic cancer. Mod Rheumatol. 2007;17:262-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Siddiqui S, Fiorillo M, Tzeng J, Fowkes M. Polymyositis as a paraneoplastic phenomenon of occult pancreatic cancer. Am J Gastroenterol. 2011;106:S220.. |
| 21. | Amroun KL, De Mestier L, Deguelte-Lardiere S, Diebold MD, Bouché O, Kianmanesh R. Pancreas cancer-associated polymyositis of the legs regressing after cephalic duodenopancreatectomy: case report and review of the literature. JOP. 2012;13:674-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 22. | Padniewski JJ, Nelson E, Mian I, Laczniak A, Ives S, Nasr R. Paraneoplastic myopathy in pancreatic cancer: a case report and literature review. J Community Hosp Intern Med Perspect. 2021;11:847-851. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 23. | Decraene F, Van De Mierop F. A rare case of paraneoplastic myositis associated with neuroendocrine carcinoma of the pancreas. Acta Gastroenterol Belg. 2022;85:640-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 24. | Ozcan G, Shaikh A, Becker E, Perosevic N. Not a Statin-Induced Myopathy: Metastatic Pancreatic Adenocarcinoma Presenting As Paraneoplastic Myositis. Cureus. 2022;14:e25016. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 25. | Pentsova E, Liu A, Rosenblum M, O'Reilly E, Chen X, Hormigo A. Gemcitabine induced myositis in patients with pancreatic cancer: case reports and topic review. J Neurooncol. 2012;106:15-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Levy MJ, Kipp BR, Milosevic D, Schneider AR, Voss JS, Avula R, Kerr SE, Henry MR, Highsmith E Jr, Liu MC, Gleeson FC. Analysis of Cell-Free DNA to Assess Risk of Tumoremia Following Endoscopic Ultrasound Fine-Needle Aspiration of Pancreatic Adenocarcinomas. Clin Gastroenterol Hepatol. 2018;16:1632-1640.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 27. | Ghate J, Katsambas A, Augerinou G, Jorizzo JL. Review article: a therapeutic update on dermatomyositis/polymyositis. Int J Dermatol. 2000;39:81-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 28. | Khan FY. Rhabdomyolysis: a review of the literature. Neth J Med. 2009;67:272-283. [PubMed] |
| 29. | Zeng X, Zhang L, Wu T, Fu P. Continuous renal replacement therapy (CRRT) for rhabdomyolysis. Cochrane Database Syst Rev. 2014;CD008566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 30. | Tomaszewski M, Stępień KM, Tomaszewska J, Czuczwar SJ. Statin-induced myopathies. Pharmacol Rep. 2011;63:859-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 131] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 31. | Allison RC, Bedsole DL. The other medical causes of rhabdomyolysis. Am J Med Sci. 2003;326:79-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 100] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 32. | Sharma U. Statin-induced delayed rhabdomyolysis. BMJ Case Rep. 2019;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 33. | Schreiber DH, Anderson TR. Statin-induced rhabdomyolysis. J Emerg Med. 2006;31:177-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 34. | Fah Lee H. I Feel Unergetic. Malays J Emerg Med. 2nd EMAS Meeting Supplementary Issue. Nov 29, 2018. [cited 29 November 2018]. Available from:. |
| 35. | Moriarty N, Moriarty J, Keating J. Contrast-Induced Rhabdomyolysis Occurring after ERCP in a Patient with Pancreatic Cancer: A Case Report. Eur J Case Rep Intern Med. 2020;7:001704. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |